Abstract
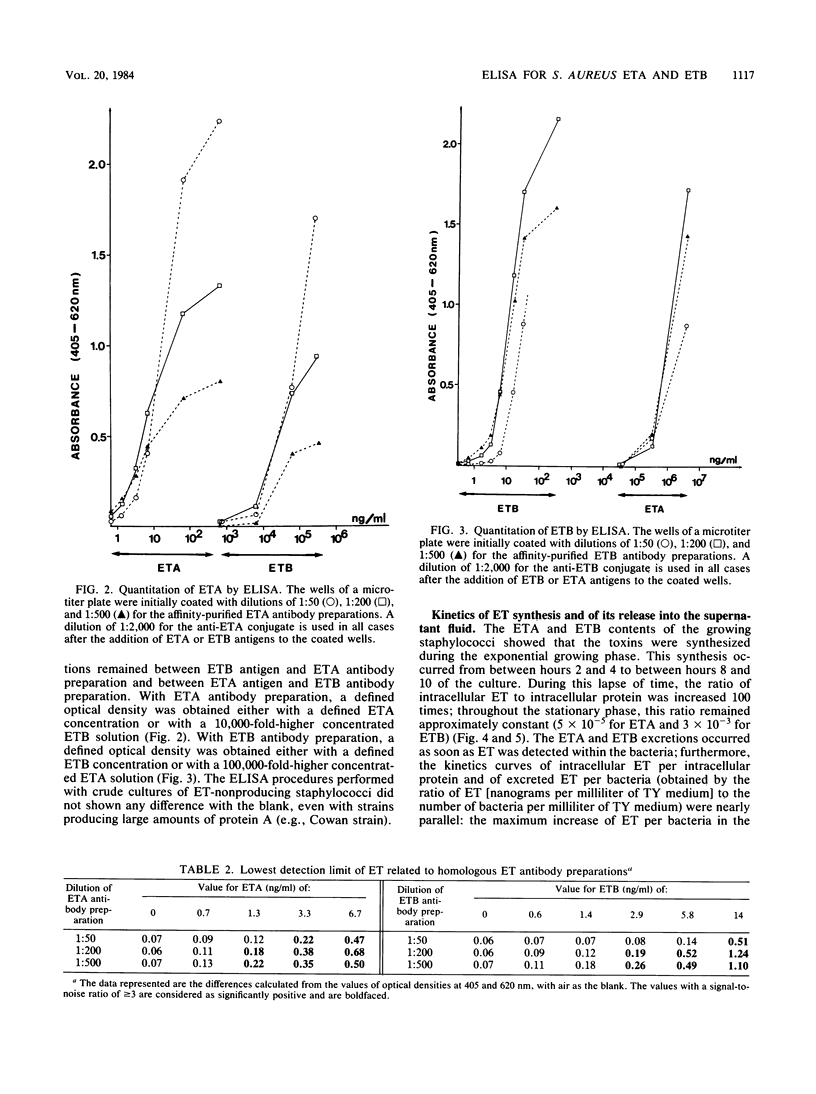
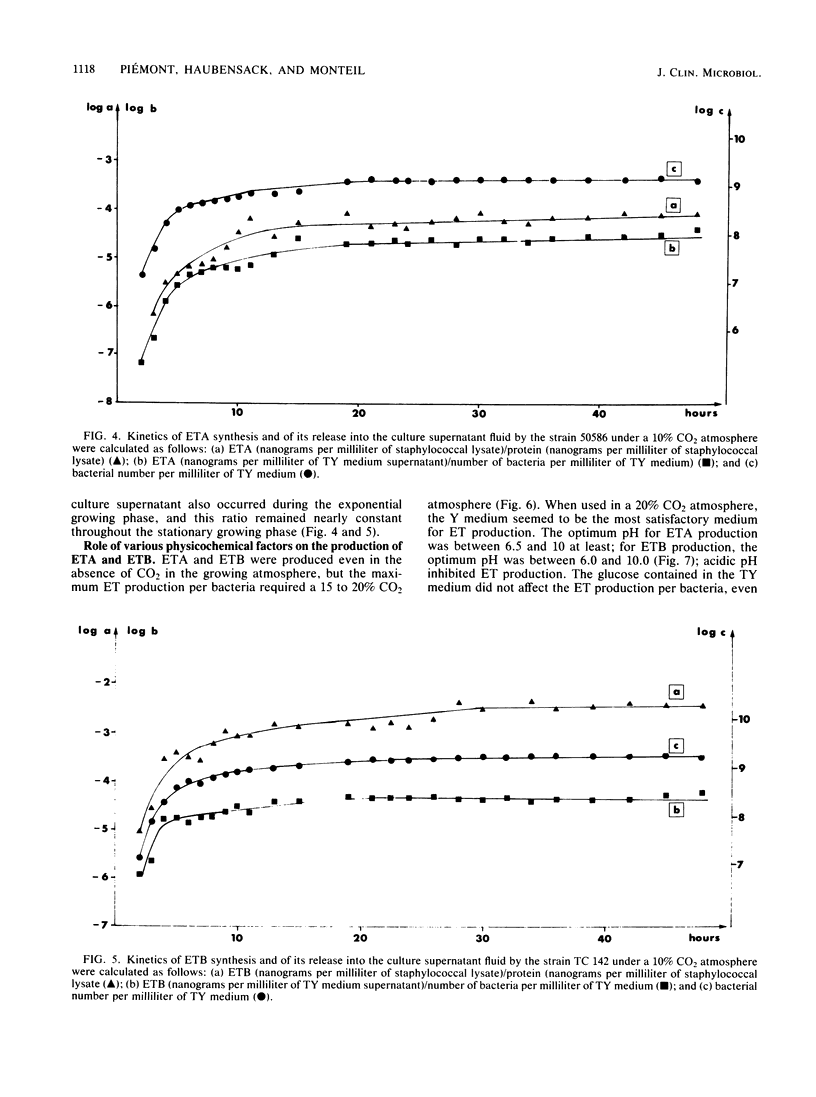
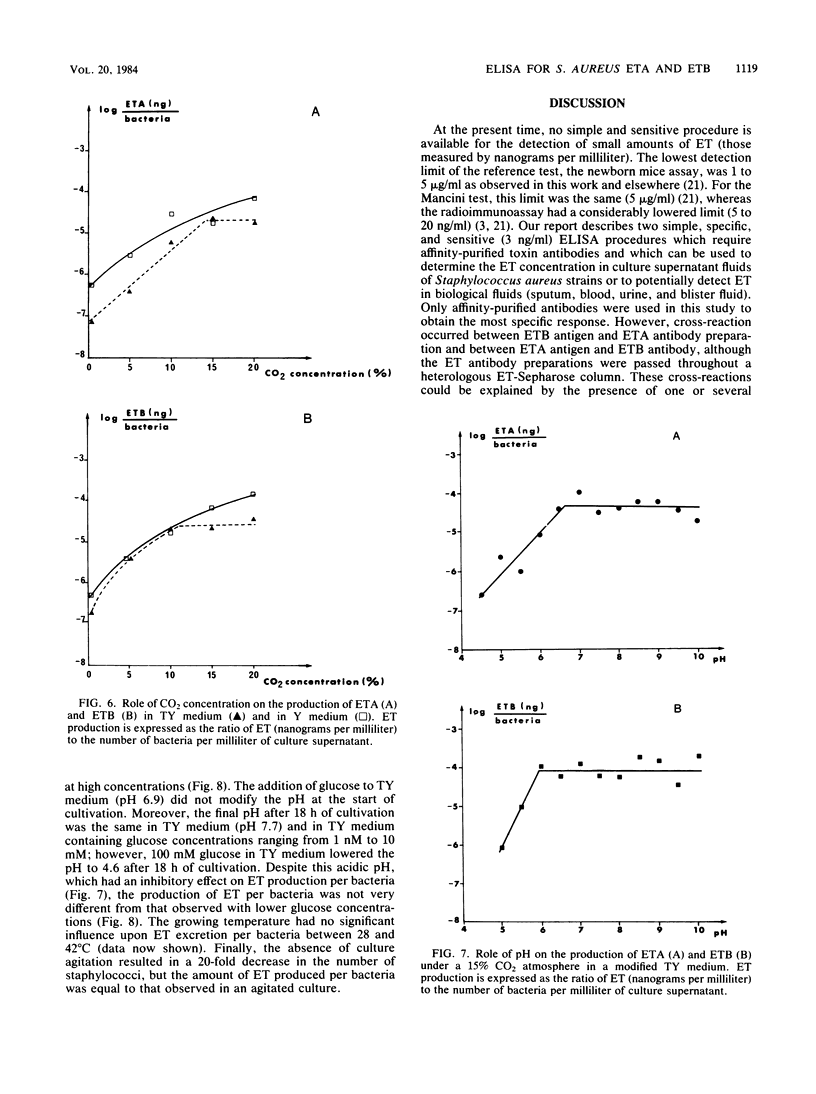
Two enzyme-linked immunosorbent assays were developed for detection of staphylococcal exfoliative toxins A and B (ETA and ETB) with a double-antibody sandwich protocol. Antibodies against both toxins were purified by affinity chromatography from sheep antisera raised against purified ETA and ETB. These affinity-purified antibodies were free of detectable amounts of antibodies to other staphylococcal antigens and neutralized the actions of ETA and ETB. Alkaline phosphatase was conjugated to these antibodies. The enzyme-linked immunosorbent assay, which could detect at least 3 ng of ETA and ETB per ml, was used to quantitate the toxins in the culture supernatant fluids of staphylococcal strains. Thus, the kinetics of ETA and ETB synthesis and of ETA and ETB release into the supernatant fluids were determined; other determinations included the roles of carbon dioxide concentration, pH, glucose concentration, temperature, and agitation on the production of ETA and ETB.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anthony B. F., Giuliano D. M., Oh W. Nursery outbreak of staphylococcal scalded skin syndrome. Rapid identification of the epidemic bacterial strain. Am J Dis Child. 1972 Jul;124(1):41–44. doi: 10.1001/archpedi.1972.02110130043006. [DOI] [PubMed] [Google Scholar]
- Arbuthnott J. P., Billcliffe B. Qualitative and quantitative methods for detecting staphylococcal epidermolytic toxin. J Med Microbiol. 1976 May;9(2):191–201. doi: 10.1099/00222615-9-2-191. [DOI] [PubMed] [Google Scholar]
- Baker D. H., Dimond R. L., Wuepper K. D. The epidermolytic toxin of Staphylococcus aureus: its failure to bind to cells and its detection in blister fluids of patients with bullous impetigo. J Invest Dermatol. 1978 Oct;71(4):274–275. doi: 10.1111/1523-1747.ep12515105. [DOI] [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme-linked immunosorbent assay (ELISA). Quantitative assay of immunoglobulin G. Immunochemistry. 1971 Sep;8(9):871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Freed R. C., Evenson M. L., Reiser R. F., Bergdoll M. S. Enzyme-linked immunosorbent assay for detection of staphylococcal enterotoxins in foods. Appl Environ Microbiol. 1982 Dec;44(6):1349–1355. doi: 10.1128/aem.44.6.1349-1355.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Metzger J. F., Spero L. Production, purification, and chemical characterization of Staphylococcus aureus exfoliative toxin. Infect Immun. 1975 Nov;12(5):1206–1210. doi: 10.1128/iai.12.5.1206-1210.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLOW L. S. A histochemical procedure for localizing and evaluating leukocyte alkaline phosphatase activity in smears of blood and marrow. Blood. 1955 Oct;10(10):1023–1029. [PubMed] [Google Scholar]
- Kapral F. A., Miller M. M. Product of Staphylococcus aureus responsible for the scalded-skin syndrome. Infect Immun. 1971 Nov;4(5):541–545. doi: 10.1128/iai.4.5.541-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I., Sakurai S., Sarai Y. Purification of exfoliatin produced by Staphylococcus aureus of bacteriophage group 2 and its physicochemical properties. Infect Immun. 1973 Aug;8(2):156–164. doi: 10.1128/iai.8.2.156-164.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Melish M. E., Glasgow L. A. The staphylococcal scalded-skin syndrome. N Engl J Med. 1970 May 14;282(20):1114–1119. doi: 10.1056/NEJM197005142822002. [DOI] [PubMed] [Google Scholar]
- Melish M. E., Glasgow L. A., Turner M. D. The staphylococcal scalded-skin syndrome: isolation and partial characterization of the exfoliative toxin. J Infect Dis. 1972 Feb;125(2):129–140. doi: 10.1093/infdis/125.2.129. [DOI] [PubMed] [Google Scholar]
- Piemont Y., Rasoamananjara D., Fouace J. M., Bruce T. Epidemiological investigation of exfoliative toxin-producing Staphylococcus aureus strains in hospitalized patients. J Clin Microbiol. 1984 Mar;19(3):417–420. doi: 10.1128/jcm.19.3.417-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piémont Y., Monteil H. Mise en évidence par électrosynérèse des deux sérotypes d'exfoliatine produits par Staphylococcus aureus. Ann Microbiol (Paris) 1983 Mar-Apr;134A(2):169–175. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard D., Monteil H., Piemont Y., Assi R., Messer J., Lavillaureix J., Minck R., Gandar R. L'exfoliatine dans les staphylococcies néonatales. Nouv Presse Med. 1982 Dec 18;11(51):3769–3771. [PubMed] [Google Scholar]
- Wuepper K. D., Baker D. H., Dimond R. L. Measurement of the staphylococcal epidermolytic toxin: a comparison of bioassay, radial immunodiffusion, and radioimmunoassay. J Invest Dermatol. 1976 Oct;67(4):526–531. doi: 10.1111/1523-1747.ep12664548. [DOI] [PubMed] [Google Scholar]