Abstract
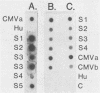
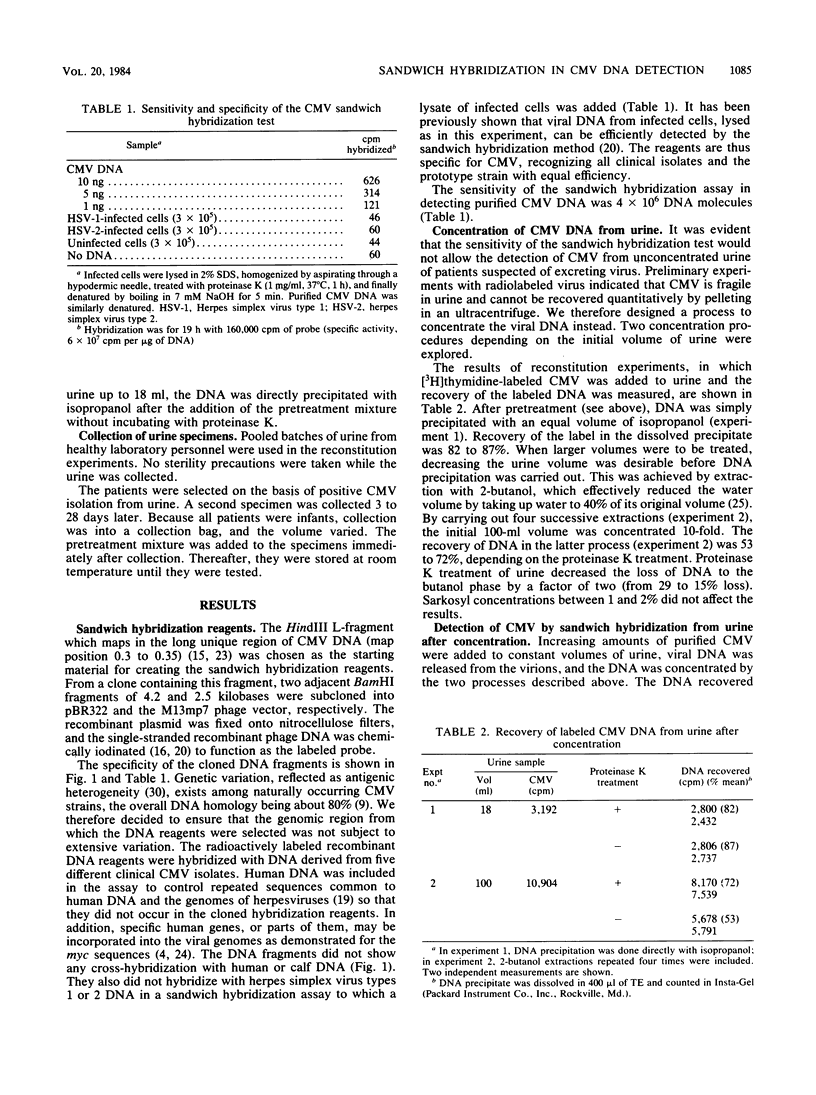
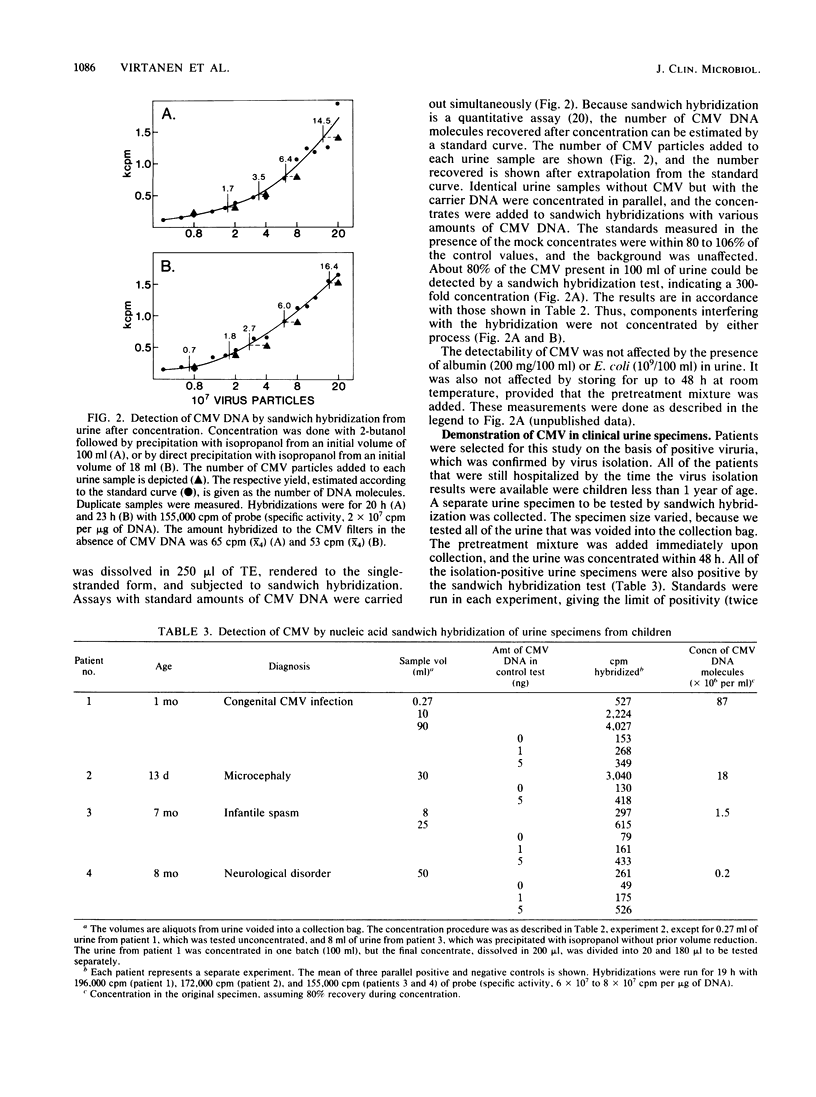
A cytomegalovirus (CMV)-specific sandwich hybridization test was constructed by using two adjacent BamHI DNA fragments of CMV DNA as reagents. The fragments were cloned into two different vectors. One of the recombinants was attached to the filter, and the other was the labeled probe. When present in the sample, CMV DNA mediated labeling of the filter by hybridizing to both the filter-bound DNA and the probe. The sandwich hybridization test was applied for the detection of CMV DNA from urine. DNA was released from virus by 2% Sarkosyl, concentrated by 2-butanol extraction and isopropanol precipitation, denatured, and finally subjected to the sandwich hybridization test. As a result, 70 to 90% of the original viral DNA could be recovered and demonstrated by the quantitative hybridization reaction. Urine could be stored at room temperature in Sarkosyl for at least 2 days without affecting the detectability of CMV. The clinical applicability of the test was evaluated by studying urine samples from four infants excreting CMV. Sandwich hybridization demonstrated the presence of CMV DNA in all of the specimens. These contained originally 10(5) to 10(8) CMV DNA molecules per ml.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou S., Merigan T. C. Rapid detection and quantitation of human cytomegalovirus in urine through DNA hybridization. N Engl J Med. 1983 Apr 21;308(16):921–925. doi: 10.1056/NEJM198304213081603. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew W. L., Conant M. A., Miner R. C., Huang E. S., Ziegler J. L., Groundwater J. R., Gullett J. H., Volberding P., Abrams D. I., Mintz L. Cytomegalovirus and Kaposi's sarcoma in young homosexual men. Lancet. 1982 Jul 17;2(8290):125–127. doi: 10.1016/s0140-6736(82)91092-3. [DOI] [PubMed] [Google Scholar]
- Gelmann E. P., Clanton D. J., Jariwalla R. J., Rosenthal L. J. Characterization and location of myc homologous sequences in human cytomegalovirus DNA. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5107–5111. doi: 10.1073/pnas.80.16.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn J. Cytomegalovirus infections following renal transplantation. Rev Infect Dis. 1981 Nov-Dec;3(6):1151–1178. doi: 10.1093/clinids/3.6.1151. [DOI] [PubMed] [Google Scholar]
- Goldstein L. C., McDougall J., Hackman R., Meyers J. D., Thomas E. D., Nowinski R. C. Monoclonal antibodies to cytomegalovirus: rapid identification of clinical isolates and preliminary use in diagnosis of cytomegalovirus pneumonia. Infect Immun. 1982 Oct;38(1):273–281. doi: 10.1128/iai.38.1.273-281.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller M., Henderson A., Kieff E. Repeat array in Epstein-Barr virus DNA is related to cell DNA sequences interspersed on human chromosomes. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5916–5920. doi: 10.1073/pnas.79.19.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang E. S., Huong S. M., Tegtmeier G. E., Alford C. Cytomegalovirus: genetic variation of viral genomes. Ann N Y Acad Sci. 1980;354:332–346. doi: 10.1111/j.1749-6632.1980.tb27976.x. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. S., Sapienza V. J., Chen C. M., Wisniewski K. Production and characterization of monoclonal antibodies specific for a glycosylated polypeptide of human cytomegalovirus. J Clin Microbiol. 1983 Aug;18(2):331–343. doi: 10.1128/jcm.18.2.331-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg B. Antiviral effects of phosphonoformate (PFA, foscarnet sodium). Pharmacol Ther. 1982;19(3):387–415. doi: 10.1016/0163-7258(82)90074-2. [DOI] [PubMed] [Google Scholar]
- Palva A. M. ompA gene in the detection of Escherichia coli and other Enterobacteriaceae by nucleic acid sandwich hybridization. J Clin Microbiol. 1983 Jul;18(1):92–100. doi: 10.1128/jcm.18.1.92-100.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham C. S., Chin K. S., Coleman J. C., Henderson K., Hurley R., Preece P. M. Cytomegalovirus infection in pregnancy: preliminary findings from a prospective study. Lancet. 1983 Jun 18;1(8338):1352–1355. doi: 10.1016/s0140-6736(83)92138-4. [DOI] [PubMed] [Google Scholar]
- Peden K., Mounts P., Hayward G. S. Homology between mammalian cell DNA sequences and human herpesvirus genomes detected by a hybridization procedure with high-complexity probe. Cell. 1982 Nov;31(1):71–80. doi: 10.1016/0092-8674(82)90406-8. [DOI] [PubMed] [Google Scholar]
- Ranki M., Palva A., Virtanen M., Laaksonen M., Söderlund H. Sandwich hybridization as a convenient method for the detection of nucleic acids in crude samples. Gene. 1983 Jan-Feb;21(1-2):77–85. doi: 10.1016/0378-1119(83)90149-x. [DOI] [PubMed] [Google Scholar]
- Ranki M., Virtanen M., Palva A., Laaksonen M., Pettersson R., Käriäinen L., Halonen P., Söderlund H. Nucleic acid sandwich hybridization in adenovirus diagnosis. Curr Top Microbiol Immunol. 1983;104:307–318. doi: 10.1007/978-3-642-68949-9_20. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Hock L., Tamashiro J. C. Cleavage maps for human cytomegalovirus DNA strain AD169 for restriction endonucleases EcoRI, BglII, and HindIII. J Virol. 1982 May;42(2):558–582. doi: 10.1128/jvi.42.2.558-582.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Vacquier J. P. Human cytomegalovirus (strain AD169) contains sequences related to the avian retrovirus oncogene v-myc. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3889–3893. doi: 10.1073/pnas.80.13.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafford D. W., Bieber D. Concentration of DNA solutions by extraction with 2-butanol. Biochim Biophys Acta. 1975 Jan 6;378(1):18–21. doi: 10.1016/0005-2787(75)90132-x. [DOI] [PubMed] [Google Scholar]
- Stagno S., Pass R. F., Alford C. A. Perinatal infections and maldevelopment. Birth Defects Orig Artic Ser. 1981;17(1):31–50. [PubMed] [Google Scholar]
- Tamashiro J. C., Hock L. J., Spector D. H. Construction of a cloned library of the EcoRI fragments from the human cytomegalovirus genome (strain AD169). J Virol. 1982 May;42(2):547–557. doi: 10.1128/jvi.42.2.547-557.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen M., Palva A., Laaksonen M., Halonen P., Söderlund H., Ranki M. Novel test for rapid viral diagnosis: detection of adenovirus in nasopharyngeal mucus aspirates by means of nucleic-acid sandwich hybridisation. Lancet. 1983 Feb 19;1(8321):381–383. doi: 10.1016/s0140-6736(83)91500-3. [DOI] [PubMed] [Google Scholar]
- Volpi A., Whitley R. J., Ceballos R., Stagno S., Pereira L. Rapid diagnosis of pneumonia due to cytomegalovirus with specific monoclonal antibodies. J Infect Dis. 1983 Jun;147(6):1119–1120. doi: 10.1093/infdis/147.6.1119. [DOI] [PubMed] [Google Scholar]
- Waner J. L., Weller T. H. Analysis of antigenic diversity among human cytomegaloviruses by kinetic neutralization tests with high-titered rabbit antisera. Infect Immun. 1978 Jul;21(1):151–157. doi: 10.1128/iai.21.1.151-157.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller T. H. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med. 1971 Jul 22;285(4):203–214. doi: 10.1056/NEJM197107222850406. [DOI] [PubMed] [Google Scholar]
- Weststrate M. W., Geelen J. L., Wertheim P. M., van der Noordaa J. Comparison of the physical maps of the DNAs of two cytomegalovirus strains. J Gen Virol. 1983 Jan;64(Pt 1):47–55. doi: 10.1099/0022-1317-64-1-47. [DOI] [PubMed] [Google Scholar]