Abstract
A potent link to dairy seems to exist for three hormone-responsive glands. Acne, breast cancer and prostate cancer have all been linked epidemiologically to dairy intake. Although mechanisms postulated here remain to be accurately defined, the likely link involves Insulin-like Growth Factor-1 as a general stimulant, synergized by the steroid hormones present in milk. The IGF-1 may be either absorbed from milk, or stimulated by its ingestion, or both. The 5alpha-reduced compound 5alpha-pregnanedione (5α-P) present in milk is a direct precursor of dihydrotestosterone and may act through that pathway in prostate cancer, but 5α-P has also recently been shown to be capable of inducing estrogen receptors in breast cancer cells, upregulating cancer cells' sensitivity to estrogen. The introduction of exogenous hormones and growth factors into tissues that have not evolved defensive feedback inhibition of their corresponding endogenous sources is postulated as a direct stimulatory threat to these organ systems, whether for hyperplasia or neoplasia.
Key words: acne, breast cancer, dairy, IGF-1, milk, 5α-pregnanedione, prostate cancer
Over the past 35 years, parts of a highly important jigsaw puzzle have gradually come to light. While there are still a few pieces missing, there is enough to suggest a working hypothesis that links dairy consumption, acne, breast cancer and prostate cancer.
Early Pieces of the Puzzle
The first piece of the puzzle was the product of an investigation in the early 1970s by Janet Darling of the Royal Hospital for Sick Children in Edinburgh, Scotland into the cause of neonatal jaundice.1 She postulated that there might be hormones involved in the pathogenesis of the disorder and this led her to develop an assay of the hormones in both human and cow milk.2 In short, she demonstrated (and quantitated within the limits of that era's technology) progesterone, 5α-androstanedione (5α-A) and 5α-pregnanedione (5α-P) in cow milk.3
This finding was of interest to me because I had been looking into the possible association of various ingestants and acne among patients in my private dermatology consulting practice. My original interest was in halogens but after a few years of patient interviews, although no suggestive relationship to iodides or bromides had appeared, the association of acne with dairy by clinical history was becoming obvious and I had begun to look for an underlying molecular cause. That took me to an article in Time magazine, published several years before Dr. Darling's discovery, which described work done by Dr. Jerome Fisher of Pasadena, California.4 He had interviewed over 1000 consecutive acne patients and had drawn the same conclusion, that dairy intake paralleled the acne severity of his patients. I contacted Dr. Fisher, he sent me the carbon copies of his paper5 (presented to the American Dermatological Association in 1966), and he told me of his suspicions of hormones in milk and of his unsuccessful attempts to identify and measure the hormones.
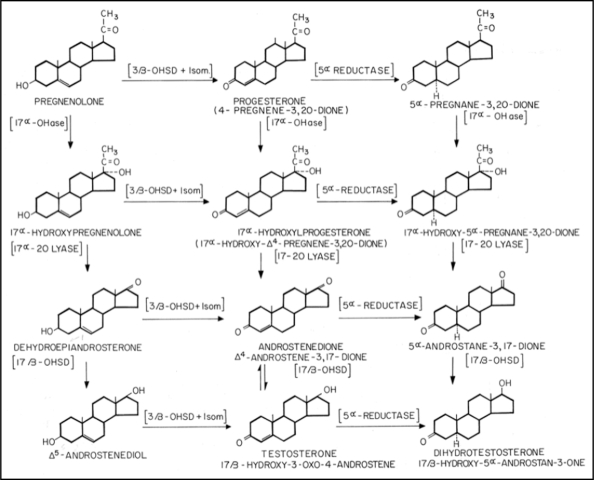
A search into the endocrine pathways that lead from cholesterol through five enzymatic steps to dihydrotestosterone (DHT) revealed that Dr. Darling's hormones (progesterone, 5α-A and 5α-P) were all part of the DHT production pathway (Fig. 1) and so could conceivably be metabolized to DHT right in the pilosebaceous unit, if the necessary enzymes were present.
Figure 1.
The location of milk-sourced steroid hormones in the dihydrotestosterone (DHT) production cascade.
In his PhD thesis for the University of Glasgow in 1970, Dr. K.C. Calman used autoradiographic techniques to localize the first of these necessary enzymes (17β-hydroxysteroid dehydrogenase) in the infundibulum and in the sebaceous gland of the pilosebaceous unit.6 This work was later expanded and partly confirmed by Dr. Diane Thiboutot, who fully described the intracrine metabolism of the sebaceous gland and confirmed the presence of the required enzymes therein but did not extend her studies to the enzymes in the infundibular epithelium.7
The Early Synthesis
By 1982 we had a source of hormones; a known metabolic pathway in which these hormones are metabolized; the presence of that pathway in the tissues that are central to acne; and a final common molecule that is generally accepted as both the most androgenic of androgens and the preferred agonist to act upon the androgen receptors that ultimately drive acne itself.
Subsequently, we have learned that anti-androgens that act at the level of the nuclear androgen receptor can block the effects of DHT, indeed spironolactone and drospirenone are in daily clinical use for acne management.8 The ability to block acne by blocking the action of DHT lends obvious weight to DHT's central role in acnegenesis.
So what is missing as ‘proof of concept’? Among others, we lack proof of absorption of these hormones from ingested dairy products in the bowel into the human bloodstream (or lymphatics) and subsequent metabolism of these molecules by the pilosebaceous unit enzymes to DHT, with a consequent increase in functional activity of the gland and the subsequent plugging of the pilary canal. It would be facile to argue that this experiment is performed in our patients every day—all one needs to do is take a history to learn that extensive back acne is almost exclusively due to excess dairy intake, but not always milk—one must consider other dairy excesses. The problem here is that science requires more exacting data, and that is where life gets difficult. The fact is that there is simply no way to perform double-blind dairy intake studies because a ‘control’ ingestant (real dairy without the offending hormones) does not exist—and what dairy substitutes do exist are not going to fool any teenage dairy lover.
In addition, one of the links in this chain of proof, the question of absorption of bovine hormones into the body in amounts necessary to have a physiological (or pharmacological) effect, is debated. Indeed lack of belief that such steroids were absorbed in this fashion led reviewers to reject Fisher's paper over 40 years ago. (Personal communication).
Proof that will satisfy all critics will have to wait until such hormone-free milk and dairy counterfeits are available for testing on an acne-prone population. And that brings up another highly important factor—genetics. There is no point in testing such experimental diets on an unselected population; patients without the genetic ability to form acne lesions are not appropriate subjects for such testing. A demonstrated ability to form comedones will be the target criterion, perhaps with studies commencing in adrenarche dealing with pre-pubertal children with the earliest signs of plugged pores. A second population might be those who have already developed active acne but could see clearing with an appropriate diet. The third population might be those cleared with isotretinoin and returned in a blinded fashion to real and hormone-depleted milk. An attempt by a Harvard team to recruit for exactly this last study failed: once cleared with isotretinoin, patients are notably and understandably reluctant to volunteer for a double-blind study that would expose them knowingly (as a necessary result of the informed consent process) to a possible acnegen. No published reference to this uncompleted study, unfortunately but understandably, is available.
Breast and Prostate Cancers
Looking into other hormone-responsive glands leads to epidemiologic and molecular research studies linking dairy to malignancies of the prostate and breast. The epidemiological evidence linking dairy and prostate is fairly strong; that linking dairy and breast cancer is still widely debated; but no direct dietary trials have been done, nor even started, for either malignancy. Nevertheless the epidemiologists continue to shed some light on the picture with a recent prospective Japanese trial adding weight to the association of dairy and prostate cancer and eliminating some of the potential etiological molecules suggested in the past.9
There are in addition some indirect lines of research and thinking that point toward the influence of hormones. The mechanism of stimulation of prostate gland activity has been well-defined by Labrie and the enzymatic pathways (the intracrine system) present in the pilosebaceous unit are also present in prostate gland,10 leaving it open to stimulation by the same milk/dairy-sourced hormones and DHT precursors that likely stimulate acne. We are learning that reducing the amount of DHT produced in a prostate cancer patient through inhibition of the action of 5α-reductase will control the disease to a certain extent.11 Dutasteride, a potent, selective, irreversible inhibitor of type 1 and type 2 5α-reductase, seems made for the job.12 But efficient though it is in reducing endogenous DHT, the 5α-P and 5α-A in dairy products provide the prostate's intracrine system with DHT precursors that are already 5α-reduced. By extension, the dairy-laden diet that stimulates acne, and which becomes an increasing proportion of the diet of aging prostate cancer victims in failing health, should be re-considered. 5α-free protein, perhaps available as soy-derived protein powder, deserves a trial in this population as a substitute for dairy.
In breast cancer, the story is somewhat more complex, but interesting data have come from re-examination of the Women's Health Initiative data and commentary. Although it was not part of the discussion and analysis in the original paper describing the reasons for stopping part of the trial,13 subsequent information revealed that the increase in breast cancer came not from a link to ‘estrogens’ alone as originally widely reported but to ‘progestins’ added to the estrogens, the former being a euphemism for the compound actually used in the study, not true progesterone USP but Ayerst-Wyeth's patented androgenic progestin marketed as medroxyprogesterone acetate (MPA) (Provera®).14
Cyclic Influences on Breast Tissue
Linking androgenic progestins to the induction and/or promotion of breast cancer is a significant leap from the constant blaming of estrogens as the cause of this malignancy, but there are some reasons to look at this possibility more closely. All progestins used in oral contraceptives, until the arrival of drospirenone, have been androgenic.15,16 Studies have demonstrated the highly androgenic nature of MPA17 and its influence on breast cancer tissue culture in mice.18 While there is a demonstrated place for the estrogen receptor in the chain of events that leads to breast cancer, the simple existence of such a receptor by itself is not likely to be adequate. It must be part of a functional stimulative system. Estrogen receptors, after all, are present in many tissues and few have had their malignancies reliably linked to estrogen levels. As a case in point, melanocytic nevi have estrogen receptors and melanomas have been linked epidemiologically to breast cancer.19
An hypothesis presented in the early 1980s proposed that an ‘estrogen window’ (actually two such windows) exists that exposes estrogen receptors in breast tissue to unopposed cyclic estrogens from the ovaries, repeatedly stimulating the breasts, inducing changes that lead to breast cancer.20–22 This left unquestioned the influence or importance of progesterone receptors and the effects of cyclic progesterone. The fact is that breast tissue stimulation is much more obvious during the progesterone load of the late luteal phase than earlier in the cycle under increasing estrogens. In short, one must wonder why we have been blaming cyclic ovarian estrogen when ovarian progesterone and its metabolites may be a more logical cause of irregularities, both benign and malignant, in breast growth. Clinically, we observe that the use of the non-androgenic and anti-androgenic progestin drospirenone smoothes out these breast swelling episodes very nicely indeed, strongly suggesting again the androgenic effect on breast activity of cyclic ovarian progesterone.23
A paper from the mid-1980s may hold the answer to this relationship. Backstrom demonstrated that the human corpus luteum secretes 5α-P, cyclically and in significant quantities.24 This is one of the 5α-reduced DHT precursors that Darling found in cow milk, and Backstrom showed that the luteal phase of each menstrual cycle was associated with about an 8-fold rise in this hormone above the follicular phase level. Thus, we have a late-cycle DHT precursor, capable of becoming an androgenic hormone, available to the circulation and thus to mammary gland tissue, for up to four decades as women pass through their reproductive lifespan.
Lack of Feedback Inhibition
A short aside needs to be taken at this point to introduce a concept likely to be very important in this whole discussion, the question of feedback inhibition. It is understood that estradiol-17-β and progesterone, the two natural ovarian hormones, operate in a classic feedback system regulated by the blood levels of the active forms of these hormones, and their feedback loops are ‘administered’ by the hypothalamus and pituitary. The level of each of these active natural human hormones is thus under constant and natural constraint.
This is, however, apparently not so for DHEAS, androstenedione, testosterone and DHT. Their control is a somewhat looser mix involving cortisol, cortisol releasing factor (CRF) and ACTH as one arm of the control acting through the adrenal cortex; luteinizing hormone (LH) acting through the ovaries and testes; and perhaps other as yet undefined mediators, perhaps even a postulated cortical androgen stimulating hormone.25
In essence, if a feedback regulatory system exists, it needs to rely on serum DHT (or perhaps T) levels for accurate modulation. No such system has been discovered to date. To function effectively, it would need to recognize when a tissue that utilizes DHT has drawn down the supply of serum T. The lower level of serum T or DHT would signal the hypothalamic production of Androtropin Releasing Factor (ARF), stimulating the release of appropriate androgen-controlling messengers such as the postulated (but unproven) pituitary Cortical Androgen Stimulating Hormone (CASH) to the adrenals. LH to the ovaries and testes would be released concurrently, and so more T would be produced by adrenal, ovaries and testes, and the need satisfied.
Conversely, if there were adequate DHT downstream from the 5α-reductase (from whatever source, including exogenous ones such as dairy), there would be no drawdown of T or DHT from the serum and so no more T production would be signaled.
So how does dairy consumption relate to the induction of abnormal cellular activity in target tissues? I suggest that a problem arises when this incomplete (or non-existent) feedback system is bypassed, for example when the system is invaded by DHT precursors in dairy products. Some of the DHT precursors are already 5α-reduced so they need not go to 5α-reductase for reduction to DHT. This supply of DHT does not rely on serum T, so endogenous serum T is not consumed, serum T stays high, and even if this steady level of serum T and DHT signals no need for further production of more T, it matters little because the cow-sourced precursors, progesterone and both 5α-A and 5α-P, can go directly to the production of more DHT without any negative feedback influence from the hypothalamus and pituitary.
Evolution By-Passed
The human endocrine system, not having evolved under the influence of ingested dairy and other exogenous hormones and growth factors, is not equipped to cope with such a ‘sneak attack’. We simply have not yet evolved a way of coping with these unexpected, unwanted, unnecessary and highly potent DHT precursors. The unprotected and non-discriminating enzymes of the intracrine systems simply do what they are programmed to do—they make more DHT when presented with precursor molecules. To make matters worse, the increase in ‘product’ stimulated by these hormones results in larger sebaceous (and other) glands and so even more of the target tissue itself, with its own increased complement of intracrine enzymes and androgen receptors, effecting an auto-multiplier influence on sebaceous, prostate and mammary glandular tissue.
This system is highly likely to remain the way it has evolved because of the antagonistic pleiotropy theory of aging, which asserts that selection for early reproductive success permits deleterious effects on fitness in later life because genes that are operative during development are also operative in later life, but exert different effects on health.26 Indeed, because the lack of negative feedback ensures maintenance of the drive to procreation mediated by testosterone, the present evolutionary state (without a mechanism of limiting free T) may already represent the desirable ultimate of evolution as far as propagation of the species is concerned.
5α-P and Breast Cancer
Now to this playing field comes a new player, a researcher who has begun to unlock the complex interactions between androgenic progestins and estrogens in the breast. Working in breast cancer cell cultures, Dr. Wiebe at the University of Western Ontario has further unraveled some of the mysteries. In essence, he has demonstrated that the estrogen receptors on breast tissue (which respond to estradiol-17β) are capable of upregulation by 5α-pregnane-3,20-dione (5α-P), which through this mechanism exhibits marked mitogenic and metastatic properties. On the other hand, progesterone metabolites, 4-pregnen-3α-ol-20-one (3α-hydroxyprogesterone or 3αHP) and 4-pregnen-20α-ol-3-one (20α-hydroxyprogesterone), oppose these actions.
Wiebe states “Current estrogen-based theories and therapies apply to only a fraction of all breast cancers; the majority (about two-thirds) of breast cancer cases are estrogen-insensitive and have lacked endocrine explanations. As the progesterone metabolites, 5α-P and 3α-HP, have been shown to act with equal efficacy on all breast cell lines tested, regardless of their tumorigenicity, estrogen sensitivity and estrogen receptor/progesterone receptor status, it is proposed that they offer a new hormonal basis for all forms of breast cancer.”27
Is this is the link between dairy 5α-P, ovarian 5α-P and breast cancer? The existence of the mitogenic and stimulative 5α-P in cow milk is only half the story—the other half is the lack of 3α-HP, the benign antagonist, in cow milk. Other questions involve learning whether a similar upregulation of (estrogen and other) receptors exists in all breast tumors, or only genetically-determined ones, in prostate tissue (normal and cancerous) and indeed in the pilosebaceous unit. Further, it would be worth learning what effect other progesterone analogs and antagonists have in this system, the ones used in clinical medicine being prime targets for such an assay. Medroxyprogesterone is already known to be mitogenic in five lines of breast tumors in mice.18 Further work along these lines is urgently needed.
It is worthy of note that specific, high affinity receptors for 5α-P are located in the plasma membrane fractions of both tumorigenic and non-tumorigenic mammary cells, making it likely that these receptors are part of the complement of the normal cell, not a phenomenon related to aberrant tumor growth. If so, one might expect to find these receptors on prostate, pilosebaceous and other tissues. That may provide the explanation for the link between breast cancer and numerous other cancers.
And what about therapy? Wiebe has shown that dutasteride (which blocks both isozymes of 5α-reductase) inhibits progesterone conversion to 5α-pregnanedione and similar molecules by greater than 95%, strongly suggesting such inhibition may be beneficial in breast cancer.28 But, as noted above, even this very effective level of inhibition will be bypassed by the exogenous source of 5α-P presented by our increasing consumption of dairy products containing this potent mitogenic molecule.
Summary
The evidence assembled here suggests that dairy-sourced hormones, not being subject to any innate feedback inhibition, may be the source of the androgenic and mitogenic progestins that drive acne, prostate and breast cancer. This is the most promising unitary hypothesis available to explain the etiology of diverse diseases that blemish, scar, shorten and take the lives of millions.
Abbreviations
- 5alpha-pregnanedione
5α-P
- 5alpha-androstanedione
5α-A
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/7124
References
- 1.Darling JA, Harkness RA. Human breast milk jaundice; estimation of steroids in milk. Arch Dis Child. 1969;44:782. doi: 10.1136/adc.44.238.782-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darling JA, Harkness RA. A method for the group analysis of steroids in milk. Acta Endocrinol (Copenh) 1973;72:391–400. doi: 10.1530/acta.0.0720391. [DOI] [PubMed] [Google Scholar]
- 3.Darling JA, Laing AH, Harkness RA. A survey of the steroids in cows' milk. J Endocrinol. 1974;62:291–297. doi: 10.1677/joe.0.0620291. [DOI] [PubMed] [Google Scholar]
- 4.Dermatology: Acne, hormones and milk. Time. 1966 Ref Type: Magazine Article. [Google Scholar]
- 5.Fisher JK. Acne Vulgaris; A Study of One Thousand Cases. JK Fisher. 2006. Available from: URL: http://www.acnemilk.com/fisher_s_original_paper.
- 6.Calman KC. The histochemical demonstration of hydroxysteroid dehydrogenase in human skin [Medicine] University of Glasgow; 1970. [Google Scholar]
- 7.Thiboutot D. Regulation of human sebaceous glands. J Invest Dermatol. 2004;123:1–12. doi: 10.1111/j.1523-1747.2004.t01-2-.x. [DOI] [PubMed] [Google Scholar]
- 8.Krunic A, Ciurea A, Scheman A. Efficacy and tolerance of acne treatment using both spironolactone and a combined contraceptive containing drospirenone. J Am Acad Dermatol. 2008;58:60–62. doi: 10.1016/j.jaad.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 9.Kurahashi N, Inoue M, Iwasaki M, Sasazuki S, Tsugane AS. Dairy product, saturated fatty acid, and calcium intake and prostate cancer in a prospective cohort of Japanese men. Cancer Epidemiol Biomarkers Prev. 2008;17:930–937. doi: 10.1158/1055-9965.EPI-07-2681. [DOI] [PubMed] [Google Scholar]
- 10.Labrie F. Current status of endocrine therapy in localized prostate cancer: cure has become a strong possibility. Int Braz J Urol. 2004;30:3–11. doi: 10.1590/s1677-55382004000100002. [DOI] [PubMed] [Google Scholar]
- 11.Musquera M, Fleshner NE, Finelli A, Zlotta AR. The REDUCE trial: Chemoprevention in prostate cancer using a dual 5α-reductase inhibitor, dutasteride. Expert Rev Anticancer Ther. 2008;8:1073–1079. doi: 10.1586/14737140.8.7.1073. [DOI] [PubMed] [Google Scholar]
- 12.Keam SJ, Scott LJ. Dutasteride: A review of its use in the management of prostate disorders. Drugs. 2008;68:463–485. doi: 10.2165/00003495-200868040-00008. [DOI] [PubMed] [Google Scholar]
- 13.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 14.NIH, author. State-of-the-Science Conference Statement on management of menopause-related symptoms. NIH Consens State Sci Statements. 2005;22:1–38. [PubMed] [Google Scholar]
- 15.Darney PD. The androgenicity of progestins. Am J Med. 1995;98:104–110. doi: 10.1016/s0002-9343(99)80067-9. [DOI] [PubMed] [Google Scholar]
- 16.Dickey RP. Managing contraceptive pill patients. 12 ed. EMIS Inc; 1998. [Google Scholar]
- 17.Luthy IA, Begin DJ, Labrie F. Androgenic activity of synthetic progestins and spironolactone in androgen-sensitive mouse mammary carcinoma (Shionogi) cells in culture. J Steroid Biochem. 1988;31:845–852. doi: 10.1016/0022-4731(88)90295-6. [DOI] [PubMed] [Google Scholar]
- 18.Lanari C, Luthy I, Lamb CA, Fabris V, Pagano E, Helguero LA, et al. Five novel hormone-responsive cell lines derived from murine mammary ductal carcinomas: In vivo and in vitro effects of estrogens and progestins. Cancer Res. 2001;61:293–302. [PubMed] [Google Scholar]
- 19.Tanemura A, van Hoesel AQ, Mori T, Yu T, Hoon DS. The role of estrogen receptor in melanoma. Expert Opin Ther Targets. 2007;11:1639–1648. doi: 10.1517/14728222.11.12.1639. [DOI] [PubMed] [Google Scholar]
- 20.Cartwright S. Breast cancer and the estrogen window. Lancet. 1980;1:929. doi: 10.1016/s0140-6736(80)90857-0. [DOI] [PubMed] [Google Scholar]
- 21.Henderson BE, Ross RK, Judd HL, Krailo MD, Pike MC. Do regular ovulatory cycles increase breast cancer risk? Cancer. 1985;56:1206–1208. doi: 10.1002/1097-0142(19850901)56:5<1206::aid-cncr2820560541>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 22.Sherman BM, Wallace RB, Bean JA. Cyclic ovarian function and breast cancer. Cancer Res. 1982;42:3286–3288. [PubMed] [Google Scholar]
- 23.Foidart JM. Added benefits of drospirenone for compliance. Climacteric. 2005;8:28–34. doi: 10.1080/13697130500330309. [DOI] [PubMed] [Google Scholar]
- 24.Backstrom T, Andersson A, Baird DT, Selstam G. The human corpus luteum secretes 5 α-pregnane-3,20-dione. Acta Endocrinol (Copenh) 1986;111:116–121. doi: 10.1530/acta.0.1110116. [DOI] [PubMed] [Google Scholar]
- 25.Parker LN, Lifrak ET, Odell WD. A 60,000 molecular weight human pituitary glycopeptide stimulates adrenal androgen secretion. Endocrinology. 1983;113:2092–2096. doi: 10.1210/endo-113-6-2092. [DOI] [PubMed] [Google Scholar]
- 26.Summers K, Crespi B. The androgen receptor and prostate cancer: A role for sexual selection and sexual conflict? Med Hypotheses. 2008;70:435–443. doi: 10.1016/j.mehy.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 27.Wiebe JP. Progesterone metabolites in breast cancer. Endocr Relat Cancer. 2006;13:717–738. doi: 10.1677/erc.1.01010. [DOI] [PubMed] [Google Scholar]
- 28.Wiebe JP, Souter L, Zhang G. Dutasteride affects progesterone metabolizing enzyme activity/expression in human breast cell lines resulting in suppression of cell proliferation and detachment. J Steroid Biochem Mol Biol. 2006;100:129–140. doi: 10.1016/j.jsbmb.2006.03.010. [DOI] [PubMed] [Google Scholar]