Abstract
The encephalopathy caused by HIV, known clinically as HIV-associated dementia (HAD) and pathologically as HIV encephalitis (HIVE), results from intense infiltration of mononuclear cells, productive replication of the virus in monocyte-derived macrophages/microglia, abortive replication in astrocytes and activation of macrophages/microglia and astrocytes leading to neuronal degeneration in the brains of infected persons. Recent findings have suggested that development of HAD is based more on the activation process than on direct evidence of virus replication in the brain. Since HAD is based on the encephalitic process, major studies have been directed to the mechanisms regulating the inflammatory process. Monocyte chemoattractant protein 1, MCP-1, is a chemokine that is implicated in this process and also in the development of activation in the brain. In this review, we have attempted to identify mechanisms that induce expression of MCP-1 in the brain and the role that it plays in recruitment of mononuclear cells from blood to brain and in the activation processes of inflammatory and neural cells that lead to development of degenerative changes in the neuronal population.
Keywords: HIV-1, HAD, Neurons, Astrocytes, Macrophages, Blood Brain Barrier, Review
2. INTRODUCTION
In recent years the incidence of HIV-associated dementia (HAD) as an AIDS-defining illness has actually increased (1–4) with more prevalence of minor cognitive/motor disorder than frank dementia (5, 6). Approximately 20% of AIDS patients develop neurological deficits severe enough to be diagnosed as dementia (1) and therefore, HAD still remains a significant independent risk factor for AIDS-related death.
The CNS has been thought to be an immunologically privileged organ. However, increasing evidence suggests that inflammation is actively involved in the pathogenesis of HAD (7–10). HIV encephalitis (HIVE), the pathologic correlate of HAD is characterized by the presence of unusually large numbers of HIV-infected macrophages in the brain, formation of multi-nucleated giant cells, activation of astrocytes and microglia, all accompanied by cytokine/chemokine dysregulation, and neuronal degeneration (7, 11–14). The highly productive infection in macrophages in the brain is also accompanied by an abortive infection in astrocytes (15–18). Although the primary cell types infected by HIV-1 in the brain are macrophages/microglia, and to a lesser extent, astrocytes but not neurons, the low numbers of infected cells in the brain do not correlate with the severity of encephalopathy. Rather, the severity of HAD/HIVE correlates with the presence of activated glial cells rather than with the presence and amount of HIV-infected cells in the brain and the current thinking about the disease is that CNS injury is mainly caused by the release of neurotoxic factors by immune-activated glial cells (7, 13). The combined infection of macrophages/microglia and astrocytes leads to excessive production of viral gene products and host immune response factors, prominent among which is the monocyte chemoattractant protein-1 (MCP-1) (19, 20). MCP-1, also known as CCL2, is produced in response to proinflammatory stimuli by a wide variety of cells, including macrophages, endothelial cells, microglia, and astrocytes (21). MCP-1 attracts monocytes, macrophages, basophils, mast cells, T lymphocytes, natural killer (NK) cells, and dendritic cells to sites of injury, both peripherally and within the brain.
Numerous studies strongly suggest increased MCP-1 in CNS to be responsible for HIV-encephalitis (22–26). It is overproduced during HIVE and accumulates in the CSF and brains of immunocompromised patients with HIV dementia (23)and HIVE (27, 28) and in macaques with SIV encephalitis (SIV-E) (26, 29, 30). Serial analysis of cerebrospinal fluid (CSF) in HIV-infected individuals (22) and in SIV-infected macaques (26) showed that MCP-1 levels increase in CSF before the onset of clinical neurological disease and thus can be used as a prognostic factor for later development of HIV-encephalitis.
In the following sections the role of MCP-1 will be examined in different aspects of the processes that lead to development of HAD.
3. REGULATION OF MCP-1 IN ASTROCYTES
Astrocytes are the predominant cell type in CNS, accounting for almost 70% cells in the brain. Although astrocytes do not support efficient replication of HIV-1, they are thought to be a substantial contributor to HIV-related neuropathogenesis. MCP-1 expression in astrocytes, the main producer of this chemokine in the CNS, is increased following infection of the cells with HIV-1 (23, 31, 32). One of the factors that have been shown to activate MCP-1 production from astrocytes is the HIV-1 transactivator viral protein, Tat. Tat has been shown to upregulate the expression of MCP-1 in human fetal astrocytes (23, 33, 34). The mechanism of this phenomenon was explored in subsequent studies (33) using a CCL2 promoter containing reporter constructs. Tat induced MCP-1 expression in this study was found to be regulated at the transcriptional level through activation of AP-1 and NF-κB transcription factors. Furthermore, in the later studies by Sheng et al, the use of a synthetic ligand against κ-opioid receptor (U50, 488) resulted in inhibition of Tat-induced MCP-1 production in primary human astrocytes (35). This suppression was mediated by down regulation of Tat-induced NF-κB activation (35). In Tat-treated human astrocytes, inhibition of protein kinase C (PKC) led to a decrease in MCP-1 production (36, 37). This data suggested that Tat may be able to influence MCP-1 expression by acting as a cytokine itself (37). Other evidence suggested that induction of MCP-1 in Tat-treated cells may be further increased by Tat’s ability to induce the expression of other cytokines that have direct effects on MCP-1 production (38–40).
We examined SHIV89.6P, an HIV/SIV chimeric that expresses HIV envelope, for its ability to induce changes in MCP-1 release in the astrocytic cell line CCF-STTG1 and found that, similar to findings reported for Tat (23, 33, 34), the infectious virus, SHIV89.6P, also caused increase in MCP-1 release from astrocytes as compared to untreated control cells (data not shown). Several intracellular pathways were activated following SHIV treatment of astrocytes; however, not all of these pathways were found to have a direct effect on MCP-1 expression. Exposure of astrocytes to SHIV89.6P or Tat resulted in activation of AKT, JNK and ERK1/2 MAP kinases (data not shown). Inhibition of these kinases with the use of pharmacological inhibitors resulted in decreased expression of MCP-1; however none of the inhibitors were able to completely abrogate the expression of MCP-1 (Figure 1A). This indicated that these pathways most likely operate together to converge on a common transcription factor such a s N F-κB, which subsequently mediates MCP-1 expression. Previous studies have alluded to the role of NF-κB in modulation of MCP-1 expression (33, 35). Our studies supported this hypothesis showing that NF-κB in astrocytes treated with SHIV89.6P translocated to the nucleus of the infected cells (Figure 1B). These findings were further confirmed by pre-treating the cells with the specific inhibitor of NF-κB prior to virus exposure. Significant inhibition of MCP-1 was observed in the presence of the pharmacological inhibitor for NF-κB, thus underscoring the role of this transcription factor in virus induced MCP-1 expression.
Figure 1.
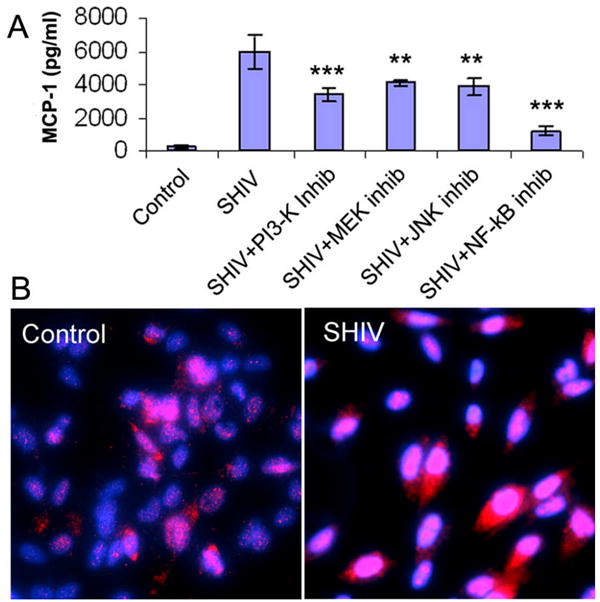
SHIV89.6P induced signaling pathways in CCF-STTG1 Astrocytes. A) Inhibition of the SHIV89.6P induced MCP-1 expression by PI3-Kinase (LY294002), MEK1/2 (U0126), JNK (JNK Inhibitor II), NFκB (TPCK) inhibitors. Serum-starved astrocytes were pre-incubated with medium alone or with 20μM inhibitor for 30 min, followed by incubation with virus for 6hrs. MCP-1 expression was then analyzed by ELISA in the collected supernatants. Values are mean + S.D. from three independent experiments (*** p<0.001 vs values with control). B) Translocation of activated NFκB into nucleus of CCF-STTG1 cells by SHIV 89.6P. Astrocytes grown on cover-slips were either untreated (left panel) or treated with SHIV89.6P for 30 min (right panel), and stained with anti-NF-κB p65 polyclonal antibody followed by treatment with Alexa Fluor 594-conjugated secondary antibody. After the final washing, the slides were mounted in Slow Fade antifade reagent (with DAPI, blue stain) and images were captured by fluorescence microscopy (Magnification 200X).
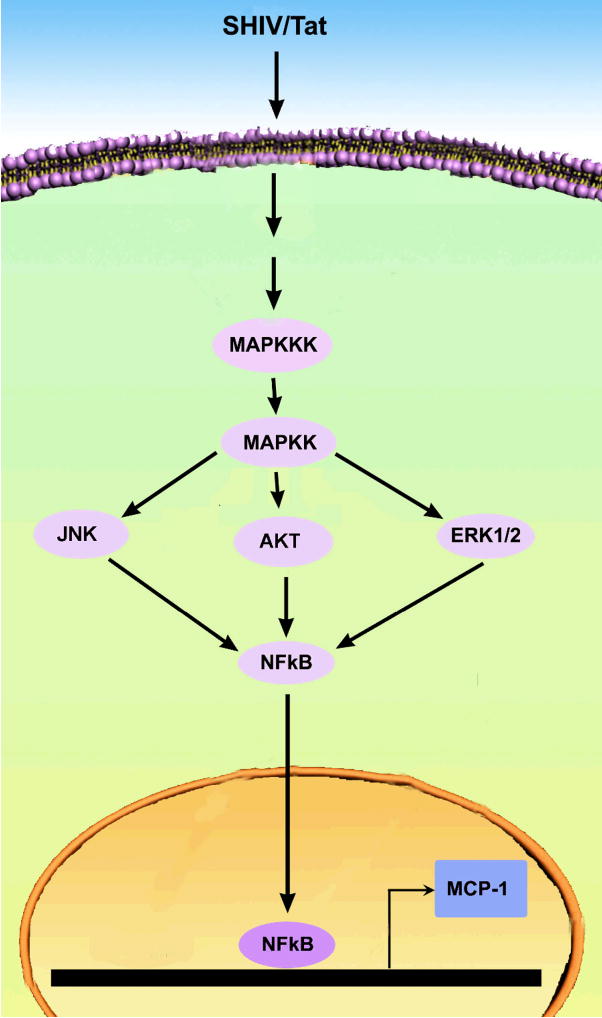
Figure 2 is a schematic illustrating the possible signaling pathway (s) that are activated by SHIV or viral protein Tat to mediate the up regulation of MCP-1 expression in astrocytes. Upon binding of SHIV/Tat there is an activation of MAP Kinase signaling which in turn can initiate the phosphorylation of JNK, ERK1/2, or the AKT pathways. All of these pathways culminate in translocation of the transcription factor NF-κB into the nucleus, its binding to the MCP-1 promoter followed by increased transcription.
Figure 2.
Schematic of various signaling pathways involved in SHIV/Tat-mediated induction of MCP-1 in astrocytes.
4. REGULATION OF MCP-1 IN MACROPHAGES
Study by Mengozzi et al on HIV-infection of human monocyte-derived macrophages demonstrated increased expression and secretion of MCP-1 by macrophages productively infected with either R5 or X4-coreceptor utilizing viruses (28). Our previous studies on infection in rhesus macrophages with SHIVKU2, an X4 virus or SIVmac251, an R5 virus, also showed that the infection in these cells led to increased production of MCP-1 (29). This was demonstrated in cell culture experiments and in brain sections where there was co-localization of markers specific for macrophages and enhanced expression of MCP-1 (30).
Exposure of macrophages or microglia to Tat protein also resulted in superinduction of MCP-1 in a concentration dependent manner (41, 42). In a study of the effects of Tat on cultured microglial cells, Eugenin et al showed that Tat caused enhanced secretion of MCP-1 and this was associated with actin rearrangement, formation of microglial processes (42) and migration of microglia to areas of viral infiltration within the brain. Not only did the infection lead to enhanced production of MCP-1 but exogenous application of MCP-1 to infected CD4+ peripheral blood mononuclear cells resulted in enhanced virus replication (43).
5. MCP-1 AND THE BLOOD BRAIN BARRIER
Macrophages are terminally differentiated cells that originate from monocytes and cannot undergo further cell division. Therefore, the large numbers of macrophages in the HIV-infected brain can only be explained by pathophysiological changes in which large numbers of monocytes are recruited into the brain from the blood, across the tight junctioned endothelial cells comprising the blood-brain barrier (BBB). MCP-1 is known to contribute in neuropathogenesis by facilitating the recruitment of infected and/or activated monocytes into the brain (20, 22, 26, 44). Enhanced transmigration of HIV-infected leukocytes across an astrocyte/endothelial cell coculture model of BBB in response to MCP-1 has been observed compared to transmigration of uninfected leukocytes (20). This increase in migration of HIV-infected leukocytes coincided with the increased expression of the MCP-1 receptor (CCR2) on HIV-infected leukocytes. MCP-1 mediated transmigration of leukocytes across BBB has also been demonstrated to be dependent upon expression of ICAM-1 and E-selectin adhesion molecules on endothelial cells (45). The enhanced expression of ICAM-1, VCAM-1 and E-selectin correlate with the extent of leukocyte infiltration in HIV-encephalitis and various other CNS inflammatory diseases (46–48).
The production of MCP-1 by macrophages (28), astrocytes (45) and brain endothelial cells (49) provided compelling evidence that this chemokine is responsible for the transmigration of lymphocytes and monocytes across the BBB into the CNS during inflammatory diseases. In addition to increased expression of MCP-1 by HIV/SIV infected macrophages, the interaction of HIV-infected macrophages with brain microvascular endothelial cells results in further up regulation of MCP-1 expression in macrophages (50). In a further study, Weiss et al, showed that treatment of cocultures on the astrocyte side with HIV-Tat results in enhanced secretion of MCP-1 from astrocytes but not from endothelial cells (51).
Recent studies indicated that MCP-1 not only functions as a contributor to enhanced infection in the brain by recruiting large number of macrophages (virus host cells) in the brain (23) but also plays a major factor in BBB disruption (52). Both in vitro and in vivo studies suggest that MCP-1 can directly alter the permeability of BBB by altering expression or redistribution of occludin, claudin-5, zona occludens (ZO)-1 and ZO-2 tight junction proteins (TJPs) (52, 53). Further, the MCP-1 mediated changes in the BBB were absent in the mice lacking the CCR2 receptor and were reduced when mice were depleted of monocyte/macrophages by clondronate liposomes (52) suggesting that recruitment of monocytes by MCP-1 might contribute to BBB disruption. This was further confirmed by a detailed in vitro study by Eugenin et al using coculture model of brain endothelial cells and astrocytes in which they demonstrated enhanced BBB permeability, reduced expression TJPs and upregulated expression of matrix metalloproteinase (MMP)-2 and MMP-9 on transmigration of HIV-infected monocytes in response to MCP-1 (20).
7. PERSPECTIVE
The encephalopathy caused by HIV is unique among neurovirulent viral infection because HIV does not cause infection in neurons. However, the multiplicity of a large number of toxic factors induced by the virus in infected macrophages and astrocytes in the brain contribute to degenerative effects in neurons. MCP-1 clearly has an important role among these various factors by contributing to neuropathogenesis by both facilitating migration of infected and/or activated monocytes into the brain where they become host cells for HIV-1 replication and by activating macrophages, microglia and astrocytes that results in release of a number of potent neurotoxins.
Acknowledgments
This work was supported by grants DA020392-01, MH62969-01, RR016443, MH-068212, and MH072355 from the National Institutes of Health.
References
- 1.Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol JID - 9216781. 1991;1:163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 2.Spencer DC, Price RW. Human immunodeficiency virus and the central nervous system. Annu Rev Microbiol. 1992;46:655–693. doi: 10.1146/annurev.mi.46.100192.003255. [DOI] [PubMed] [Google Scholar]
- 3.McArthur JC, Hoover DR, Bacellar H, Miller EN, Cohen BA, Becker JT, Graham NM, McArthur JH, Selnes OA, Jacobson LP. Dementia in AIDS patients: incidence and risk factors. Multicenter AIDS Cohort Study. Neurology. 1993;43:2245–2252. doi: 10.1212/wnl.43.11.2245. [DOI] [PubMed] [Google Scholar]
- 4.Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, Becker JT, Cohen B, McArthur JC. HIV-associated neurologic disease incidence changes:: Multicenter AIDS Cohort Study, 1990–1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 6.Albright AV, Soldan SS, Gonzalez-Scarano F. Pathogenesis of human immunodeficiency virus-induced neurological disease. J Neurovirol. 2003;9:222–7. doi: 10.1080/13550280390194073. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- 8.Kaul M, Lipton SA. Mechanisms of neuronal injury and death in HIV-1 associated dementia. Curr HIV Res. 2006;4:307–18. doi: 10.2174/157016206777709384. [DOI] [PubMed] [Google Scholar]
- 9.Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–92. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka M, Bradley WG, Shapshak P, Nagano I, Stewart RV, Xin KQ, Srivastava AK, Nakamura S. Role of immune activation and cytokine expression in HIV-1-associated neurologic diseases. Adv Neuroimmunol. 1995;5:335–58. doi: 10.1016/0960-5428(95)00012-q. [DOI] [PubMed] [Google Scholar]
- 11.McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, Sacktor N. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 12.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–35. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 13.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 14.Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–60. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Wesselingh SL, Griffin DE, McArthur JC, Johnson RT, Glass JD. Localization of HIV-1 in human brain using polymerase chain reaction/in situ hybridization and immunocytochemistry. Ann Neurol. 1996;39:705–11. doi: 10.1002/ana.410390606. [DOI] [PubMed] [Google Scholar]
- 16.Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, Golding K, Cvetkovich TA, Blumberg BM. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–81. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- 17.Trillo-Pazos G, Diamanturos A, Rislove L, Menza T, Chao W, Belem P, Sadiq S, Morgello S, Sharer L, Volsky DJ. Detection of HIV-1 DNA in microglia/macrophages, astrocytes and neurons isolated from brain tissue with HIV-1 encephalitis by laser capture microdissection. Brain Pathol. 2003;13:144–54. doi: 10.1111/j.1750-3639.2003.tb00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranki A, Nyberg M, Ovod V, Haltia M, Elovaara I, Raininko R, Haapasalo H, Krohn K. Abundant expression of HIV Nef and Rev proteins in brain astrocytes in vivo is associated with dementia. Aids. 1995;9:1001–8. doi: 10.1097/00002030-199509000-00004. [DOI] [PubMed] [Google Scholar]
- 19.El-Hage N, Wu G, Wang J, Ambati J, Knapp PE, Reed JL, Bruce-Keller AJ, Hauser KF. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia. 2006;53:132–46. doi: 10.1002/glia.20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu L, Rutledge B, Fiorillo J, Ernst C, Grewal I, Flavell R, Gladue R, Rollins B. In vivo properties of monocyte chemoattractant protein-1. J Leukoc Biol. 1997;62:577–80. doi: 10.1002/jlb.62.5.577. [DOI] [PubMed] [Google Scholar]
- 22.Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A JID - 7505876. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelder W, McArthur JC, Nance-Sproson T, McClernon D, Griffin DE. Beta-chemokines MCP-1 and RANTES are selectively increased in cerebrospinal fluid of patients with human immunodeficiency virus-associated dementia. Ann Neurol JID - 7707449. 1998;44:831–835. doi: 10.1002/ana.410440521. [DOI] [PubMed] [Google Scholar]
- 25.Sozzani S, Introna M, Bernasconi S, Polentarutti N, Cinque P, Poli G, Sica A, Mantovani A. MCP-1 and CCR2 in HIV infection: regulation of agonist and receptor expression. J Leukoc Biol. 1997;62:30–33. doi: 10.1002/jlb.62.1.30. [DOI] [PubMed] [Google Scholar]
- 26.Zink MC, Coleman GD, Mankowski JL, Adams RJ, Tarwater PM, Fox K, Clements JE. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J Infect Dis. 2001;184:1015–1021. doi: 10.1086/323478. [DOI] [PubMed] [Google Scholar]
- 27.Sanders VJ, Pittman CA, White MG, Wang G, Wiley CA, Achim CL. Chemokines and receptors in HIV encephalitis. AIDS JID - 8710219. 1998;12:1021–1026. [PubMed] [Google Scholar]
- 28.Mengozzi M, De Filippi C, Transidico P, Biswas P, Cota M, Ghezzi S, Vicenzi E, Mantovani A, Sozzani S, Poli G. Human immunodeficiency virus replication induces monocyte chemotactic protein-1 in human macrophages and U937 promonocytic cells. Blood. 1999;93:1851–1857. [PubMed] [Google Scholar]
- 29.Hicks A, Potula R, Sui YJ, Villinger F, Pinson D, Adany I, Li Z, Long C, Cheney P, Marcario J, Novembre F, Mueller N, Kumar A, Major E, Narayan O, Buch S. Neuropathogenesis of lentiviral infection in macaques: roles of CXCR4 and CCR5 viruses and interleukin-4 in enhancing monocyte chemoattractant protein-1 production in macrophages. Am J Pathol. 2002;161:813–822. doi: 10.1016/S0002-9440(10)64241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buch S, Sui Y, Potula R, Pinson D, Adany I, Li Z, Huang M, Li S, Dhillon N, Major E, Narayan O. Role of interleukin-4 and monocyte chemoattractant protein-1 in the neuropathogenesis of X4 simian human immunodeficiency virus infection in macaques. J Neurovirol. 2004;10(Suppl 1):118–24. doi: 10.1080/753312763. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, Gelbard HA, Su ZZ, Kang DC, Brooks AI, Fisher PB, Volsky DJ. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10(Suppl 1):25–32. doi: 10.1080/753312749. [DOI] [PubMed] [Google Scholar]
- 32.Dou H, Morehead J, Bradley J, Gorantla S, Ellison B, Kingsley J, Smith LM, Chao W, Bentsman G, Volsky DJ, Gendelman HE. Neuropathologic and neuroinflammatory activities of HIV-1-infected human astrocytes in murine brain. Glia. 2006;54:81–93. doi: 10.1002/glia.20358. [DOI] [PubMed] [Google Scholar]
- 33.Lim SP, Garzino-Demo A. The human immunodeficiency virus type 1 Tat protein up-regulates the promoter activity of the beta-chemokine monocyte chemoattractant protein 1 in the human astrocytoma cell line U-87 MG: role of SP-1, AP-1, and NF-kappaB consensus sites. J Virol. 2000;74:1632–40. doi: 10.1128/jvi.74.4.1632-1640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pu H, Tian J, Flora G, Lee YW, Nath A, Hennig B, Toborek M. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol Cell Neurosci. 2003;24:224–37. doi: 10.1016/s1044-7431(03)00171-4. [DOI] [PubMed] [Google Scholar]
- 35.Sheng WS, Hu S, Lokensgard JR, Peterson PK. U50,488 inhibits HIV-1 Tat-induced monocyte chemoattractant protein-1 (CCL2) production by human astrocytes. Biochem Pharmacol. 2003;65:9–14. doi: 10.1016/s0006-2952(02)01480-6. [DOI] [PubMed] [Google Scholar]
- 36.Conant K, Ma M, Nath A, Major EO. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-kappa B binding and protein kinase C activity in primary human astrocytes. J Virol. 1996;70:1384–9. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park IW, Wang JF, Groopman JE. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood. 2001;97:352–8. doi: 10.1182/blood.v97.2.352. [DOI] [PubMed] [Google Scholar]
- 38.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–21. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rautonen N, Rautonen J, Martin NL, Wara DW. HIV-1 Tat induces cytokine synthesis by uninfected mononuclear cells. Aids. 1994;8:1504–6. [PubMed] [Google Scholar]
- 40.Chen P, Mayne M, Power C, Nath A. The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases. J Biol Chem. 1997;272:22385–8. doi: 10.1074/jbc.272.36.22385. [DOI] [PubMed] [Google Scholar]
- 41.D’Aversa TG, Yu KO, Berman JW. Expression of chemokines by human fetal microglia after treatment with the human immunodeficiency virus type 1 protein Tat. J Neurovirol. 2004;10:86–97. doi: 10.1080/13550280490279807. [DOI] [PubMed] [Google Scholar]
- 42.Eugenin EA, Dyer G, Calderon TM, Berman JW. HIV-1 tat protein induces a migratory phenotype in human fetal microglia by a CCL2 (MCP-1)-dependent mechanism: possible role in NeuroAIDS. Glia. 2005;49:501–10. doi: 10.1002/glia.20137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vicenzi E, Alfano M, Ghezzi S, Gatti A, Veglia F, Lazzarin A, Sozzani S, Mantovani A, Poli G. Divergent regulation of HIV-1 replication in PBMC of infected individuals by CC chemokines: suppression by RANTES, MIP-1alpha, and MCP-3, and enhancement by MCP-1. J Leukoc Biol. 2000;68:405–12. [PubMed] [Google Scholar]
- 44.Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol JID -0113724. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–903. [PubMed] [Google Scholar]
- 46.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–35. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 47.Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–95. [PubMed] [Google Scholar]
- 48.Washington R, Burton J, Todd RF, 3rd, Newman W, Dragovic L, Dore-Duffy P. Expression of immunologically relevant endothelial cell activation antigens on isolated central nervous system microvessels from patients with multiple sclerosis. Ann Neurol. 1994;35:89–97. doi: 10.1002/ana.410350114. [DOI] [PubMed] [Google Scholar]
- 49.Harkness KA, Sussman JD, Davies-Jones GA, Greenwood J, Woodroofe MN. Cytokine regulation of MCP-1 expression in brain and retinal microvascular endothelial cells. J Neuroimmunol. 2003;142:1–9. doi: 10.1016/s0165-5728(03)00251-0. [DOI] [PubMed] [Google Scholar]
- 50.Boven LA, Middel J, Breij EC, Schotte D, Verhoef J, Soderland C, Nottet HS. Interactions between HIV-infected monocyte-derived macrophages and human brain microvascular endothelial cells result in increased expression of CC chemokines. J Neurovirol. 2000;6:382–9. doi: 10.3109/13550280009018302. [DOI] [PubMed] [Google Scholar]
- 51.Weiss JM, Nath A, Major EO, Berman JW. HIV-1 Tat induces monocyte chemoattractant protein-1-mediated monocyte transmigration across a model of the human blood-brain barrier and up-regulates CCR5 expression on human monocytes. J Immunol. 1999;163:2953–9. [PubMed] [Google Scholar]
- 52.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, Andjelkovic AV. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 53.Song L, Pachter JS. Monocyte chemoattractant protein-1 alters expression of tight junction-associated proteins in brain microvascular endothelial cells. Microvasc Res. 2004;67:78–89. doi: 10.1016/j.mvr.2003.07.001. [DOI] [PubMed] [Google Scholar]