Abstract
Chromatin boundaries regulate gene expression by modulating enhancer–promoter interactions and insulating transcriptional influences from organized chromatin. However, mechanistic distinctions between these two aspects of boundary function are not well understood. Here we show that SF1, a chromatin boundary located in the Drosophila Antennapedia complex (ANT-C), can insulate the transgenic miniwhite reporter from both enhancing and silencing effects of surrounding genome, a phenomenon known as chromosomal position effect or CPE. We found that the CPE-blocking activity associates with different SF1 sub-regions from a previously characterized insulator that blocks enhancers in transgenic embryos, and is independent of GAF-binding sites essential for the embryonic insulator activity. We further provide evidence that the CPE-blocking activity cannot be attributed to an enhancer-blocking activity in the developing eye. Our results suggest that SF1 contains multiple non-overlapping activities that block diverse transcriptional influences from embryonic or adult enhancers, and from positive and negative chromatin structure. Such diverse insulating capabilities are consistent with the proposed roles of SF1 to functionally separate fushi tarazu (ftz), a non-Hox gene, from the enhancers and the organized chromatin of the neighboring Hox genes.
INTRODUCTION
Chromatin organization has long been known to affect gene activity [for reviews see (1–4)]. Expression of integrated transgenes is also influenced by chromatin organization of the surrounding genome. For example, Drosophila carrying the transgene miniwhite marker display a wide variety of eye colors depending on the transgene insertion site, a phenomenon referred to as chromosomal position effect (CPE) (5,6). In vertebrates, integrated transgenes are often progressively silenced by the neighboring chromatin in an insertion-site-dependent fashion (7,8). Chromatin boundaries are specialized DNAs located between genomic domains of distinct chromatin structure and function. They can block communication between gene promoter and regulatory enhancers, and protect integrated reporter genes from positive or negative effects of the surrounding chromatin (9–21) [see (22–28) for reviews]. Indeed, some of the best-characterized boundary elements were initially identified by their ability to protect reporter transgenes. For example, the Drosophila scs and scs′ elements were shown to protect miniwhite against CPE, resulting in more consistent and lighter eye colors (11). The vertebrate β-globin cHS4 boundary can also protect reporter genes from the silencing effect of the genome (10).
Recent studies indicated that the activity that impedes the spread of silent chromatin within the β-globin boundary, called a barrier, depends on different cis- and trans-factors from the activity that block enhancers. The barrier recruits histone-modification enzymes to establish centers of active chromatin (12,29). These results indicate that the two aspects of boundary function are mediated by distinct mechanisms (13,30). Related mechanisms have also been proposed for certain boundaries in the yeast telomeres or silent mating loci (15,31–36). Although parallels have been drawn between the fly CPE-blocking activity and vertebrate barriers, separation of CPE-blocking and enhancer-blocking activities has not been reported in Drosophila. In particular, the Drosophila Gypsy suHw boundary appears to support both of its enhancer-blocking and CPE-blocking activities through the same DNA sequence and the same zinc finger protein SUHW (37). These observations are inconsistent with a common mechanism underlying all ‘position effects’ in different organisms, and consequently, a common mechanism for all ‘barrier-like’ activities.
To address these questions we have probed the CPE-blocking activity associated with SF1, a 2.4-kb boundary element located in the intergenic region between the non-Hox gene ftz and the homeotic gene Sex comb reduced (Scr) in the Drosophila ANT-C homeotic complex. We have previously shown that SF1 contains a potent embryonic enhancer-blocking activity (38). In this study, we report that SF1 can protect the miniwhite reporter from the influences of organized chromatin surrounding the transgene insertion site. We show that the DNA regions within SF1 that support CPE block is different from the element that mediates enhancer block; and that GAF sites, critical for the latter, is dispensable for the former. Importantly, we provide evidence that the CPE-blocking activity cannot be attributed to a potential enhancer-blocking activity in the developing eye. Our findings suggest that the Drosophila SF1 boundary contains multiple non-overlapping activities that block enhancers or chromatin-mediated effects. These functional properties of SF1 may be important for the insulation of the non-homeotic ftz gene from neighboring enhancers and repressive chromatin associated with homeotic genes. Our results also suggest the diverse mechanisms may underlie ‘chromosomal position effect’, as well as the activities that impede them.
METHODS
Construction of CPE-blocking transgenes
The full-length SF1 and its sub-fragments SF1a–SF1c were generated by PCR using primers containing Not I site and cloned into pCRII/TOPO vector (Invitrogen). The resulting constructs were digested with Not I or Nsi I and the DNA inserts were gel extracted, purified and ligated into the respective sites flanking the miniwhite reporter in the pCaSpeR transformation vector. Site-directed mutagenesis of the two GAF sites in SF1c was performed using the single-stranded DNA method as described previously (38). The base substitution in the GAF sites was done using the following oligonucleotides: 5′ ACAATGAACAGGATCCTGATGAATTA 3′ and 5′ GTTGTGATGCAGATCTGCTTACTTAG 3′.
Construction of enhancer-blocking transgenes
The G5 enhancers (provided by Jumin Zhou) were digested with Bam HI, purified, and ligated into the unique Bam HI site of the CaSpeR vector, resulting in the CA-G5 plasmids. SF1 or suHw insulators were inserted into the unique Not I site (converted from the original Eco RI site) in the CA-G5 plasmids. Details of the embryonic enhancer-blocking assay in Figure 2 are described previously (38).
Figure 2.
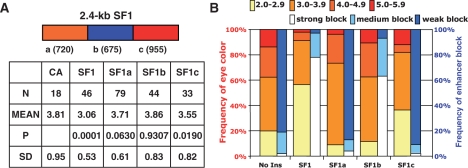
SF1 contains separate enhancer-blocking and CPE-blocking activities. (A) Analysis of CPE-blocking activity in SF1 sub-fragments. Top: A schematic of the three sub-fragments within the SF1 boundary, with size of each fragment in base pairs shown in parentheses. Bottom: A table summarizing the total numbers of lines (N), mean eye color (MEAN), SD and the probability distribution (P) for each group against CA was calculated by chi-square test. (B) Comparison of CPE- and enhancer-blocking activities in SF1 and its sub-fragments. The yellow-orange bar graph summarizes the CPE-blocking activity of no insulator (No ins, CA), SF1 and three SF1 sub-fragments using the assay outlined in Figure 1C. The percentage of lines displaying eye colors within the designated range, as shown on top of bar graph, is indicated on the left y-axis. The white-blue bar graph summarizes the embryonic enhancer-blocking activity in the corresponding DNA elements (38). The percentage of embryos showing strong (70–100%), medium (30–70%) or weak (0–30%) block, as shown on top of bar graph, is indicated on the right y-axis.
P-element-mediated germline transformation
P-element mediated transformation was carried out as described previously (43,44) (Rainbow Transgenic Inc, California). The y1w67c23 and w1118 Drosophila strains were used to generate transgenic lines. Eighteen or more independent lines were generated for all CPE-blocking tests. Five or more independent transgenic lines were obtained and characterized for each enhancer-blocking construct.
Eye color assessment and pigment measurement
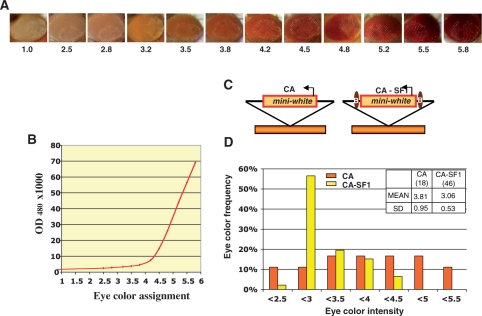
The eye color of 5–7 days old heterozygous females was assigned and color level by visual assessment according to a 12-point scale of progressively darker color shown in Figure 1A, under 10× objective and intermediate illumination with NCL150 cold light source. Eye pigment was extracted from 20 7-day old flies of indicated eye color as described previously (45): flies were homogenized in 100 µl AEA buffer (30% EtOH, 0.1% concentrated HCl) and brought to 1 ml by adding 900 µl AEA. The samples were then vortexed for 30 min and spun for 10 min in a microcentrifuge. Twenty microliters of 0.5% hydrogen peroxide was added to the supernatant to oxidize the extracted pigment. The samples were mixed, spun and measured for absorbance at OD480 using Genova Life Analyzer spectrophotometer. Each OD480 reading was repeated three times and the mean value was used to generate the chart in Figure 1B.
Figure 1.
The CPE-blocking activity of SF1. (A) Eye color intensity standard shown by eyes of w1118 and transgenic flies showing increasing eye color. The number below each eye indicates the color designation, with 1 being the parental strain w1118 and 5.8 being the darkest eye color observed. (B) OD480 absorbance of eye pigment extracted from flies in each eye color category (see Methods section). (C) Schematic representation of unprotected (CaSpeR, or CA) or SF1-flanked (CaSpeR-SF1, or CA-SF1) miniwhite randomly integrated into genome (brown bars). Arrows represent miniwhite promoter and ovals represent the SF1 boundary. (D) Bar graph showing eye color distribution of CA and CA-SF1 transgenic lines. Each independent line was assigned an eye color score according to chart in B. The Y-axis indicates the percentage of lines displaying eye color within the indicated range (shown in X-axis). The inset table provides sample number (N, in parentheses), eye color mean (MEAN) and SD for CA and CA-SF1 transgenes.
Statistical analysis
The P value in Figure 2A is calculated by Chi-square test using an on-line calculator from QuickCalcs (GraphPad Software, Inc., La Jolla, CA), where number of lines in each eye color category was compared to that of the control (CA). Using color distribution expected from CA transgenic lines, the probability (P) of observing eye color distribution as seen in CA-SF1 lines is <0.0001 (χ2 = 16.767 with 1 degree of freedom). Similar calculation was done for CA-SF1a (P < 0.0630); CA-SF1b (P < 0.9307); and CA-SF1c (P < 0.0190). For eye enhancer-blocking assays in Figure 4 with no insulator, suHw or SF1, eye color of transgenic lines was scored according to the color standard in Figure 1A. Data compilation and statistical analyses, except otherwise indicated, were done using the Microsoft excel software.
Figure 4.
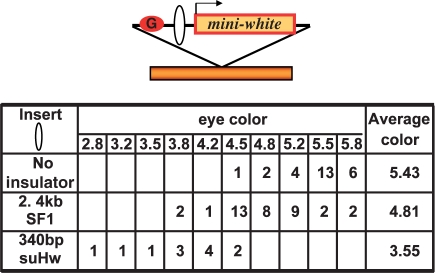
The SF1 contains little enhancer-blocking activity in the developing eye. Top: diagram of eye enhancer-blocking transgenes. The transgene containing G5 enhancer (G, red circle) and the miniwhite transgene (arrow), separated by insert DNA (open oval), is randomly integrated in the chromosome (orange bar). Table: rows from top to bottom, number of lines in each eye color category for G5-miniwhite transgene containing no insert, 2.4-kb SF1 or 340-bp suHw insulators, respectively.
RESULTS
SF1 contains a CPE-blocking activity
Transgenic flies carrying the miniwhite reporter exhibit wide range of eye colors depending on the site of transgene insertion (Figure 1A). Such variation, known as CPE, is attributed to the influences of the surrounding chromatin on an otherwise weak miniwhite promoter (5,6,46). Since the effect of CPE on miniwhite expression has been evaluated by visual assessment of eye color in previous studies, we categorized the eye color of transgenic flies carrying miniwhite into 12 intensity levels, and use them to evaluate the CPE-blocking results reported below (Figure 1A). However, we also defined the eye color standard by measuring the absorbance (OD480) of the eye pigment extracted from these flies (Figure 1B, see ‘Methods’ section). Our measurements indicate that the CPE-caused variation in the miniwhite expression, as measured by OD480, can range up to 70-fold, and much of it is above the sensitive range of the human eye.
To test the ability of SF1 to protect miniwhite from CPE, transgenic flies carrying the miniwhite reporter with or without the protection of flanking SF1 were assigned an eye color intensity level according to the color standard (Figure 1C). We found that fly lines carrying unprotected miniwhite in the pCaSpeR vector (CA) exhibit a wide range of eye colors with a comparable number of lines in each color category from 2.5 to 5.5 (Figure 1C and D). This is indicative of a strong CPE. In contrast, fly lines carrying miniwhite protected by the full-length SF1 (brown ovals, CA-SF1) display predominantly yellow to light orange eye colors (Figure 1C and D). Among 46 CA-SF1 lines, 78% exhibited eye colors between 3.0 and 3.5 (compared to 27% among unprotected CA lines) with few lines exhibiting extremely light or dark colors. In addition to decreasing eye color variation, shown by lower standard deviation (SD, Figure 1D), flanking SF1 also appeared to reduce the average eye color (MEAN, Figure 1D). Both such effects have been previously reported for the scs and suHw insulators and have been attributed to the insulation of primarily positive influences of the surrounding chromatin (11,37). The average eye color in SF1 protected lines appears to be lighter than those in suHw and scs protected lines. This could be due to the slight variances in the assay parameters, such as the color standard, the inclusion of a yellow marker in the P-element in the previous studies, or to potential repressive effects of SF1 (37). Taken together, our results indicate that SF1 contains a potent CPE-blocking activity.
Molecular dissection of the SF1 CPE-blocking activity
To identify and characterize the CPE-blocking activity within SF1, we dissected the 2.4-kb full-length boundary into three fragments of comparable sizes (SF1a, SF1b and SF1c, Figure 2A), and tested them individually for CPE-blocking activity. For each SF1 sub-fragment, a large number of independent lines were scored for eye colors (N, Figure 2A and B). Compared to the no insulator controls (No ins, CA in Figure 1C and D), SF1a- and SF1c-containing flies showed more lines with yellow and light-orange eyes, and/or fewer lines with dark-red eyes (Figure 2B). This is indicative of CPE-blocking activity associated with these two elements, although both appeared significantly weaker than that of the full-length SF1. In contrast, the eye color variation among SF1b lines was similar to those of the CaSpeR control (Figure 2B). Statistical analysis of sample groups indicates that SF1, SF1a and SF1c, but not SF1b, showed significant difference in the eye color distribution from the CaSpeR control (see P-value in Figure 2A).
The lack of CPE-blocking activity in the SF1b region is somewhat unexpected because this element was previously found to exhibit about 80% of the enhancer-blocking activity of full-length SF1 in a transgenic embryo enhancer-blocking assay [for comparison see blue bars in Figure 2B (38)]. In contrast, the SF1a and SF1c showed little insulator activity in the enhancer-blocking assay. This result suggests that the SF1 boundary may contain two potent and non-overlapping activities: one that blocks embryonic enhancers and the other insulates against positive and negative CPE in the developing eye.
CPE-blocking activity is independent of GAF sites and TATA-like motifs
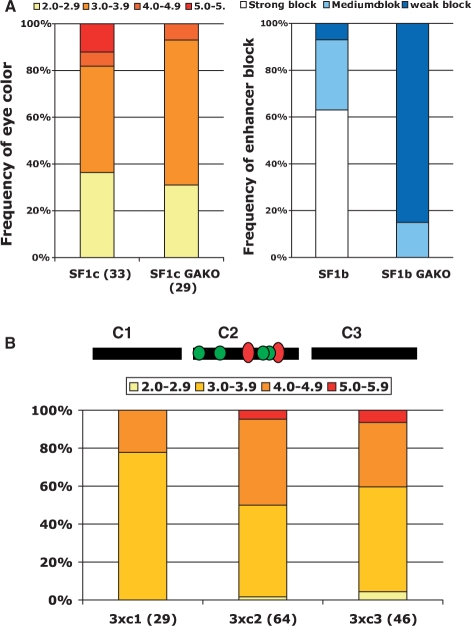
To further analyze the distinctions between the enhancer- and CPE-blocking activities within SF1, we tested whether they require different sequence motifs, including binding sites for insulator protein GAF, a BTB/zinc finger protein. Our previous study showed that mutations in GAF binding sites in the SF1b element abolished its enhancer-blocking activity (Figure 3A, right panel) (38,47). To test whether GAF sites are also required for the CPE-blocking activity, we replaced the two GAF sites in SF1c with unrelated sequences. As shown in Figure 3A, mutations in the GAF sites in SF1c (SF1cGAKO) did not compromise the CPE-blocking activity. In fact, the proportion of SF1cGAKO lines with medium to light eye colors is slightly higher. This could be due to the loss of binding by GAF, which is also known to mediate transcription activation. Our results suggest that the CPE-blocking activity in SF1c and the insulator activity in SF1b depend on distinct cis- and trans-components (Figure 3A).
Figure 3.
CPE-blocking activity is independent of GAF sites and TATA-like motifs in SF1c. (A) Effect of GAF site mutations on CPE-blocking activity of SF1c and enhancer-blocking activity of SF1b. Left panel: bar graph summarizing CPE-blocking activity of SF1c (left) and SF1cGAKO (right). The percentage of lines displaying eye colors within the designated range, as shown on top of bar graph, is indicated on the left y-axis. The positions of the GAF sites (red ovals) within SF1c are indicated in diagram on top in (B). Right panel: bar graph summarizing enhancer-blocking activity of SF1b (left) and SF1bGAKO [right, (38)]. (B) CPE-blocking activity of SF1c sub-fragments. Top: diagram of three SF1c sub-regions (SF1c1-c3), with positions of GAF sites (red ovals) and TATA-like motifs (green circles) indicated. Bottom: bar graph summarizing CPE-blocking activity of three tandem copies of SF1c1-3. The percentage of lines displaying eye colors within the designated range, as shown on top of bar graph, is indicated on the left y-axis.
In addition to GAF sites, SF1c also contains multiple TATA-like motifs in its central region (green circles, Figure 3B). Similar AT-rich motifs and other promoter-like sequences are found in several other boundary elements, prompting the hypothesis that these cis-elements could serve as a sink of regulatory influences (48). To further define the cis-requirement of CPE-blocking activity in SF1c and to test whether TATA-motifs contribute to the CPE-blocking activity, we dissected the SF1c region further into three sub-fragments and tested each in three tandem copies in miniwhite-protection assay (Figure 3B). Our results indicate that the central region of SF1c (3c2) does not contain higher level of CPE-blocking activity than the neighboring regions, suggesting that GAF and TATA-like motifs do not contribute significantly to the CPE-blocking function of SF1c.
The CPE-blocking activity is distinct from a late eye enhancer-blocking activity in SF1
The Drosophila CPE has often been compared to the vertebrate position effect, which is the gradual silencing of integrated reporters by the genome or chromatin surrounding the transgene. However, key differences exist between the two phenomena. First, the Drosophila CPE appears to be both negative and positive in nature, as shown by the decrease of both extreme light and extreme dark eye colors in boundary-protected transgenic lines. Second, the Drosophila CPE is more dramatically manifested in the behavior of the miniwhite reporter, which is also used in most boundary protection studies (11,37). Other features, including a lack of time-dependence in the Drosophila CPE, also suggest mechanistic differences between the two effects. An alternative explanation for the Drosophila CPE suggests the action of eye-specific enhancers or silencers around transgene insertion sites. If this were true, the two non-overlapping activities in SF1 would represent two enhancer-blocking activities, one that functions in the embryo and the other in the developing eye.
To distinguish between these two mechanisms, we tested SF1 for its ability to block enhancers in the same tissue and developmental stage as in the CPE-blocking assay. We used the eye-specific glass multiple repeat (G5) and the miniwhite reporter to perform the blocking tests (Figure 4) (49,50). As controls, we also made G5-miniwhite transgene with no insulator, or with the suHw insulator, which has been shown to contain enhancer-blocking activity in the adult eye. We found that the G5 enhancer without intervening insulator can strongly activate miniwhite expression in majority of transgenic lines, resulting an average eye color of 5.43, which corresponds to OD480 level of 53 (Figure 4, no insulator). The 340-bp suHw placed between G5 and miniwhite strongly reduced the average eye color and shifted the peak of eye color distribution to the lighter range, with an average eye color of 3.55 and OD480 level of 4 (Figure 4, suHw insulator). The 93% reduction in the OD480 level is consistent with the enhancer-blocking activity of suHw in the eye tissue. However, the 2.4-kb SF1 only weakly reduced average eye color, with an average eye color of 4.81 and OD480 level of 30 (Figure 4, SF1 insulator). This result suggests the SF1 contains much weaker or little enhancer-blocking activity in the adult eye, especially considering the 2.4-kb linear distance that separates the G5 enhancer from miniwhite due to the insertion of SF1. This is in strong contrast to the strong CPE-blocking activity SF1 exhibited in the same tissue. It is also in strong contrast to its potent activity in blocking diverse embryonic enhancers (Figure 2B) (38). Taken together, our results do not support the hypothesis that an eye-specific enhancer-blocking activity is responsible for the CPE-blocking behavior of SF1. Our results also indicate that the ability of SF1 to block enhancers in the eye is weak, suggesting that the boundary element may be regulated in a stage-specific and/or tissue-specific fashion.
DISCUSSION
In this study we have characterized the CPE-blocking activity associated with the Drosophila SF1 boundary. Our results suggest that SF1 contains at least two non-overlapping boundary activities, a strong embryonic enhancer-blocking activity associated with SF1b element, and strong CPE- blocking activities associated with SF1a and SF1c elements. Mutagenesis and dissection studies indicate that the CPE-blocking activity depends on different cis and trans components from the embryonic enhancer-blocking activity. We further showed that the CPE-blocking activity is unlikely to be attributed to a late stage enhancer-blocking activity in the developing eye.
Drosophila CPE, manifested predominantly by the enhancement or suppression of miniwhite, was thought to result from the active or repressive chromatin around the transgene insertion sites.
CPE-blocking activity, therefore, has been compared to the vertebrate barrier activity and long used as a defining feature for chromatin boundaries in Drosophila (11,48). However, the ability of Drosophila boundaries to block both positive and negative CPE argues against a shared mechanism between these elements and the vertebrate barriers such as the β-globin barrier, which counter the progression of silent chromatin by establishing centers of active chromatin (13,29).
An alternative explanation for the Drosophila CPE invokes the action of enhancers or silencers near the integrated transgenes. This model is consistent with the ability of boundaries to block both positive and negative effects. It also accommodates the fact that for some Drosophila boundaries the CPE-blocking activity depends on the same cis- and trans- components as the enhancer-blocking activity (14,37,39,40,51). However, this hypothesis would predict widespread presence of eye-specific enhancers and silencers in the genome to account for the prevalence of the CPE effect.
Our analysis of the SF1 boundary provides the first evidence that the CPE-blocking activity can be separated from the enhancer-blocking activity, suggesting that these two insulating functions may be mediated through distinct mechanisms in Drosophila. It is possible that the CPE-blocking activities in Drosophila form structures that are transcriptionally ‘neutral’, and able to insulate the weak miniwhite promoter from the effect of local chromatin. It is unclear, however, whether such local chromatin effect can compare, in range or strength, to that of constitutive heterochromatin, or whether such effect influences Drosophila gene promoters in general. A previous study showed that human MAR sequence could facilitate CPE blocking either arranged to flank the reporter or placed upstream in tandem copies (41). This is distinct from the CPE-blocking behavior of Drosophila boundaries such as suHw and scs, further demonstrating the diverse mechanisms that could influence the regulation of the miniwhite reporter.
The SF1 boundary is located in the Scr-ftz genomic interval in the Drosophila ANT-C, which differs from other Hox clusters in that it contains both homeotic and non-homeotic genes. Proper regulation of these genes requires modulation of enhancer traffic as well as insulation of chromatin-mediated effects. The SF1 compound boundary fulfills both requirements: the SF1b element can restrict long-range enhancers from interfering with the ftz and Scr promoter (38); and the SF1a and SF1c elements may protect the non-Hox ftz gene from chromatin-mediated regulation, such as the PRE/TRE maintenance of the neighboring Hox genes. Separation and selective association of different types of boundary activities could determine the regulatory role of compound boundaries and provide flexibility in their function.
FUNDING
Funding for open access charge: National Institutes of Health GM058458.
Conflict of interest statement. None declared.
Footnotes
The authors wish it to be known that, in their opinion, the first two authors should be regarded as joint First Authors.
REFERENCES
- 1.Bell A, Boyes J, Chung J, Pikaart M, Prioleau MN, Recillas F, Saitoh N, Felsenfeld G. The establishment of active chromatin domains. Cold Spring Harb. Symp. Quant. Biol. 1998;63:509–514. doi: 10.1101/sqb.1998.63.509. [DOI] [PubMed] [Google Scholar]
- 2.Richards EJ, Elgin SC. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell. 2002;108:489–500. doi: 10.1016/s0092-8674(02)00644-x. [DOI] [PubMed] [Google Scholar]
- 3.Grewal SI, Moazed D. Heterochromatin and epigenetic control of gene expression. Science. 2003;301:798–802. doi: 10.1126/science.1086887. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad K, Henikoff S. Epigenetic consequences of nucleosome dynamics. Cell. 2002;111:281–284. doi: 10.1016/s0092-8674(02)01081-4. [DOI] [PubMed] [Google Scholar]
- 5.Levis R, Hazelrigg T, Rubin GM. Effects of genomic position on the expression of transduced copies of the white gene of Drosophila. Science. 1985;229:558–561. doi: 10.1126/science.2992080. [DOI] [PubMed] [Google Scholar]
- 6.Pirrotta V, Steller H, Bozzetti MP. Multiple upstream regulatory elements control the expression of the Drosophila white gene. EMBO J. 1985;4:3501–3508. doi: 10.1002/j.1460-2075.1985.tb04109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Festenstein R, Sharghi-Namini S, Fox M, Roderick K, Tolaini M, Norton T, Saveliev A, Kioussis D, Singh P. Heterochromatin protein 1 modifies mammalian PEV in a dose- and chromosomal-context-dependent manner. Nat. Genet. 1999;23:457–461. doi: 10.1038/70579. [DOI] [PubMed] [Google Scholar]
- 8.Ayyanathan K, Lechner MS, Bell P, Maul GG, Schultz DC, Yamada Y, Tanaka K, Torigoe K, Rauscher FJ., 3rd. Regulated recruitment of HP1 to a euchromatic gene induces mitotically heritable, epigenetic gene silencing: a mammalian cell culture model of gene variegation. Genes Dev. 2003;17:1855–1869. doi: 10.1101/gad.1102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell AC, West AG, Felsenfeld G. The protein CTCF is required for the enhancer blocking activity of vertebrate insulators. Cell. 1999;98:387–396. doi: 10.1016/s0092-8674(00)81967-4. [DOI] [PubMed] [Google Scholar]
- 10.Chung JH, Whiteley M, Felsenfeld G. A 5′ element of the chicken beta-globin domain serves as an insulator in human erythroid cells and protects against position effect in Drosophila. Cell. 1993;74:505–514. doi: 10.1016/0092-8674(93)80052-g. [DOI] [PubMed] [Google Scholar]
- 11.Kellum R, Schedl P. A position-effect assay for boundaries of higher order chromosomal domains. Cell. 1991;64:941–950. doi: 10.1016/0092-8674(91)90318-s. [DOI] [PubMed] [Google Scholar]
- 12.Recillas-Targa F, Pikaart MJ, Burgess-Beusse B, Bell AC, Litt MD, West AG, Gaszner M, Felsenfeld G. Position-effect protection and enhancer blocking by the chicken beta-globin insulator are separable activities. Proc. Natl Acad. Sci. USA. 2002;99:6883–6888. doi: 10.1073/pnas.102179399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West AG, Huang S, Gaszner M, Litt MD, Felsenfeld G. Recruitment of histone modifications by USF proteins at a vertebrate barrier element. Mol. Cell. 2004;16:453–463. doi: 10.1016/j.molcel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Geyer PK, Corces VG. DNA position-specific repression of transcription by a Drosophila zinc finger protein. Genes Dev. 1992;6:1865–1873. doi: 10.1101/gad.6.10.1865. [DOI] [PubMed] [Google Scholar]
- 15.Donze D, Adams CR, Rine J, Kamakaka RT. The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev. 1999;13:698–708. doi: 10.1101/gad.13.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai H, Levine M. Modulation of enhancer-promoter interactions by insulators in the Drosophila embryo. Nature. 1995;376:533–536. doi: 10.1038/376533a0. [DOI] [PubMed] [Google Scholar]
- 17.Morcillo P, Rosen C, Dorsett D. Genes regulating the remote wing margin enhancer in the Drosophila cut locus. Genetics. 1996;144:1143–1154. doi: 10.1093/genetics/144.3.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao S, Osborne CS, Bharadwaj RR, Pasceri P, Sukonnik T, Pannell D, Recillas-Targa F, West AG, Ellis J. Retrovirus silencer blocking by the cHS4 insulator is CTCF independent. Nucleic Acids Res. 2003;31:5317–5323. doi: 10.1093/nar/gkg742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J, Ashe H, Burks C, Levine M. Characterization of the transvection mediating region of the abdominal- B locus in Drosophila. Development. 1999;126:3057–3065. doi: 10.1242/dev.126.14.3057. [DOI] [PubMed] [Google Scholar]
- 20.Hagstrom K, Muller M, Schedl P. Fab-7 functions as a chromatin domain boundary to ensure proper segment specification by the Drosophila bithorax complex. Genes Dev. 1996;10:3202–3215. doi: 10.1101/gad.10.24.3202. [DOI] [PubMed] [Google Scholar]
- 21.Golovnin A, Birukova I, Romanova O, Silicheva M, Parshikov A, Savitskaya E, Pirrotta V, Georgiev P. An endogenous Su(Hw) insulator separates the yellow gene from the Achaete-scute gene complex in Drosophila. Development. 2003;130:3249–3258. doi: 10.1242/dev.00543. [DOI] [PubMed] [Google Scholar]
- 22.Celniker SE, Drewell RA. Chromatin looping mediates boundary element promoter interactions. Bioessays. 2007;29:7–10. doi: 10.1002/bies.20520. [DOI] [PubMed] [Google Scholar]
- 23.Wallace JA, Felsenfeld G. We gather together: insulators and genome organization. Curr. Opin. Genet. Dev. 2007;17:400–407. doi: 10.1016/j.gde.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei GH, Liu de P, Liang CC. Chromatin domain boundaries: insulators and beyond. Cell Res. 2005;15:292–300. doi: 10.1038/sj.cr.7290298. [DOI] [PubMed] [Google Scholar]
- 25.Cai HN. In: Gene Expression and Regulation. Ma J, editor. Beijing: Higher Education Press; 2006. [Google Scholar]
- 26.Sipos L, Gyurkovics H. Long-distance interactions between enhancers and promoters. FEBS J. 2005;272:3253–3259. doi: 10.1111/j.1742-4658.2005.04757.x. [DOI] [PubMed] [Google Scholar]
- 27.Valenzuela L, Kamakaka RT. Chromatin insulators. Annu. Rev. Genet. 2006;40:107–138. doi: 10.1146/annurev.genet.39.073003.113546. [DOI] [PubMed] [Google Scholar]
- 28.Gaszner M, Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat. Rev. Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- 29.Mutskov VJ, Farrell CM, Wade PA, Wolffe AP, Felsenfeld G. The barrier function of an insulator couples high histone acetylation levels with specific protection of promoter DNA from methylation. Genes Dev. 2002;16:1540–1554. doi: 10.1101/gad.988502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang S, Li X, Yusufzai TM, Qiu Y, Felsenfeld G. USF1 recruits histone modification complexes and is critical for maintenance of a chromatin barrier. Mol. Cell Biol. 2007;27:7991–8002. doi: 10.1128/MCB.01326-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrari S, Simmen KC, Dusserre Y, Muller K, Fourel G, Gilson E, Mermod N. Chromatin domain boundaries delimited by a histone-binding protein in yeast. J. Biol. Chem. 2004;279:55520–55530. doi: 10.1074/jbc.M410346200. [DOI] [PubMed] [Google Scholar]
- 32.Bi X, Broach JR. Chromosomal boundaries in S. cerevisiae. Curr. Opin. Genet. Dev. 2001;11:199–204. doi: 10.1016/s0959-437x(00)00179-9. [DOI] [PubMed] [Google Scholar]
- 33.Oki M, Kamakaka RT. Blockers and barriers to transcription: competing activities? Curr. Opin. Cell Biol. 2002;14:299–304. doi: 10.1016/s0955-0674(02)00327-7. [DOI] [PubMed] [Google Scholar]
- 34.Donze D, Kamakaka RT. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J. 2001;20:520–531. doi: 10.1093/emboj/20.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bi X, Braunstein M, Shei GJ, Broach JR. The yeast HML I silencer defines a heterochromatin domain boundary by directional establishment of silencing. Proc. Natl Acad. Sci. USA. 1999;96:11934–11939. doi: 10.1073/pnas.96.21.11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bi X, Broach JR. UASrpg can function as a heterochromatin boundary element in yeast. Genes Dev. 1999;13:1089–1101. doi: 10.1101/gad.13.9.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roseman RR, Pirrotta V, Geyer PK. The su(Hw) protein insulates expression of the Drosophila melanogaster white gene from chromosomal position-effects. EMBO J. 1993;12:435–442. doi: 10.1002/j.1460-2075.1993.tb05675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belozerov VE, Majumder P, Shen P, Cai HN. A novel boundary element may facilitate independent gene regulation in the Antennapedia Complex of Drosophila. EMBO J. 2003;22:3113–3121. doi: 10.1093/emboj/cdg297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ip YT, Levine M, Small SJ. The bicoid and dorsal morphogens use a similar strategy to make stripes in the Drosophila embryo. J. Cell Sci. Suppl. 1992;16:33–38. doi: 10.1242/jcs.1992.supplement_16.5. [DOI] [PubMed] [Google Scholar]
- 40.Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science. 1982;218:348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- 41.Cai HN, Zhang Z, Adams JR, Shen P. Genomic context modulates insulator activity through promoter competition. Development. 2001;128:4339–4347. doi: 10.1242/dev.128.21.4339. [DOI] [PubMed] [Google Scholar]
- 42.Gindhart JG, Jr., Kaufman TC. Identification of Polycomb and trithorax group responsive elements in the regulatory region of the Drosophila homeotic gene Sex combs reduced. Genetics. 1995;139:797–814. doi: 10.1093/genetics/139.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levis R, O'Hare K, Rubin GM. Effects of transposable element insertions on RNA encoded by the white gene of Drosophila. Cell. 1984;38:471–481. doi: 10.1016/0092-8674(84)90502-6. [DOI] [PubMed] [Google Scholar]
- 44.Farkas G, Gausz J, Galloni M, Reuter G, Gyurkovics H, Karch F. The Trithorax-like gene encodes the Drosophila GAGA factor. Nature. 1994;371:806–808. doi: 10.1038/371806a0. [DOI] [PubMed] [Google Scholar]
- 45.Kuhn EJ, Geyer PK. Genomic insulators: connecting properties to mechanism. Curr. Opin. Cell Biol. 2003;15:259–265. doi: 10.1016/s0955-0674(03)00039-5. [DOI] [PubMed] [Google Scholar]
- 46.Moses K, Rubin GM. Glass encodes a site-specific DNA-binding protein that is regulated in response to positional signals in the developing Drosophila eye. Genes Dev. 1991;5:583–593. doi: 10.1101/gad.5.4.583. [DOI] [PubMed] [Google Scholar]
- 47.Ellis MC, O'Neill EM, Rubin GM. Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development. 1993;119:855–865. doi: 10.1242/dev.119.3.855. [DOI] [PubMed] [Google Scholar]
- 48.Patton JS, Gomes XV, Geyer PK. Position-independent germline transformation in Drosophila using a cuticle pigmentation gene as a selectable marker. Nucleic Acids Res. 1992;20:5859–5860. doi: 10.1093/nar/20.21.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mallin DR, Myung JS, Patton JS, Geyer PK. Polycomb group repression is blocked by the Drosophila suppressor of Hairy-wing [su(Hw)] insulator. Genetics. 1998;148:331–339. doi: 10.1093/genetics/148.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerasimova TI, Gdula DA, Gerasimov DV, Simonova O, Corces VG. A Drosophila protein that imparts directionality on a chromatin insulator is an enhancer of position-effect variegation. Cell. 1995;82:587–597. doi: 10.1016/0092-8674(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 51.Namciu SJ, Fournier RE. Human matrix attachment regions are necessary for the establishment but not the maintenance of transgene insulation in Drosophila melanogaster. Mol. Cell Biol. 2004;24:10236–10245. doi: 10.1128/MCB.24.23.10236-10245.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]