Abstract
A requirement for scaffolding complexes containing internalized G protein-coupled receptors and β-arrestins in the activation and subcellular localization of extracellular signal-regulated kinases 1 and 2 (ERK1/2) has recently been proposed. However, the composition of these complexes and the importance of this requirement for function of ERK1/2 appear to differ between receptors. Here we report that substance P (SP) activation of neurokinin-1 receptor (NK1R) stimulates the formation of a scaffolding complex comprising internalized receptor, β-arrestin, src, and ERK1/2 (detected by gel filtration, immunoprecipitation, and immunofluorescence). Inhibition of complex formation, by expression of dominant-negative β-arrestin or a truncated NK1R that fails to interact with β-arrestin, inhibits both SP-stimulated endocytosis of the NK1R and activation of ERK1/2, which is required for the proliferative and antiapoptotic effects of SP. Thus, formation of a β-arrestin-containing complex facilitates the proliferative and antiapoptotic effects of SP, and these effects of SP could be diminished in cells expressing truncated NK1R corresponding to a naturally occurring variant.
The neuropeptide substance P (SP) interacts with the neurokinin-1 receptor (NK1R) to activate members of the mitogen-activated protein kinase (MAPK) cascade, including extracellular signal-regulated kinases 1 and 2 (ERK1/2) and p38MAPK. These pathways are often activated under different conditions and can lead to both growth and apoptosis (1, 2). The mechanism by which this signal specificity is conveyed is poorly understood, although an emerging role for scaffolding protein complexes that determine the subcellular localization and consequent specificity of signaling proteins may provide an explanation (3–5).
The most commonly studied mechanism by which G protein-coupled receptors (GPCRs) activate MAPK is the release of G protein βγ subunits (3). G protein βγ subunits recruit components of the ras-dependent cascade, such as shc, grb2, and src, leading to the activation of raf-1 and MAP kinase kinase 1, a specific activator of ERK1/2 (6). A component of the MAPK signaling pathway has recently been identified. β-arrestin, originally thought only to mediate receptor uncoupling and internalization, is required for activation of ERK1/2 by a number of GPCRs (4, 5, 7, 8). In the case of proteinase-activated receptor 2 (PAR2), β-arrestin forms a complex with the internalized receptor, raf-1, and ERK1/2, retaining the activated kinases in the cytosol (4). Formation of this complex prevents the typical proliferative effects associated with translocation of ERK1/2 to the nucleus, thereby promoting phosphorylation of cytosolic substrates. Thus, scaffolding complexes can determine the subcellular location and specificity of ERK1/2 and thereby govern the mitogenic potential of a given signal. A different β-arrestin complex, containing the β2-adrenergic receptor (β2-AR) and the tyrosine kinase src, also leads to ERK1/2 activation (5), but this signaling pathway mediates a distinct set of cellular responses, possibly because of different subcellular localization of the activated kinases.
In view of these newly emerging roles for β-arrestin, and its established involvement in endocytosis of NK1R (9, 10), we hypothesized that the ability of SP to activate ERK1/2 depends on the formation of β-arrestin-containing scaffolding complexes. A naturally occurring truncated variant of the NK1R (NK1Rδ325) exhibits impaired SP-induced desensitization and endocytosis, possibly because of an inability to interact with β-arrestin (11, 12). Therefore, we also compared the ability of wild type and truncated NK1R to activate ERK1/2. Our aims were to (i) determine the effect of expressing a dominant β-arrestin and truncated NK1R on SP-mediated ERK1/2 activation; (ii) identify the β-arrestin-containing scaffolding complex; (iii) determine the subcellular localization of activated ERK1/2; and (iv) investigate the physiological consequences of SP-induced ERK1/2 activation.
Materials and Methods
Materials.
Most reagents have been reported (4, 10, 12–14). Cell Death Kit was from Boehringer Mannheim. Dithiobis(succinimidyl propionate) (DSP, crosslinker that is disrupted by reducing SDS/PAGE to allow detection of monomers) was from Pierce. Antibodies to BAX-1 and Bcl2 were from Oncogene Science. β-arrestin1 cDNA was from R. J. Lefkowitz (Duke University, Durham, NC). Other reagents were from Sigma.
Cell Lines.
Kirsten sarcoma virus-transformed rat kidney epithelial cells (KNRK) were from American Type Culture Collection. The following stable cell lines, which have been fully described (10, 12), were used: KNRK-NK1R, expressing rat NK1R with N-terminal Flag; KNRK-NK1Rδ325, expressing FlagNK1R truncated at residue 325; KNRK-NK1R + ARR, coexpressing FlagNK1R and β-arrestin1 with C-terminal enhanced green fluorescent protein (GFP) and KNRK-NK1R + ARR319–418, expressing FlagNK1R and dominant-negative βarrestin319–418 with C-terminal GFP. Human dermal microvascular endothelial cells (HDMEC; J. Ansel, Emory University, Atlanta, GA), which naturally express the NK1R, were maintained for <6 passages (15, 16).
MAPK Assays.
Confluent cells were maintained in MEM without serum overnight and incubated with 10–100 nM SP or [Sar9MetO211]SP for 0–60 min at 37°C. To examine the mechanism of ERK1/2 activation, cells were preincubated with vehicle (control), 100 nM GF109203X, 10 μM genistein, or 20 μM tyrphostin 25 for 20 min. Activation of ERK1/2 was assessed in whole-cell, nuclear, or cytosolic extracts by measuring kinase activity with myelin basic protein as a substrate or by Western blotting using antibodies to phosphorylated ERK1/2 (pERK1/2) (4).
Immunoprecipitation.
Cells were preincubated with 1 mM DSP in PBS for 1 h on ice, neutralized in 200 mM Tris⋅HCl, pH 8.0, for 5 min, and immunoprecipitated as described (4).
Gel Filtration.
Cells were lysed in RIPA buffer (0.15 M NaCl/0.05 M Tris⋅HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS) and fractionated on a column (3 × 125 cm, 375 ml) of S300 Sephacryl preequilibrated in PBS (4). Proteins were eluted in PBS at 1.1 ml/min, and fractions were collected every 5 min. Proteins were precipitated with methanol/chloroform and analyzed by 10% SDS/PAGE followed by Western blotting with antibodies to NK1R (Flag M2 or 94168, to NK1R C terminus), β-arrestin1, src, and ERK1/2 (4, 14). Partition coefficients (σ) and Stoke's radii were calculated (17).
Immunofluorescence and Confocal Microscopy.
Cells were incubated with 10–100 nM SP or fluorescent Alexa 594-SP and fixed in 4% paraformaldehyde (9, 10, 12–14). The NK1R was detected by using Alexa-SP, β-arrestins with GFP, and src by immunofluorescence (1:500, overnight, 4°C). Cells were observed by confocal microscopy (12).
Proliferation Assays.
Cells (104 cells per well in 24-well plates) were grown to 80% confluence, maintained in serum-free medium for 16 h, and incubated with 10–100 nM SP or [Sar9MetO211]SP or 20% FCS for 48 h at 37°C. During the last 20 h, 1 μCi (1 Ci = 37 GBq) [3H]thymidine was added. Incorporation of [3H]thymidine into DNA and cell number were determined (4).
Apoptosis Assays.
Cells were serum-starved for 16 h and incubated in serum-free medium for 24 h with 10–100 nM SP, [Sar9MetO211]SP, or 20 μM U0126. Apoptotic double-stranded DNA fragmentation was determined by terminal deoxynucleotidyltransferase-mediated UTP end labeling (TUNEL) assay according to the manufacturer's instructions for microscopy and flow cytometry. Apoptotic protein expression was determined by Western blotting of cell lysates with antibodies to BAX-1 (1:1,000), Bcl-2 (1:1,000), or actin (1:2,000; loading control) (4).
Statistical Analyses.
Results are expressed as mean ± standard error. Differences were examined by ANOVA and Student–Newman–Keul's test, with P < 0.05 considered significant.
Results
NK1R Activates ERK1/2 by β-Arrestin and Tyrosine Kinase-Dependent Pathways.
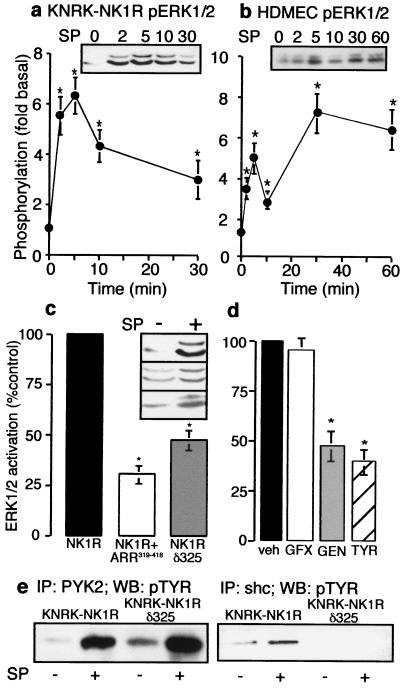
In KNRK-NK1R cells, SP maximally stimulated ERK1/2 phosphorylation by 6.0 ± 0.8-fold (Fig. 1a) and ERK1/2 activity by 5.5 ± 1.0-fold (data not shown) over basal at 5 min. In HDMEC, the NK1R selective agonist [Sar9MetO211]-SP maximally stimulated ERK1/2 phosphorylation by 6.8 ± 0.4-fold at 5 min (Fig. 1b). Thus, NK1R agonists stimulate ERK1/2 in transfected cells and in cells that naturally express the NK1R.
Figure 1.
NK1R-mediated activation of ERK1/2. (a and b) Cells were incubated with 10 nM SP (KNRK) or 100 nM [Sar9MetO211]SP (SM-SP, HDMEC) for 0–60 min. Phosphorylation of ERK1/2 in KNRK-NK1R cells (a) and HDMEC cells (b). (Inset) representative pERK Western blot. *, P < 0.05, as compared with untreated controls, n = 4. (c) KNRK-NK1R, KNRK-NK1R + ARR319–418, and KNRK-NK1Rδ325 cells were treated with 10 nM SP for 5 min and pERK1/2 was determined. (Inset) pERK Western blots of untreated and SP-treated cells (Top, NK1R; Middle, NK1R+ARR319–418; Bottom, NK1R, δ325). *, P < 0.05 as compared with SP-treated KNRK-NK1R cells, n = 3. (d) KNRK-NK1R cells were treated with 10 nM SP for 5 min with vehicle for inhibitors (veh), or after pretreatment with 100 nM GF103209X (GFX), 20 μM genistein (GEN), or 20 μM tyrphostin 25 (TYR) for 20 min, and pERK1/2 was determined. *, P < 0.05 for cells treated with SP-treated controls, n = 3. (e) KNRK-NK1R and KNRK-NK1Rδ325 cells were treated with or without 10 nM SP for 5 min and immunoprecipitated with either PYK2 antibody (Left) or shc antibody (Right), followed by Western blotting with phosphotyrosine (pTYR) antibody.
Expression of β-arrestin319–418 inhibits SP-induced endocytosis of the NK1R, and NK1Rδ325 is endocytosis-defective (10, 12). We investigated the requirement of β-arrestin-dependent endocytosis for SP-stimulated ERK1/2 activation by comparing KNRK-NK1R, KNRK-NK1R + ARR319–418, and KNRK-NK1Rδ325 cells. In comparison to KNRK-NK1R cells, peak ERK1/2 phosphorylation (5 min) was reduced by 75 ± 5% in KNRK-NK1R + ARR319–418, and by 60 ± 2% in KNRK-NK1Rδ325 cells (Fig. 1c). Expression of ARR-GFP did not affect SP-mediated ERK1/2 activation (data not shown). Thus, maximal activation of ERK1/2 at early times requires β-arrestin-dependent internalization. And a truncated, internalization-defective variant of the NK1R, which fails to interact with β-arrestin (N.W.B., unpublished observation), does not fully activate ERK1/2. At later time points (30 min), when ERK1/2 activation was diminished in cells expressing NK1R, ERK1/2 phosphorylation was reduced by 50 ± 4% in KNRK-NK1R + ARR319–418 and by 47 ± 3% in KNRK-NK1Rδ325 cells (data not shown). This diminished difference suggests a β-arrestin-independent mechanism of ERK1/2 activation at later times.
GPCRs activate ERK1/2 by several mechanisms (18–20), including transactivation of receptor tyrosine kinases (20–22) and activation of proline rich tyrosine kinase 2 (PYK2), a tyrosine kinase that leads to shc phosphorylation and ras-activation (19, 23–25). To determine the mechanism used by NK1R, we examined the sensitivity of SP-stimulated ERK activation to GF103209X, a nonspecific protein kinase C inhibitor, genistein, a nonspecific tyrosine kinase inhibitor, and tyrphostin 25, an inhibitor of receptor tyrosine kinases. Genistein and tyrphostin 25 inhibited SP-stimulated ERK1/2 activation at 5 min by 53% and 65%, respectively, but GF109203X had no effect (Fig. 1d). Similar results were observed with NK1Rδ325 (data not shown).
We hypothesized that ERK1/2 activation by SP might involve activation of PYK2 and phosphorylation of the small adaptor protein shc, and that the requirement for internalization might lie downstream of these events. In KNRK-NK1R and KNRK-NK1Rδ325 cells, SP stimulated an ≈3-fold increase in PYK2 phosphorylation at 5 min (Fig. 1e). In cells expressing wild-type NK1R, but not in cells expressing NK1Rδ325, SP stimulated shc phosphorylation (Fig. 1e). Thus, NK1R may use two mechanisms to activate the src/ras pathway leading to ERK1/2 activity, with only one being dependent on shc phosphorylation and receptor internalization.
SP Induces the Formation of a Complex Comprising NK1R, β-Arrestin, src, and ERK1/2.
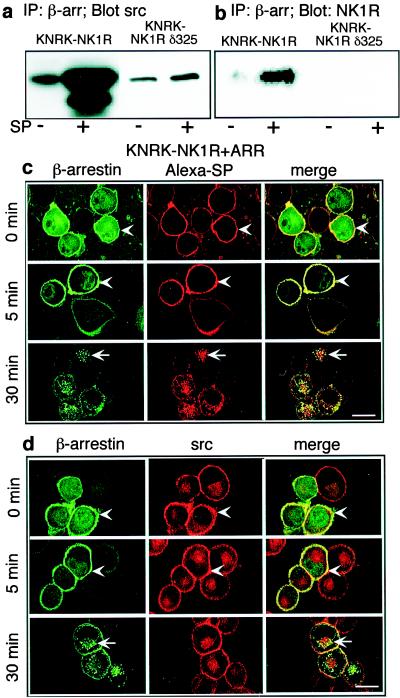
Agonists of PAR2 induce formation of a complex comprising internalized receptor, β-arrestin, raf-1, and pERK1/2, that sequesters activated kinases in the cytosol (4). Agonists of the β2-AR induce formation of a different complex of the receptor, β-arrestin, and src (5). Thus, agonists of different GPCRs lead to the formation of different β-arrestin-containing complexes that could direct substrate specificities in a receptor-dependent fashion. We examined whether SP induced association of β-arrestin with either raf-1 or src by coimmunoprecipitation. When KNRK-NK1R cells were treated with SP for 5 min followed by crosslinking with DSP, src and NK1R could be coprecipitated with β-arrestin (Fig. 2 a and b). β-arrestin and src also coprecipitated to a minor extent in unstimulated cells. In KNRK-NK1Rδ325 cells, SP-induced association of both src and NK1R with β-arrestin was strongly inhibited, suggesting a requirement for receptor internalization in this process (Fig. 2 a and b). Coprecipitation of raf-1 with β-arrestin was not observed (data not shown), suggesting the complex formed in response to SP differs from that observed with activation of PAR2, but resembles that observed for the β2-AR (4, 5)
Figure 2.
(a and b) Association of β-arrestin with src and NK1R. KNRK-NK1R and KNRK-NK1Rδ325 cells were treated with or without 10 nM SP for 5 min, crosslinked with DSP, and immunoprecipitated with β-arrestin (β-arr) antibody, followed by Western blotting with src (a) or NK1R (b) antibodies. (c and d) Localization of β-arrestin, src, and NK1R. KNRK-NK1R + ARR cells were incubated with 100 nM Alexa-SP (c) or 10 nM SP (d) for 60 min at 4°C, washed, and incubated at 37°C for 0–30 min. NK1R was localized by Alexa-SP, β-arrestin with GFP, and src by immunofluorescence with a Texas-red-conjugated secondary antibody. Colocalization is shown in yellow in the merged images. Arrowheads indicate plasma membrane, and arrows indicate intracellular localization. (Scale bar = 10 μm.)
To confirm these findings, and to determine the subcellular distribution of this putative complex, we used confocal microscopy. In unstimulated KNRK-NK1R + ARR cells, NK1R and src were at the plasma membrane and β-arrestin1 was in the cytosol (data not shown and see refs. 9 and 10). SP induced translocation of β-arrestin1 to the plasma membrane (at 0 and 5 min) where it colocalized with Alexa-SP (and thus the NK1R) and src (Fig. 2 c and d). At 30 min, β-arrestin1 and NK1R were confined to the same endosomes, whereas src remained at the plasma membrane. In KNRK-NK1Rδ325, SP did not induce membrane translocation of β-arrestin or rapid endocytosis of the NK1R (data not shown). These results suggest that the putative complex forms at or near the plasma membrane, similar to observations made with the β-arrestin complex formed on activation of PAR2 (4).
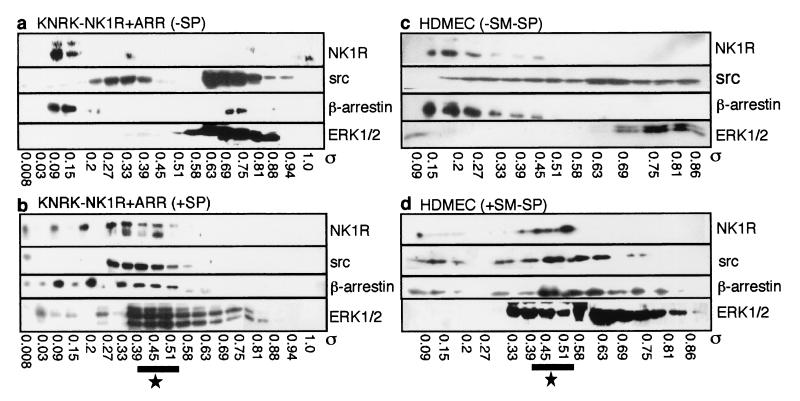
To examine the formation of a scaffolding complex, we treated KNRK-NK1R cells and HDMEC with SP or [Sar9MetO211]SP, respectively, crosslinked proteins with DSP, and fractionated lysates by gel filtration. In the absence of SP, NK1R, β-arrestin, src, and ERK1/2 did not all coelute (Fig. 3 a and c). SP induced a redistribution in the elution profiles of these proteins such that NK1R, β-arrestin, src, and ERK1/2 coeluted between σ 0.33–0.45 in KNRK-NK1R cells and 0.39–0.41 in HDMEC (Fig. 3 b and d). This range corresponds to a Stoke's radius of ≈6 nm or a molecular mass of ≈300 kDa. The size of this complex is considerably larger than would be predicted from the sum of these four proteins, suggesting the presence of other proteins, as observed for PAR2 (4). Both β-arrestin and src eluted in regions other than those containing the complex, suggesting their involvement in other complexes. SP also induced the aggregation of src into very large complexes that eluted in the void volume (data not shown), which explains the apparent quantitative loss of src.
Figure 3.
Gel filtration analysis. Elution profiles from an S300-Sephacryl column of NK1R, src, β-arrestin, and ERK1/2 from KNRK-NK1R + ARR cells (a and b) and HDMEC (c and d). Cells were incubated with (b and d) or without (a and c) 10 nM SP (KNRK cells) or 100 nM [Sar9MetO211]SP (SM-SP, HDMEC) for 5 min, crosslinked with DSP, and fractionated. Western blots of all four proteins in eluted fractions within partition coefficients (σ) of 0.09–0.86 are shown. The region where all four proteins coelute after treatment with NK1R agonists is designated by the star.
SP Induces Nuclear Translocation of Activated ERK1/2 and Proliferation.
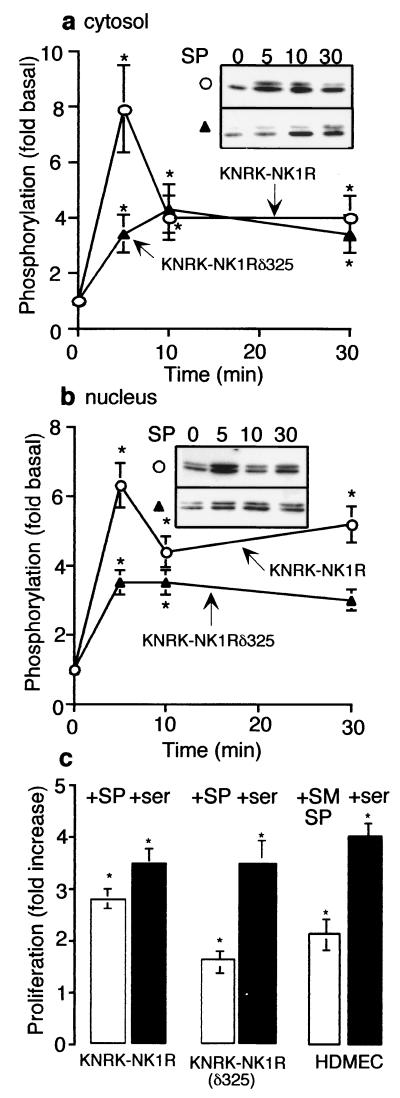
The varying role of β-arrestin in the activation of ERK1/2 by different receptors may ensure the appropriate subcellular localization and substrate specificity of the activated kinases. Therefore, we examined whether the formation of this NK1R/β-arrestin/src complex might direct the subcellular localization of activated ERK1/2 by assaying ERK1/2 after subcellular fractionation. In KNRK-NK1R cells, SP stimulated ERK1/2 phosphorylation in both cytosolic and nuclear fractions (Fig. 4 a and b, ○). In KNRK-NK1Rδ325 cells, nuclear and cytosolic ERK1/2 phosphorylation was reduced by ≈50% at 5 min (Fig. 4 a and b, ▴), similar to that observed in total cellular lysates. Although this difference in the cytosol was lost after 30 min, it persisted in the nucleus. Thus, in contrast to PAR2, the NK1R/β-arrestin/src complex does not sequester activated ERK1/2 in the cytosol (4).
Figure 4.
Nuclear translocation of ERK1/2. (a and b) pERK1/2 in KNRK-NK1R (○) and KNRK-NK1Rδ325 (▴) cells after treatment with 10 nM SP for 0–30 min in cytosolic fractions (a) and nuclear fractions (b). (Inset) pERK Western blots. (c) -Fold increase in [3H]thymidine incorporation into newly synthesized DNA of KNRK-NK1R, KNRK-NK1Rδ325, and HDMEC cells after 48 h with 10 nM SP (KNRK cells), 100 nM [Sar9MetO211]SP (SM-SP, HDMEC), or 20% serum (ser), as compared with untreated cells. *, P < 0.05 as compared with untreated cells, n = 3 (a and b) and 4 (c).
Because nuclear translocation of ERK1/2 may induce transcription of mitogenic genes, we examined whether SP stimulated [3H]thymidine incorporation into newly synthesized DNA and cell proliferation in KNRK-NK1R and KNRK-NK1Rδ325 cells. In both cell lines, serum stimulated an ≈3-fold increase in proliferation (Fig. 4c) and cell number (data not shown). However, whereas SP stimulated a 2.8 ± 0.4-fold increase in [3H]thymidine incorporation and a 3.0 ± 0.6-fold increase in cell number in KNRK-NK1R cells, SP stimulated only a 1.6 ± 0.4-fold increase in [3H]thymidine incorporation and a 1.5 ± 0.2-fold increase in cell number in KNRK-NK1Rδ325 cells (Fig. 4c). Because KNRK cells are ras-transformed, we confirmed the proliferative effects of [Sar9MetO211]SP in HDMEC. Treatment of HDMEC with [Sar9MetO211]SP resulted in a 2.1 ± 0.3-fold increase in [3H]thymidine incorporation and a 2.0 ± 0.3-fold increase in cell number, suggesting that the observed proliferative effects of SP are not artifacts of a ras-transformed cell line.
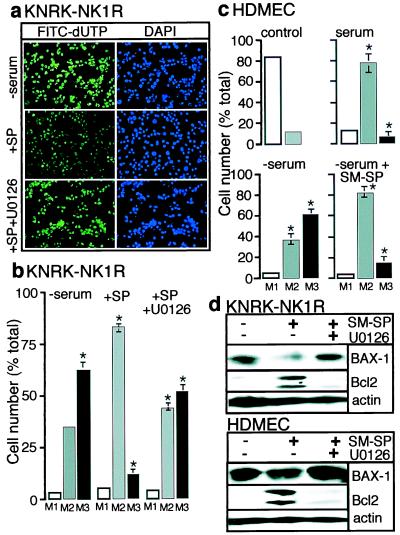
Although SP stimulates proliferation of endothelial cells, nondividing cells such as neurons also express the NK1R. Therefore, we addressed the possibility that ERK1/2 activation by NK1R might serve to protect cells from apoptosis, using a TUNEL assay to determine DNA fragmentation. KNRK-NK1R cells were serum-starved for 16 h and then maintained without serum for another 24 h in the presence or absence of SP or the MAP kinase kinase 1 inhibitor, U0126. Whereas prolonged serum withdrawal induced apoptosis (Fig. 5a Top), SP decreased the apoptotic response (Fig. 5a Middle). U0126 reversed the protective effect of SP (Fig. 5a Bottom), suggesting this effect is mediated by activation of ERK1/2. DAPI staining of total nuclei (Fig. 5 Right) showed that the observed decrease in FITC-labeled nuclei was not caused by a decrease in cell number. We used flow cytometry to quantify apoptosis. In this analysis, M1 represents background fluorescence, M2 the basal fluorescence of control cells grown with serum, and M3 the apoptotic cells as determined by a shift in the mean fluorescence with respect to the controls. In the presence of serum, >80% of cells were in M2 (data not shown). After serum withdrawal, 70 ± 5% of the cells shifted to M3, indicating apoptosis (Fig. 5b). SP reduced the number of cells in M3 by 60 ± 5%. This effect was reversed by U0126. This protective ability of NK1R agonists was confirmed in HDMEC. Serum starvation induced a 50% increase in apoptosis that was reversed by [Sar9MetO211]SP (Fig. 5c).
Figure 5.
Apoptosis assays. (a) KNRK-NK1R cells were serum-starved for 16 h, followed by continued serum starvation for 24 h in the absence (Top) or presence (Middle) of 10 nM SP, or in the presence of 10 nM SP and 20 μM U0126 (Bottom). TUNEL assay shows FITC-labeled Br-dUTP incorporation into apoptotic nuclei of cells (Left), and the 4′6-diamidino-2-phenylindole (DAPI) staining reveals nuclei (Right). (b) Fluorescence-activated cell sorter (FACS) analysis of data shown in a as a percentage of total cells (y axis) in each of three groups: M1 (no fluorescence), M2 (basal fluorescence, healthy cells), and M3 (high fluorescence, apoptotic cells). (c) HDMEC were serum-starved for 24 h in the absence or presence of 100 nM[Sar9MetO211]SP (SM-SP). FACS analysis was as in b. Control shows background fluorescence. (d) KNRK-NK1R cells or HDMEC were treated as described in a and c, and expression of proapoptotic BAX-1, antiapoptotic Bcl-2, and actin (control) was determined by Western blotting. *, P < 0.05, as compared with serum controls, n = 3 (b and d).
We examined the possibility that SP might regulate the expression of anti- and proapoptotic protein such as Bcl-2 and Bax-1, respectively, in an ERK1/2-dependent fashion (26–29). In KNRK-NK1R cells and HDMEC, serum starvation resulted in low levels of Bcl-2 expression and high expression of BAX-1 (Fig. 5d). In KNRK-NK1R cells, SP stimulated Bcl-2 expression and inhibited BAX-1 expression, and U0126 prevented these effects. In HDMEC, [Sar9MetO211]SP stimulated Bcl-2 expression and slightly inhibited BAX-1 expression, and U0126 prevented these effects (Fig. 5d). Actin expression levels remained constant, showing that this effect on apoptotic gene expression was not attributable to an effect on overall protein levels. Thus, SP induces expression of antiapoptotic genes and protects against apoptosis in an ERK1/2-dependent manner.
Discussion
Our results using transfected KNRK cells and endothelial cells that naturally express the NK1R show that NK1R agonists strongly activate ERK1/2 by a β-arrestin-dependent mechanism. SP induces the formation of a multiprotein complex containing NK1R, β-arrestin, src, and ERK1/2, which forms close to the plasma membrane. Once activated, ERK1/2 translocate to the nucleus to induce proliferation and to protect from apoptosis. A truncated, desensitization- and internalization-defective mutant of the NK1R that does not interact with β-arrestin and that may correspond to a naturally occurring variant shows impaired ability to activate ERK1/2. Thus, the capacity of SP to affect proliferation and apoptosis in vivo may depend on whether cells express full length or truncated variants of the NK1R.
SP Induces the Formation of a Multiprotein Scaffolding Complex.
SP induced the formation of a complex comprising NK1R, β-arrestin, src, and ERK1/2, identified by immunoprecipitation, gel filtration, and confocal microscopy. This complex resembles that formed in HEK293 cells expressing the β2-AR (5). In contrast, the complex that forms in response to PAR2 agonists comprises PAR2, β-arrestin, raf, and pERK, and results in cytosolic retention of ERK1/2 (4). β-arrestin is an integral component of these complexes and contains domains that bind phosphorylated receptors, clathrin, src, and, possibly, raf (4, 5). However, the requirement for β-arrestin-dependent endocytosis in ERK1/2 activation differs between receptor types. This variation is independent of cell type, because the two receptors (NK1R and PAR2) expressed in the same cell line (KNRK) induce the formation of distinct scaffolding complexes.
SP-Induced ERK1/2 Activation Is Facilitated by β-Arrestin-Mediated Receptor Endocytosis.
Our results suggest that the NK1R couples to two pathways of ERK1/2 activation. One pathway depends on β-arrestin-mediated receptor endocytosis, as SP-stimulated ERK1/2 activation was inhibited by expression of dominant-negative β-arrestin or internalization-defective NK1Rδ325. Activation of ERK1/2 by both wild-type and truncated NK1R also depended on tyrosine kinases and transactivation of receptor tyrosine kinases, but was independent of protein kinase C, and resulted in PYK2 phosphorylation. However, both of these events were diminished in cells expressing NK1Rδ325. The truncated receptor was also incapable of forming a complex with β-arrestin and src, suggesting that this complex might serve to link the receptor to the ras-dependent pathway of ERK1/2 activation, perhaps potentiating its activation and nuclear translocation. The activation of nuclear ERK1/2 was delayed in the case of NK1Rδ325, which is consistent with the concept of two separate mechanisms of activation. The pathway used by this naturally occurring truncated receptor to activate ERK1/2 and the physiological outcomes of this activation remain to be determined.
Functional Consequences of SP-Mediated ERK1/2 Activation.
An important question with respect to the present studies is: What is the functional significance of the newly emerging role for β-arrestin-dependent scaffolding complexes in ERK1/2 activation? The experiments shown here, and studies on the β2-AR, suggest that the formation of a receptor/β-arrestin/src complex directs activation and nuclear translocation of ERK1/2, leading to the proliferative effects associated with activation of some GPCRs (30). In the case of PAR2, formation of a receptor/β-arrestin/raf/ERK1/2 complex leads to cytosolic retention of activated kinases and promotes the nonproliferative effects associated with that receptor (4), including actin–cytoskeleton rearrangements (K.A.D. and N.W.B., unpublished observations). In contrast to the NK1R, where an internalization- and desensitization-defective mutant exhibits diminished ability to activate ERK1/2, a comparable PAR2 mutant strongly activates ERK1/2, resulting in nuclear translocation and proliferation (4). Although the function of the complex formed in the case of PAR2 may be cytosolic retention of ERK1/2, the purpose of the complex formed in the case of the NK1R seems to be potentiation of the tyrosine kinase-dependent pathway of ERK1/2 activation. Dimerization is a prerequisite for nuclear translocation of ERK1/2 (31), an event required for proliferative effects of ERK1/2 activation (32). In SP-treated cells, a considerable portion of the ERK1/2 redistributed to partition coefficients of ≈0.6 nm Stoke's radius, which would be consistent with the formation of ERK1/2 dimers, and complex formation may promote dimerization. Because the C termini of PAR2 and NK1R are important for complex formation, perhaps by serving as scaffolds for the assembly of other proteins, it will be of interest to determine whether the C-terminal tails of GPCRs can confer substrate specificity onto activated ERK1/2.
For the full-length NK1R, formation of a β-arrestin-containing complex allows nuclear translocation of ERK1/2, proliferation, and protection from apoptosis. In support of these findings, agonists of the NK1R stimulate proliferation of endothelial cells and protect thymocytes from glucocorticoid-induced apoptosis in vitro and in vivo (33, 34). These events may be of importance during neurogenic inflammation, when SP is released from the peripheral endings of primary spinal afferent neurons. However, SP is less able to activate these pathways in cells expressing truncated NK1R, suggesting that the effects of SP depend on whether cells express full-length or truncated NK1R. Moreover, the antiapoptotic effects of SP occur only when cells are arrested in G1/S and an opposing MAPK cascade is activated in actively dividing cells, leading to a proapoptotic effect (K.A.D. and N.W.B., unpublished observations). Future studies will need to investigate the possibility that a β-arrestin scaffolding complex can serve as a molecular switch for activation of proliferative or apoptotic pathways under different conditions.
Acknowledgments
This paper is dedicated to our friend and colleague John Walsh (University of California, Los Angeles), who died on June 14, 2000. We thank Drs. J. Ansel (Emory University) for HDMEC, R. J. Lefkowitz (Duke University) for antibodies to β-arrestin and β-arrestin cDNA, J. Zalevsky for help with gel filtration analysis, S. K. Böhm for preparing KNRK-NK1Rδ325 cells, and Paul Dazin for help with flow cytometry. This work was supported by National Institutes of Health Grant DK39957.
Abbreviations
- SP
substance P
- GPCR
G protein-coupled receptor
- NK1R
neurokinin-1 receptor
- PAR2
proteinase-activated receptor 2
- β2-AR
β2-adrenergic receptor
- MAPK
mitogen-activated protein kinase
- ERK1/2
extracellular signal-regulated kinases 1 and 2
- pERK
phosphorylated ERK
- KNRK
Kirsten sarcoma virus-transformed rat kidney epithelial cells
- HDMEC
human microvascular dermal endothelial cells
- GFP
green fluorescent protein
- DSP
dithiobis(succinimidyl proprionate)
- PYK2
proline rich tyrosine kinase 2
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.190276697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.190276697
References
- 1.Horstmann S, Kahle P J, Borasio G D. J Neurosci Res. 1998;52:483–490. doi: 10.1002/(SICI)1097-4547(19980515)52:4<483::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Xia Z, Dickens M, Raingeaud J, Davis R J, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 3.Daaka Y, Luttrell L M, Lefkowitz R J. Nature (London) 1997;390:88–91. doi: 10.1038/36362. [DOI] [PubMed] [Google Scholar]
- 4.DeFea K A, Zalevsky J, Thoma M S, Déry O, Mullins R D, Bunnett N W. J Cell Biol. 2000;148:1267–1282. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luttrell L M, Ferguson S S, Daaka Y, Miller W E, Maudsley S, Della Rocca G J, Lin F, Kawakatsu H, Owada K, Luttrell D K, et al. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 6.van Biesen T, Hawes B E, Luttrell D K, Krueger K M, Touhara K, Porfiri E, Sakaue M, Luttrell L M, Lefkowitz R J. Nature (London) 1995;376:781–784. doi: 10.1038/376781a0. [DOI] [PubMed] [Google Scholar]
- 7.Ignatova E G, Belcheva M M, Bohn L M, Neuman M C, Coscia C J. J Neurosci. 1999;19:56–63. doi: 10.1523/JNEUROSCI.19-01-00056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vögler O, Nolte B, Voss M, Schmidt M, Jakobs K H, van Koppen C J. J Biol Chem. 1999;274:12333–12338. doi: 10.1074/jbc.274.18.12333. [DOI] [PubMed] [Google Scholar]
- 9.McConalogue K, Corvera C U, Gamp P D, Grady E F, Bunnett N W. Mol Biol Cell. 1998;9:2305–2324. doi: 10.1091/mbc.9.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McConalogue K, Déry O, Lovett M, Wong H, Walsh J H, Grady E F, Bunnett N W. J Biol Chem. 1999;274:16257–16268. doi: 10.1074/jbc.274.23.16257. [DOI] [PubMed] [Google Scholar]
- 11.Li H, Leeman S E, Slack B E, Hauser G, Saltsman W S, Krause J E, Blusztajn J K, Boyd N D. Proc Natl Acad Sci USA. 1997;94:9475–9480. doi: 10.1073/pnas.94.17.9475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Böhm S K, Khitin L M, Smeekens S P, Grady E F, Payan D G, Bunnett N W. J Biol Chem. 1997;272:2363–2372. doi: 10.1074/jbc.272.4.2363. [DOI] [PubMed] [Google Scholar]
- 13.Grady E F, Garland A G, Gamp P D, Lovett M, Payan D G, Bunnett N W. Mol Biol Cell. 1995;6:509–524. doi: 10.1091/mbc.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grady E F, Baluk P, Böhm S, Gamp P, Wong H, Payan D G, Ansel J, Portbury A L, Furness J B, McDonald D M, et al. J Neurosci. 1996;16:6975–6986. doi: 10.1523/JNEUROSCI.16-21-06975.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinlan K S, Song I S, Naik S M, Letran E L, Olerud J E, Bunnett N W, Armstrong C A, Caughman S W, Ansel J C. J Immunol. 1999;162:1656–1661. [PubMed] [Google Scholar]
- 16.Quinlan K L, Song I S, Bunnett N W, Letran E, Steinhoff M, Harten B, Olerud J E, Armstrong C A, Wright Caughman S, Ansel J C. Am J Physiol. 1998;275:C1580–C1590. doi: 10.1152/ajpcell.1998.275.6.C1580. [DOI] [PubMed] [Google Scholar]
- 17.Chun P W, Kim S J, Stanley C A, Ackers G K. Biochemistry. 1969;8:1625–1632. doi: 10.1021/bi00832a044. [DOI] [PubMed] [Google Scholar]
- 18.Della Rocca G J, van Biesen T, Daaka Y, Luttrell D K, Luttrell L M, Lefkowitz R J. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 19.Della Rocca G J, Maudsley S, Daaka Y, Lefkowitz R J, Luttrell L M. J Biol Chem. 1999;274:13978–13984. doi: 10.1074/jbc.274.20.13978. [DOI] [PubMed] [Google Scholar]
- 20.Luttrell L M, van Biesen T, Hawes B E, Koch W J, Krueger K M, Touhara K, Lefkowitz R J. Adv Second Messenger Phosphoprotein Res. 1997;31:263–277. [PubMed] [Google Scholar]
- 21.Schönwasser D C, Marais R M, Marshall C J, Parker P J. Mol Cell Biol. 1998;18:790–798. doi: 10.1128/mcb.18.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zwick E, Wallasch C, Daub H, Ullrich A. J Biol Chem. 1999;274:20989–20996. doi: 10.1074/jbc.274.30.20989. [DOI] [PubMed] [Google Scholar]
- 23.Blaukat A, Ivankovic-Dikic I, Grönroos E, Dolfi F, Tokiwa G, Vuori K, Dikic I. J Biol Chem. 1999;274:14893–14901. doi: 10.1074/jbc.274.21.14893. [DOI] [PubMed] [Google Scholar]
- 24.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. Nature (London) 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 25.Eguchi S, Iwasaki H, Inagami T, Numaguchi K, Yamakawa T, Motley E D, Owada K M, Marumo F, Hirata Y. Hypertension. 1999;33:201–206. doi: 10.1161/01.hyp.33.1.201. [DOI] [PubMed] [Google Scholar]
- 26.Basu A, Haldar S. Mol Hum Reprod. 1998;4:1099–1109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- 27.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 28.Park D S, Stefanis L, Yan C Y I, Farinelli S E, Greene L A. J Biol Chem. 1996;271:21898–21905. doi: 10.1074/jbc.271.36.21898. [DOI] [PubMed] [Google Scholar]
- 29.Deckwerth T L, Elliott J L, Knudson C M, Johnson E M, Jr, Snider W D, Korsmeyer S J. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 30.Gutkind J S. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 31.Khokhlatchev A V, Canagarajah B, Wilsbacher J, Robinson M, Atkinson M, Goldsmith E, Cobb M H. Cell. 1998;93:605–615. doi: 10.1016/s0092-8674(00)81189-7. [DOI] [PubMed] [Google Scholar]
- 32.Lenormand P, Brondello J M, Brunet A, Pouysségur J. J Cell Biol. 1998;142:625–633. doi: 10.1083/jcb.142.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ziche M, Morbidelli L, Pacini M, Geppetti P, Alessandri G, Maggi C A. Microvasc Res. 1990;40:264–268. doi: 10.1016/0026-2862(90)90024-l. [DOI] [PubMed] [Google Scholar]
- 34.Dimri R, Sharabi Y, Shoham J. J Immunol. 2000;164:2479–2486. doi: 10.4049/jimmunol.164.5.2479. [DOI] [PubMed] [Google Scholar]