Craniopharyngiomas are the most common extraneural tumors of the CNS in children.1 Because craniopharyngiomas usually grow along the anatomic midline, the tumors or their treatments (surgical excision and radiation) frequently lead to hypopituitarism, visual field defects, obesity, sleep abnormalities, and daytime hypersomnolence (DH).2,3 DH persists despite hormone replacement or treatment of commonly associated obstructive sleep apnea (OSA). We hypothesized that disrupted sleep patterns in patients with craniopharyngioma result from dysfunction of the hypothalamic circadian pacemaker located in the suprachiasmatic nucleus, which controls the timing of the daily sleep propensity rhythm. Daily variations in levels of the pineal hormone melatonin serve as a marker of the function of this system. Low salivary melatonin has been documented in obese craniopharyngioma survivors and melatonin supplementation has proved beneficial in some cases; however, a detailed analysis of circadian rhythms in this patient population had not been performed.4
Methods.
Subjects older than 8 years of age with self-reported DH requiring daytime stimulant medication were recruited from a pool of 42 craniopharyngioma survivors treated at the Children’s Hospital Boston (CHB)/Dana-Farber Cancer Institute Brain Tumor Program between 1990 and 2002 by a single neurologist (S.L.P.). Four subjects were enrolled after informed consent was obtained in accordance with the Committee on Clinical Research. Three subjects (2 female, 1 male) ages 15, 15, and 22 completed the study. All had undergone both surgical extirpation and radiotherapy, were morbidly obese (BMI 41–54; normal 17–25), had panhypopituitarism requiring hormone supplementation, and had mild REM-related OSA, and 1 had a seizure disorder. Wrist actigraphy was obtained for 2 to 3 weeks prior to admission to the General Clinical Research Center sleep laboratory of CHB. During the ambulatory baseline weeks 1 and 2, subjects were not given any specific instructions regarding their sleep/wake schedules; during week 3, instructions to maintain strict and consistent sleep and waking times were given. Stimulant medications were discontinued in the third week but hormonal supplementation was continued throughout. Actigraphy data were analyzed to estimate sleep and wake periods in the 2 to 3 weeks before hospitalization (ACTION-W V2.0 and ACT-Millennium, Ambulatory Monitoring Inc., Ardsley, NJ). Subjects were then admitted to the hospital for 72 hours. Plasma melatonin was measured under dim light conditions (less than 10 lux) approximately every 20 minutes via indwelling IV catheter. IV access was lost in subject 1 following 40 hours of data collection. Two consecutive nights of 20-channel polysomnography (PSG) (Biologic Systems Corp., Mundelein, IL) were obtained. PSG records were scored according to standard scoring criteria.
Results.
Actigraphy demonstrated rhythmic rest/activity patterns that were in general alignment with a 24-hour light-dark cycle; however, irregular bedtimes, frequent nighttime activity, and inappropriate daytime episodes of rest were observed despite a protocol of scheduled bedtimes and wake times (figure, A). In contrast, PSGs showed preservation of normal sleep architecture with confirmation of mild OSA in all subjects (data not shown). There were strikingly low mean 24-hour plasma melatonin levels in all subjects (2.7 ± 0.07, 0.82 ± 0.12, and 0.61 ± 0.02 pg/mL, respectively) compared to historical controls of 8 pg/mL (daytime hours) to 50 pg/mL (evening hours).5 More importantly, average nocturnal melatonin levels (between 10 pm and 7 am) were also markedly decreased (0.84 ± 0.82, 0.74 ± 0.29, 1.22 ± 1.27, respectively) as compared to historical controls (50–60 pg/mL; figure, B).6
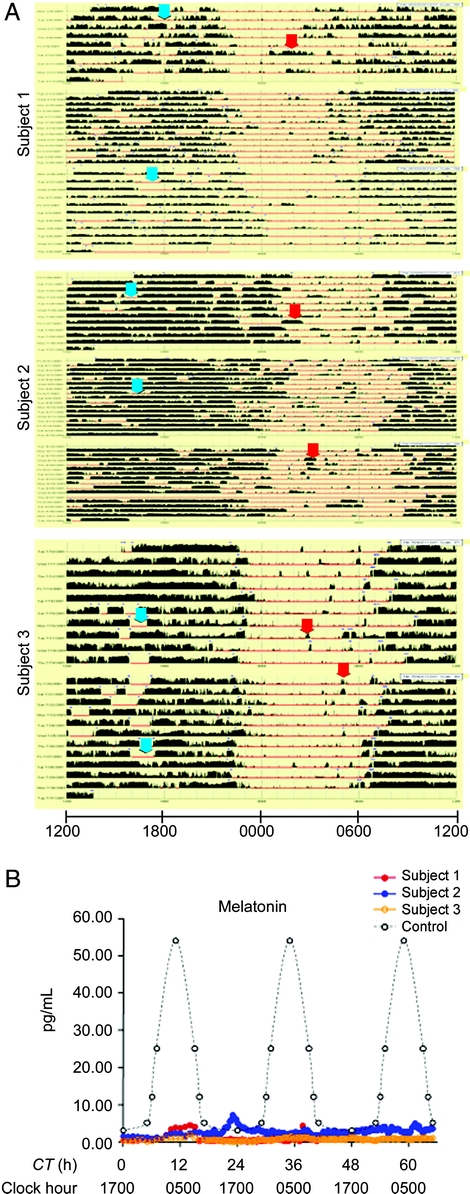
Figure Abnormal circadian behavior and melatonin levels in craniopharyngioma survivors
(A) Ambulatory baseline recordings. Dark bars represent movement detected by wrist actigraphy; absence of dark bars indicates presumed sleeping bouts. Horizontal lines represent consecutive 24-hour periods. Clock hour is indicated on the x-axis. Arrows indicate examples of inappropriate presumed daytime sleep (red) or nocturnal arousal (blue). (B) Plasma melatonin profiles measured in craniopharyngioma subjects (colored circles) and controls (open circles). Clock hour is indicated on the x-axis. CT = circadian time in hours from onset of inpatient monitoring.
Discussion.
Sleep/wake cycles are hypothesized to result from a balance between both circadian and homeostatic influences. We have obtained evidence for both melatonin deficiency and irregular circadian function, as evidenced by substantial abnormalities in serially measured plasma melatonin levels in 3 survivors of craniopharyngioma. Our results are consistent with previous reports that have demonstrated normal PSGs in craniopharyngioma survivors.3 Because melatonin rhythms serve as a marker for the functionality of the human circadian pacemaker, our data are suggestive of profound dysfunction in this system and consequent melatonin deficiency. We speculate that the complete loss of the circadian melatonin rhythm may reflect a disruption of daytime circadian arousal mechanisms, leaving homeostatic sleep drive unopposed and thus contributing to DH and sleep disruption in these patients. The observed absence of the nocturnal peak of melatonin suggests that symptoms in these patients may respond to exogenously administered melatonin during nighttime hours. Previous studies have demonstrated low salivary melatonin in obese craniopharyngioma patients with some improvement of self-reported DH with supplemental melatonin.4,7 A multifocused approach to improve both DH and consolidation of sleep/wake rhythms could routinely include aggressive weight reduction, proper treatment of OSA, exposure to bright light upon awakening, and a 24-hour urinary measurement of the melatonin metabolite 6-hydroxymelatonin sulfate. Patients with melatonin deficiency may respond to exogenous melatonin supplementation.
Supported by grants M01RR02172 and M01RR02635 from the National Center for Research Resources to Children’s Hospital Boston and the Brigham and Women’s Hospital.
Disclosure: Dr. Jonathan Lipton, Dr. J. Thomas Megerian, Dr. Sanjeev V. Kothare, Dr. Yoon-Jae Cho, Dr. Theresa Shanahan, Hilary Chart, Dr. Richard Ferber, Lisa Adler-Golden, and Dr. Laurie E. Cohen report no disclosures. Dr. Charles A. Czeisler has received consulting fees from Actelion, Ltd., Axon Labs, Cephalon, Inc., Eli Lilly and Co., Koninklijke Philips Electronics, N.V., Republic of Ireland Garda Síochána Inspectorate, Respironics, Inc., Sepracor, Inc., Somnus Therapeutics, Inc., Takeda Pharmaceuticals, and Vanda Pharmaceuticals, Inc.; owns an equity interest in Axon Labs, Somnus Therapeutics, Inc., and Vanda Pharmaceuticals, Inc.; and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, Dr. Czeisler has served as an expert witness on various legal cases related to sleep and/or circadian rhythms. Dr. Czeisler’s laboratory has also received clinical trial research grants and contracts from Cephalon, Inc., Merck & Co., Inc., and Pfizer, Inc.; education funds and/or support for research expenses from Cephalon, Inc., Koninklijke Philips Electronics, N.V., Takeda Pharmaceuticals, ResMed, Sanofi-Aventis, Inc., and Sepracor, Inc.; and Dr. Czeisler is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. Dr. Scott L. Pomeroy reports no disclosures.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Received October 28, 2008. Accepted in final form March 10, 2009.
Address correspondence and reprint requests to Dr. Jonathan Lipton, Department of Neurology, Children’s Hospital Boston, Harvard Medical School, 300 Longwood Avenue, CLS 13-060, Boston, MA 02115; jonathan.lipton@childrens.harvard.edu
&NA;
- 1.Prabhu VC, Brown HG. The pathogenesis of craniopharyngiomas. Childs Nerv Syst 2005;21:622–627. [DOI] [PubMed] [Google Scholar]
- 2.Palm L, Nordin V, Elmqvist D, Blennow G, Persson E, Westgren U. Sleep and wakefulness after treatment for craniopharyngioma in childhood; influence on the quality and maturation of sleep. Neuropediatrics 1992;23:39–45. [DOI] [PubMed] [Google Scholar]
- 3.van der Klaauw AA, Biermasz NR, Pereira AM, et al. Patients cured from craniopharyngioma or non-functioning pituitary macroadenoma suffer similarly from increased daytime somnolence despite normal sleep patterns compared to healthy controls. Clin Endocrinol (Oxf) 2008;69:769–774. [DOI] [PubMed] [Google Scholar]
- 4.Muller HL, Handwerker G, Wollny B, Faldum A, Sorensen N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J Clin Endocrinol Metab 2002;87:3993–3996. [DOI] [PubMed] [Google Scholar]
- 5.Bojkowski CJ, Arendt J, Shih MC, Markey SP. Melatonin secretion in humans assessed by measuring its metabolite, 6-sulfatoxymelatonin. Clin Chem 1987;33:1343–1348. [PubMed] [Google Scholar]
- 6.Arendt J. Melatonin: characteristics, concerns, and prospects. J Biol Rhythms 2005;20:291–303. [DOI] [PubMed] [Google Scholar]
- 7.Muller HL, Handwerker G, Gebhardt U, et al. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control 2006;17:583–589. [DOI] [PubMed] [Google Scholar]


