Abstract
Aims
Severe heart failure (HF) is often associated with cachexia that reverses post-heart transplantation (HTx) with frequent development of obesity. Ghrelin is a novel appetite-stimulating hormone. The aim was to determine the role of ghrelin in regulating appetite, food intake, and body composition in HF and post-HTx.
Methods and results
We measured serial ghrelin, hunger sensation, caloric intake, and body composition in 12 HF patients awaiting HTx, 12 patients 12.7 ± 8.6 months post-HTx, and 7 controls. Seven of 12 HF patients were followed for longitudinal analysis post-HTx. Body mass index was 23.1 ± 3.1 in HF and 31.5 ± 5.5 post-HTx (P < 0.001). Heart transplantation patients had gained 18.0 ± 7.7 kg since HTx. Ghrelin area under the curve between controlled meals (control: 186 ± 39; HF: 264 ± 71; HTx: 194 ± 47 ng min/mL, P < 0.007) was higher in HF, but test meal caloric intake (control: 1185 ± 650; HF: 391 ± 103; HTx: 831 ± 309 kcal, P < 0.008) was lower in HF. The longitudinal analysis confirmed these findings.
Conclusion
Heart failure may be associated with resistance to the appetite-stimulating effects of ghrelin, which may contribute to cachexia. Heart transplantation may be associated with resolution of ghrelin resistance, which may contribute to weight gain. These findings are preliminary and should be confirmed in larger trials.
Keywords: Ghrelin, Heart failure, Cachexia, Heart transplantation, Weight gain, Appetite
Introduction
Cardiac cachexia is well described in severe heart failure (HF).1,2 Heart transplantation (HTx) is associated with weight gain and the frequent development of obesity. Although this weight gain has been attributed to glucocorticoid therapy, it is more dramatic than after other solid organ transplants, despite similar steroid regimens, and also unrelated to steroid dose,3 suggesting that other mechanisms are responsible.
Ghrelin is an appetite-stimulating hormone that is released from the stomach in response to fasting and weight loss and is inhibited by food intake.4,5 Ghrelin was originally identified as the endogenous ligand for the growth hormone (GH) secretagogue receptor and partially acts by stimulating GH release.4 Ghrelin receptors are widely distributed in the heart and vessels,6 and its administration may improve left ventricular function, decrease muscle wasting, and improve exercise capacity in HF.7 In one study, ghrelin was elevated in cachectic HF.8 Ghrelin has not been studied post-HTx.
We hypothesized that cachexia in severe HF may be in part due to resistance to the appetite-stimulating and anabolic effects of ghrelin and that weight gain post-HTx may be in part due to resolution of this resistance. Accordingly, we examined the hormonal regulation of appetite, caloric intake, and body composition in HF, HTx, and matched healthy controls.
Methods
We performed a cross-sectional study in 12 patients with New York Heart Association functional class IV HF awaiting HTx, 12 patients 12.7 ± 8.6 months post-HTx, and 7 control subjects. We then performed a longitudinal study of 7 of 12 HF patients 7.4 ± 4.4 months after HTx. Of the remaining five HF patients, three patients declined repeat testing after HTx, one died before HTx, and one became too ill for HTx. Subjects were age and gender matched. Heart failure and HTx patients were matched by aetiology, and controls were matched by body mass index (BMI) to HTx (Table 1). The protocol complied with the Declaration of Helsinki and was approved by the institutional review board of Columbia University Medical Center, and written informed consent was obtained from all patients.
Table 1.
Baseline characteristics of the cross-sectional cohort
| Group | Control (n = 7) | HF (n = 12) | HTx (n = 12) | P-value (overall) |
|---|---|---|---|---|
| Age (years) | 42 ± 14 | 52 ± 16 | 47 ± 18 | >0.2 |
| Gender n (%) | ||||
| Male | 5 (71%) | 10 (83%) | 11 (92%) | >0.2 |
| Female | 2 (29%) | 2 (17%) | 1 (8%) | |
| BMI (kg/m2) | 27.8 ± 4.2 | 23.1 ± 3.1 | 31.5 ± 5.5 | <0.001 |
| Cachexia presenta | NA | 7 (58%) | NA | NA |
| Diabetes | 0 | 3 (25%) | 2 (17%) | NA |
| Weight gain since HTx (kg) | NA | NA | 18.0 ± 7.7 | NA |
| Time since HTx (months) | NA | NA | 12.7 ± 8.6 | NA |
| Aetiology | ||||
| Ischaemic | NA | 4 (33%) | 6 (50%) | >0.2 |
| Dilated | 6 (50%) | 5 (42%) | ||
| Valvular | 2 (17%) | 1 (8%) | ||
| HF drug therapy | ||||
| Loop diuretic | NA | 12 (100%) | NA | NA |
| ACE-inhibitor | 12 (100%) | |||
| Beta-blocker | 5 (42%) | |||
| Inotropes | 7 (58%) | |||
| HTx drug therapy | ||||
| Prednisone (mg) | NA | NA | 6 ± 4 | NA |
| Cyclosporine A | 9 (75%) | |||
| Tacrolimus | 3 (25%) | |||
| Mycophenolate mofetil | 12 (100%) | |||
DEXA scanning to analyse body composition was performed with a QDR 4500 A Delphi W densitometer (Hologic Inc., Bedford, USA). Waist circumference was measured in duplicate at the level of the umbilicus. Measurement of resting energy expenditure (REE) and cardiopulmonary exercise testing with measurement of peak oxygen consumption (peak VO2) was performed using a metabolic cart (Medical Graphics, Minneapolis, USA).
Subjects reported to the laboratory at 8 a.m. in the fasting state and underwent blood sampling, completed a visual analogue (0–10) assessing hunger, and then consumed a yoghurt-based breakfast test meal where they were encouraged to eat until absolute satiety. Eating until absolute satiety ensured that post-prandial assessment would not be affected by differences in baseline food intake and satiety. Post-prandial serial blood testing and hunger assessment was performed hourly for 5 h. Subjects were then given a lunch meal of regular food of their choosing and were neither encouraged nor dissuaded to eat. Caloric intake at each meal was estimated using computerized software NDS-R 4.05-33 (University of Minnesota, Minneapolis, USA).
Blood was collected in chilled EDTA tubes; 2 mL blood was mixed with 0.1 mL aprotonin (for ghrelin but not GH) and centrifuged at 2500 g for 15 min at 4°C. Plasma was stored at −70°C. Ghrelin was measured by radioimmunoassay (Phoenix Pharmaceuticals, Belmont, USA) and GH was measured by a two-site immunoradiometric assay (Diagnostic Systems Laboratories, Inc., Webster, USA).
Continuous variables were compared by one-way analysis of variance. Pair-wise comparisons between cross-sectional groups were done by Fisher's least significance test. Paired comparisons between longitudinal groups were done by Student's t-test. Serial data over time were used to calculate area under the curve (AUC) for ghrelin. Ghrelin and GH were plotted against each other and Pearson's correlation calculated. A P < 0.05 was considered statistically significant. All results are reported as mean ± standard deviation.
Results
Clinical characteristics and medical regimens of the cross-sectional population are summarized in Table 1. Cachexia, defined as non-oedematous weight loss ≥7.5% over the preceding 6 months,8,9 was present in 7 of 12 HF patients. Heart transplantation patients had gained 18.0 ± 7.7 kg over 12.7 ± 8.6 months since HTx. The mean prednisone dose in the HTx patients was 6 ± 4 mg/day (range 2–15). The seven HF patients followed longitudinally (Table 3) had similar age, gender, and medical regimens compared with the 12 cross-sectional HTx patients (Table 1).
Table 3.
Baseline characteristics of the longitudinal cohort
| Group | HF long (n = 7) | HTx long (n = 7) | P-value |
|---|---|---|---|
| Age at time of respective study (years) | 47 ± 19 | 48 ± 19 | NA |
| Gender n (%) | |||
| Male | 5 (71%) | Same | NA |
| Female | 2 (29%) | ||
| BMI (kg/m2) | 23.4 ± 3.3 | 26.4 ± 4.9 | <0.009 |
| Diabetes n (%) | 2 (29%) | 2 (29%) | NA |
| Weight gain since HTx (kg) | NA | 9.6 ± 6.2 | NA |
| Time since HTx (months) | NA | 7.4 ± 4.4 | NA |
| Aetiology | |||
| Ischaemic | 1 (14%) | Same | NA |
| Dilated | 5 (71%) | ||
| Valvular | 1 (14%) | ||
| HF drug therapy | |||
| Loop diuretic | 7 (100%) | NA | NA |
| ACE-inhibitor | 7 (100%) | ||
| Beta-blocker | 3 (43%) | ||
| Inotropes | 5 (71%) | ||
| HTx drug therapy | |||
| Prednisone (mg) | NA | 6 ± 4 | NA |
| Cyclosporine A | 3 (43%) | ||
| Tacrolimus | 4 (57%) | ||
| Mycophenolate mofetil | 6 (86%) | ||
NA, not applicable.
Bold text denotes P ≤ 0.05.
Results of the cross-sectional study are depicted in Table 2. Heart failure had the lowest BMI, total body mass, lean mass, and fat mass (P < 0.01 for all except lean mass). The per cent body fat, waist circumference, and abdominal fat mass were greater in HTx than in HF and controls (all P < 0.01).
Table 2.
Body composition, resting energy expenditure, exercise tolerance, hormones, appetite, and caloric intake in the cross-sectional cohort
| Group | Control (n = 7) | HF cross-sectional (n = 12) | HTx cross-sectional (n = 12) | P-value (overall) | P-value (control vs. HF) | P-value (control vs. HTx) | P-value (HF vs. HTx) |
|---|---|---|---|---|---|---|---|
| BMI (kg/m2) | 27.8 ± 4.2 | 23.1 ± 3.1 | 31.5 ± 5.5 | <0.001 | 0.03 | 0.08 | <0.001 |
| Total mass (kg) | 87 ± 15 | 69 ± 6 | 92 ± 15 | <0.001 | 0.004 | >0.2 | <0.001 |
| Fat mass (kg)a | 24 ± 9 | 17 ± 2 | 31 ± 9 | 0.006 | <0.13 | 0.10 | <0.002 |
| Lean mass (kg)a | 66 ± 8 | 55 ± 4 | 61 ± 11 | 0.12 | 0.04 | >0.2 | 0.17 |
| Fat% | 26 ± 7 | 23 ± 2 | 33 ± 7 | <0.009 | >0.2 | <0.03 | <0.004 |
| Abdominal fat mass (kg) | 11.7 ± 5 | 8.4 ± 2 | 16.8 ± 6 | <0.01 | >0.2 | <0.06 | <0.004 |
| Waist circumference (cm) | 99 ± 10 | 86 ± 4 | 111 ± 13 | <0.001 | <0.10 | <0.05 | <0.001 |
| REE (kcal/day) | 1710 ± 384 | 1812 ± 352 | 2036 ± 528 | >0.2 | >0.2 | <0.13 | >0.2 |
| REE (kcal/m2/h) | 35 ± 5 | 41 ± 9 | 41 ± 10 | >0.2 | <0.14 | <0.15 | >0.2 |
| Peak VO2 (mL/kg/min)a | 27 ± 9 | 11 ± 2 | 15 ± 3 | <0.001 | <0.001 | <0.001 | 0.18 |
| GH (ng/mL) | 0.21 ± 0.25 | 1.13 ± 1.19 | 0.07 ± 0.13 | <0.006 | <0.02 | >0.2 | <0.003 |
| Ghrelin (pg/mL) | 669 ± 85 | 917 ± 336 | 690 ± 194 | 0.05 | 0.04 | >0.2 | <0.04 |
| Hunger fasting (0–10) | 5.8 ± 2.5 | 5.0 ± 2.4 | 3.4 ± 1.3 | <0.05 | >0.2 | 0.02 | 0.07 |
| Breakfast (kcal) | 907 ± 260 | 832 ± 364 | 1238 ± 421 | 0.03 | >0.2 | <0.07 | 0.01 |
| Ghrelin AUC (ng min/mL) | 186 ± 39 | 264 ± 71 | 194 ± 47 | <0.007 | <0.007 | >0.2 | <0.006 |
| Hunger before lunch (0–10) | 7.3 ± 1.6 | 6.6 ± 2.8 | 6.1 ± 2.7 | >0.2 | >0.2 | >0.2 | >0.2 |
| Lunch (kcal) | 1185 ± 650 | 391 ± 103 | 831 ± 309 | <0.008 | <0.002 | 0.13 | <0.07 |
aPeak VO2 and DEXA scans were not performed in the seven HF patients on inotropic therapy.
Bold text denotes P ≤ 0.05.
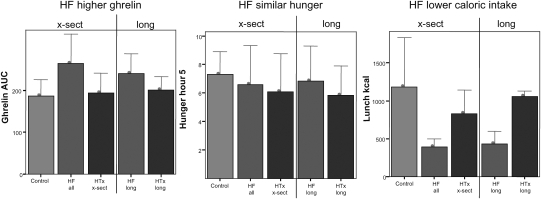
Fasting ghrelin and GH were substantially greater in HF (P = 0.05 and P < 0.006, respectively). Ghrelin correlated with GH in HF (Pearson's R = 0.76, P = 0.01) and overall (Pearson's R = 0.74, P < 0.001). At the encouraged breakfast, HF and controls had similar but HTx had higher caloric intake (P = 0.03). After eating to satiety, HF still released more ghrelin than did HTx and control, as judged by the AUC of ghrelin over a 5 h period (P < 0.007, Table 2 and Figure 1). Despite this increased exposure to ghrelin in HF, 5 h after the test meal the sensation of hunger was similar in all groups (Table 2 and Figure 1). Despite higher ghrelin and similar hunger levels, HF patients ingested dramatically less calories at the ad lib meal than HTx and control (P < 0.008, Table 2 and Figure 1). The REE was similar between the groups crudely and after adjusting for body surface area.
Figure 1.
Ghrelin area under the curve, hunger, and caloric intake in the longitudinal and cross-sectional cohorts. All subjects consumed a breakfast until absolute satiety to ensure that post-prandial assessments were not affected by differences in baseline food intake or satiety. For 5 h thereafter, the ghrelin area under the curve (AUC, ng min/mL) was significantly higher in HF than in control or HTx. Despite higher ghrelin, hunger was no different in HF compared with control or HTx. At the ad lib lunch meal, when subjects were neither encouraged nor dissuaded to eat, caloric intake was significantly and dramatically lower in HF than in control or HTx, despite significantly higher ghrelin. Hunger is measured on a 0–10 visual analogue scale. x-sect, cross-sectional; long, longitudinal.
The seven longitudinal patients were studied before and 7.4 ± 4.4 months after HTx and had gained 9.6 ± 6.2 kg since HTx. The longitudinal (Tables 3 and 4) exhibited the same patterns as the cross-sectional (Tables 1 and 2) study. Weight gain after HTx and BMI exhibited the same pattern, although the changes were smaller in the longitudinal cohort, as the time since HTx was less (Tables 1 and 3).
Table 4.
Hormones, appetite, and caloric intake in the longitudinal cohort
| Group | HF long (n = 7) | HTx long (n = 7) | P-value |
|---|---|---|---|
| BMI (kg/m2) | 23.4 ± 3.3 | 26.4 ± 4.9 | <0.009 |
| GH (ng/mL) | 1.21 ± 0.64 | 0.17 ± 0.15 | 0.03 |
| Ghrelin fasting (pg/mL) | 792 ± 188 | 683 ± 125 | 0.15 |
| Hunger fasting (0–10) | 5.4 ± 2.1 | 6.9 ± 2.0 | >0.2 |
| Breakfast (kcal) | 750 ± 345 | 773 ± 279 | >0.2 |
| Ghrelin AUC (ng min/mL) | 239 ± 48 | 200 ± 32 | <0.04 |
| Hunger before lunch (0–10) | 6.6 ± 2.5 | 5.8 ± 2.1 | >0.2 |
| Lunch (kcal) | 428 ± 166 | 1058 ± 71 | <0.17 |
| REE (kcal/day) | 1669 ± 354 | 1701 ± 122 | >0.2 |
| REE (kcal/m2/h) | 38 ± 9 | 37 ± 4 | >0.2 |
Bold text denotes P ≤ 0.05.
Discussion
Cardiac cachexia, defined as a weight loss of ≥7.5% over the preceding 6 months,8,9 is harmful regardless of BMI.2 All HF patients had a BMI less than 30 and 7 of 12 fulfilled criteria for cardiac cachexia, similar to reported prevalences of cardiac cachexia of 16–68%.1,10,11 Heart transplantation patients had gained 18 kg since HTx, both lean tissue and fat, consistent with previous reports.3,12 Peak VO2 was dramatically impaired in HF, consistent with the severity of the HF. The mechanisms underlying cachexia in HF have been suggested to be partially due to neurohormonal and cytokine activation and GH resistance,9,13 but other mechanisms are possible.
Ghrelin has received attention as an appetite stimulant, but receptors are found in heart and vessels6 and numerous cardiovascular effects have been described.14,15 In human HF, ghrelin administration may improve left ventricular function and cardiac output, decrease muscle wasting, and improve exercise capacity.7,14,15 Growth hormone may decrease ghrelin in a negative-feedback loop.16 One study of ghrelin in HF demonstrated elevated ghrelin in cachectic but not in non-cachectic HF, as well as a correlation between GH and ghrelin.8 Here, we show elevated ghrelin in HF regardless of BMI and confirm the correlation between GH and ghrelin in both HF and the overall cohort. Ghrelin post-HTx has not previously been studied. We show that fasting ghrelin levels and ghrelin AUC normalize after HTx.
The mechanism for elevated ghrelin levels in HF as in other weight reduced states5,17,18 is not clear. It may be a physiological compensation for reduced weight in an effort to increase appetite and caloric intake.17,18 Despite elevated fasting ghrelin, HF patients had no higher hunger than the other groups and actually had lower caloric intake than HTx. Ghrelin levels are known to fall after food intake.5 Following the test breakfast meal, ghrelin levels fell more slowly and remained higher, as reflected by a higher AUC, in HF than in control or HTx, suggesting that food intake does not appropriately feedback on ghrelin secretion in HF. Though the AUC of ghrelin was greatest in HF, hunger before lunch was equal among the groups, and ad lib caloric intake at lunch was significantly lower in HF than in HTx and control. At breakfast, all subjects were actively encouraged to consume as much as possible, which may be responsible for the caloric intake in HF being similar to control and only one-third less than HTx. In contrast, the lunch meal was neither encouraged nor un-encouraged, and HF patients consumed considerably less. Resting energy expenditure was similar in all groups and thus does not explain different caloric intakes. These data suggest that HF subjects are resistant to the orexigenic effects of ghrelin, similar to patients with anorexia nervosa who have high ghrelin levels without appetite stimulation or weight gain.17,18 These data also suggest that ghrelin resistance resolves after HTx.
However, since ghrelin was also elevated in non-cachectic HF and did not correlate with BMI in this study, other explanations for elevated ghrelin are possible. The beneficial cardiovascular effects of ghrelin suggest that elevations in ghrelin could be a compensatory response to low-cardiac output and maladaptive neurohormonal features of HF. Ghrelin's action as a GH secretagogue raises the possibility that ghrelin could be elevated in response to GH resistance, which occurs in severe HF.13,19 The fall in ghrelin coupled with increased caloric intake post-HTx may reflect resolution of resistance to the orexigenic effects of ghrelin. Again, however, other explanations are possible, including resolution of HF and its associated neurohormonal activation and/or resolution of GH resistance.13
The small patient population is the major limitation of the study. However, the consistency of findings and magnitude of differences in the cross-sectional and longitudinal analysis support the conclusions. The complexity of the measurements made it difficult to involve other HTx centres in the study. We cannot rule out that steroids affect weight gain and ghrelin regulation post-HTx, but steroid doses were relatively low and we have previously shown that weight gain post-HTx is unrelated to steroid dose.3 Our findings need to be confirmed in a larger study population in a multi-centre trial. The potential relationship between appetite and ghrelin should be analysed with caution. Recent reports suggest that ghrelin exists both in proforms and in active (acylated) and inactive (non-acylated) forms.20 We measured total ghrelin, which reflects both. However, measurement of active ghrelin is difficult and current assays may be inaccurate.
Conclusion
In this pilot study, we show that HF may be associated with resistance to the appetite-stimulating effects of ghrelin and that post-HTx, ghrelin levels fall and caloric intake increases, suggesting resolution of ghrelin resistance. These states may contribute to cachexia in HF and weight gain post-HTx. These findings are preliminary and should be confirmed in larger trials.
Funding
This work was supported by Stockholms Läns Landsting, Astrid och David Hageléns Stiftelse, Magnus Bergvalls Stiftelse, and the Swedish Heart-Lung Foundation, all in Stockholm, Sweden, in the form of grants to L.H.L., and by the Division of Research Resources, General Clinical Research Centres Program, National Institutes of Health [5 MO1 RR00645], Bethesda, MD, the Foundation for Cardiac Therapies (FACT Fund), New, York, NY and the Altman Fund, New York, NY, in the form of grants to D.M.M.
Conflict of interest: none declared.
References
- 1.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 2.Pocock SJ, McMurray JJ, Dobson J, Yusuf S, Granger CB, Michelson EL, Ostergren J, Pfeffer MA, Solomon SD, Anker SD, Swedberg KB. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008;29:2641–2650. doi: 10.1093/eurheartj/ehn420. [DOI] [PubMed] [Google Scholar]
- 3.Williams JJ, Lund LH, LaManca J, Kunavarapu C, Cohen DJ, Heshka S, Heymsfield SB, Mancini DM. Excessive weight gain in cardiac transplant recipients. J Heart Lung Transplant. 2006;25:36–41. doi: 10.1016/j.healun.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 5.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 6.Papotti M, Ghe C, Cassoni P, Catapano F, Deghenghi R, Ghigo E, Muccioli G. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab. 2000;85:3803–3807. doi: 10.1210/jcem.85.10.6846. [DOI] [PubMed] [Google Scholar]
- 7.Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W, Ueno K, Kitakaze M, Miyatake K, Kangawa K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674–3679. doi: 10.1161/01.CIR.0000149746.62908.BB. [DOI] [PubMed] [Google Scholar]
- 8.Nagaya N, Uematsu M, Kojima M, Date Y, Nakazato M, Okumura H, Hosoda H, Shimizu W, Yamagishi M, Oya H, Koh H, Yutani C, Kangawa K. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: relationships between ghrelin and anabolic/catabolic factors. Circulation. 2001;104:2034–2038. doi: 10.1161/hc4201.097836. [DOI] [PubMed] [Google Scholar]
- 9.Anker SD, Chua TP, Ponikowski P, Harrington D, Swan JW, Kox WJ, Poole-Wilson PA, Coats AJ. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96:526–534. doi: 10.1161/01.cir.96.2.526. [DOI] [PubMed] [Google Scholar]
- 10.Carr JG, Stevenson LW, Walden JA, Heber D. Prevalence and hemodynamic correlates of malnutrition in severe congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1989;63:709–713. doi: 10.1016/0002-9149(89)90256-7. [DOI] [PubMed] [Google Scholar]
- 11.Mancini DM, Walter G, Reichek N, Lenkinski R, McCully KK, Mullen JL, Wilson JR. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–1373. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 12.Baker AM, Levine TB, Goldberg AD, Levine AB. Natural history and predictors of obesity after orthotopic heart transplantation. J Heart Lung Transplant. 1992;11:1156–1159. [PubMed] [Google Scholar]
- 13.Lund LH, Freda P, Williams JJ, Lamanca JJ, Lejemtel TH, Mancini DM. Growth hormone resistance in severe heart failure resolves after cardiac transplantation. Eur J Heart Fail. 2009;11:525–528. doi: 10.1093/eurjhf/hfp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagaya N, Kojima M, Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of cardiopulmonary-associated cachexia. Intern Med. 2006;45:127–134. doi: 10.2169/internalmedicine.45.1402. [DOI] [PubMed] [Google Scholar]
- 15.Enomoto M, Nagaya N, Uematsu M, Okumura H, Nakagawa E, Ono F, Hosoda H, Oya H, Kojima M, Kanmatsuse K, Kangawa K. Cardiovascular and hormonal effects of subcutaneous administration of ghrelin, a novel growth hormone-releasing peptide, in healthy humans. Clin Sci (Lond) 2003;105:431–435. doi: 10.1042/CS20030184. [DOI] [PubMed] [Google Scholar]
- 16.Vestergaard ET, Dall R, Lange KH, Kjaer M, Christiansen JS, Jorgensen JO. The ghrelin response to exercise before and after growth hormone administration. J Clin Endocrinol Metab. 2007;92:297–303. doi: 10.1210/jc.2006-1435. [DOI] [PubMed] [Google Scholar]
- 17.Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, Heiman ML, Lehnert P, Fichter M, Tschop M. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145:669–673. [PubMed] [Google Scholar]
- 18.Scacchi M, Ida Pincelli A, Cavagnini F. Nutritional status in the neuroendocrine control of growth hormone secretion: the model of anorexia nervosa. Front Neuroendocrinol. 2003;24:200–224. doi: 10.1016/s0091-3022(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 19.Anker SD, Volterrani M, Pflaum CD, Strasburger CJ, Osterziel KJ, Doehner W, Ranke MB, Poole-Wilson PA, Giustina A, Dietz R, Coats AJ. Acquired growth hormone resistance in patients with chronic heart failure: implications for therapy with growth hormone. J Am Coll Cardiol. 2001;38:443–452. doi: 10.1016/s0735-1097(01)01385-7. [DOI] [PubMed] [Google Scholar]
- 20.Gualillo O, Lago F, Casanueva FF, Dieguez C. One ancestor, several peptides post-translational modifications of preproghrelin generate several peptides with antithetical effects. Mol Cell Endocrinol. 2006;256:1–8. doi: 10.1016/j.mce.2006.05.007. [DOI] [PubMed] [Google Scholar]