Abstract
Background: Although walking is the most popular leisure-time activity for adults, few long-term, longitudinal studies have examined the association between walking, an affordable and accessible form of physical activity, and weight gain.
Objective: The objective was to evaluate the association between changes in leisure-time walking and weight gain over a 15-y period.
Design: Prospective data from the Coronary Artery Risk Development in Young Adults (CARDIA) Study of 4995 men and women aged 18–30 y at baseline (1985–1986) from 4 US cities and reexamined 2, 5, 7, 10, and 15 y later. Sex-stratified, repeated-measures, conditional regression modeling with data from all 6 examination periods (n = 23,633 observations) was used to examine associations between walking and annualized 15-y weight change, with control for 15-y nonwalking physical activity, baseline weight (and their interaction), marital status, education, smoking, calorie intake, and baseline age, race, and field center.
Results: Mean (±SE) baseline weights were 77.0 ± 0.3 kg (men) and 66.2 ± 0.3 kg (women), weight gain was ≈1 kg/y, and the mean duration of walking at baseline was <15 min/d. After accounting for nonwalking physical activity, calorie intake, and other covariates, we found a substantial association between walking and annualized weight change; the greatest association was for those with a larger baseline weight. For example, for women at the 75th percentile of baseline weight, 0.5 h of walking/d was associated with 8 kg less weight gain over 15 y compared with women with no leisure time walking.
Conclusion: Walking throughout adulthood may attenuate the long-term weight gain that occurs in most adults.
See corresponding editorial on page 15.
INTRODUCTION
Walking, a relatively inexpensive and easily accessible form of physical activity, has been shown to be acceptable for adults of all ages (1, 2). Because it is suitable for most people, walking is generally reported as the most popular leisure-time physical activity for adults (3–5) and has been specifically promoted as a targeted activity to achieve national physical activity recommendations (1, 6). For most adults, walking 60 min/d at a brisk pace is sufficient to meet the Institute of Medicine's physical activity guidelines for avoiding weight gain (7–9).
Many studies have reported a decreased physical activity and an increased prevalence of overweight/obesity across all sex, age, and race/ethnic groups examined in the past 2 decades (10–13). Walking may contribute to the longitudinal change in overall activity patterns over time. Findings from the first 7 y of the Coronary Artery Risk Development in Young Adults (CARDIA) Study indicate an average decline in the physical activity score of ≈50% from age 18 to 37 y across all race-sex groups, with declines in most moderate and vigorous activities, including walking and hiking (11).
Research findings suggest an inverse relation between walking and adiposity (14, 15). However, very little data have been published on longitudinal trends in walking, how such trends might impact weight change over the course of adulthood, and whether changes in walking behavior and weight outcomes differ by sex. Of particular relevance is whether walking, a relatively low-intensity activity, can play a positive role in the reduction of long-term weight gain. In this study we used longitudinal data from the CARDIA Study spanning 15 y and 6 measurement occasions to investigate the association of longitudinal changes in walking, total physical activity, and weight change over a 15-y follow-up.
SUBJECTS AND METHODS
Study sample
The CARDIA Study is a population-based prospective epidemiologic study of the determinants and evolution of cardiovascular disease risk factors among young adults. At baseline (1985–1986), 5115 eligible participants aged 18–30 y were enrolled with balance according to race (black and white), sex, education (high school or less and more than high school), and age (18–24 and 25–30 y) from the populations of Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. Specific recruitment procedures were described elsewhere (16). Follow-up examinations were conducted in 1987–1988 (year 2), 1990–1991 (year 5), 1992–1993 (year 7), 1995–1996 (year 10), and 2000–2001 (year 15), with retention rates of 90%, 86%, 81%, 79%, and 74%, respectively.
Of the initial 5115 participants, there were a total possible 30,690 observations across the 6 examination periods. We excluded observations for women who were pregnant at the time of examination (n = 257 observations; including period-specific nonpregnant observations), observations with missing adjacent weight measures to derive weight change (6188 observations), and missing covariate data (n = 612 observations). The final sample for analysis included all available exposure, outcome, and covariate data across the 6 examination periods, totaling 23,633 observations for 4995 individuals. The current secondary data analysis was approved by the CARDIA Steering Committee and the Institutional Review Board of University of North Carolina at Chapel Hill.
Exposure and outcome measures
Weight change
Weight was measured to the nearest 0.1 kg with a calibrated balance-beam scale from participants wearing light indoor clothing and no shoes. Weight change was treated as a continuous variable, calculated as the difference between measurements. In statistical models, we used annualized weight change to correct for the unequal time between observations. In addition, we created a 3-level categorical weight change measure based on changes between each examination period (ti−ti-1): weight maintenance was defined as a change within ± 1 kg based on distribution of the sample (approximate mean annualized weight change +1 kg/y), weight gain as an increase >1 kg, and weight loss as a loss >1 kg.
Walking patterns and trends
At each examination, self-reported physical activity was ascertained with the use of an interviewer-administered questionnaire designed for the CARDIA Study. Participants were asked about the frequency of participation in 13 different activity categories (8 vigorous and 5 moderate) of recreational sports, exercise, leisure, and occupational activities over the previous 12 mo. Vigorous activities included running, racquet sports, bicycling faster than 10 miles/h, swimming, vigorous exercise classes, sports (eg, basketball and football), heavy lifting, carrying or digging on the job, and home activities such as snow shoveling or lifting heavy objects. Moderate activities included nonstrenuous sports (eg, softball), walking, bowling, golf, home maintenance (eg, gardening and raking), and calisthenics. Physical activity scores are expressed in exercise units (EU) computed by multiplying the frequency of participation by the intensity of the activity separately for heavy (ie, vigorous) and moderate activities and summing the 2 subscores for a total physical activity score. As an example, a score of 100 EU is roughly equivalent to participation in a vigorous activity 2–3 h/wk for 6 mo of the year. The reliability and validity of the instrument is comparable with that of other activity questionnaires (17, 18).
We created a specific walking score derived from walking items in the physical activity questionnaire described above. We used a continuous walking score, ranging from 0 to 144 units, 144 representing regular walking ≥4 h/wk over 12 mo at 3 metabolic equivalents (METs) (72 EU is equivalent to ≈2 h/wk, and 36 EU is equivalent to ≈1 h/wk). We also categorized walking: period-specific nonwalkers (walking score: 0), tertiles within walking scores ranging from 1 to 143 [level 1 (score: 1–24), level 2 (score: 24–48), and level 3 (score: 49–143)], and consistent (level 4) walkers (score: 144). A nonwalking physical activity score (total physical activity score minus walking score) was also created.
Although period-specific nonwalkers were included, consistent nonwalkers (scores of 0 for walking at all examination years) were excluded (n = 241) to allow investigation of time-varying shifts in walking scores among those who walked at any point during the 15 y. Comparisons (t tests) between consistent nonwalkers and the analysis sample indicated no statistically significant differences in weight changes and total nonwalking physical activity between excluded and included individuals in any of the examination years, although a greater proportion of consistent nonwalkers were black men (P < 0.01).
Additional covariates
Time-invariant measures included race/ethnicity, sex, and baseline clinic study center. Time-varying measures included age, educational attainment, marital status, smoking status (current, former, or never), and calorie intake, calculated from the participants dietary questionnaires at baseline and at the year 7 examinations.
The CARDIA diet-history questionnaire collected information about usual dietary practices and quantitative food frequency over the previous 28 d (19), with reliability and validity based on correlations between daily nutrient intakes and calorie-adjusted nutrient values from 2 histories ranging from 0.50 to 0.80 for whites and from 0.30 to 0.70 for blacks (20). To make use of the temporal data, we used the baseline measure for examination years 0–5 and the year 7 measure for examination years 7–15. Given the fair to moderate tracking of calorie intake shown in the literature, regardless of cohort age, study duration, or diet collection method (21–23), we used the baseline and year 7 measures as a rough control for calorie intake.
Statistical analysis
Statistical analyses were conducted by using Stata software (version 9.2; Stata Corp, College Station, TX) (24). Descriptive statistics were computed for walking and physical activity scores, weight status, calorie intake, smoking, and sociodemographic factors. Percentages were calculated for categorical variables. Continuous variables are presented either as means and SEs or as medians and interquartile ranges (for skewed measures).
We used longitudinal, repeated-measures conditional regression modeling to estimate the longitudinal association between walking and weight change. These models, conditioned on the subject, do not estimate parameters for variables constant within subjects (ie, race, sex, and study center), but have the advantage of adjusting for potential confounding by all measured and unmeasured characteristics of individuals (or within-person effects). The models adjust for the correlation between repeated observations taken in the same subject and have the advantage of handling longitudinal data on subjects with varying number and unequally spaced observations, thereby allowing for inclusion of the maximum number of data points (25–27). These models used all available data across 15 y and 6 examination periods.
We regressed annualized 15-y weight change (ti−ti-1) (continuous) on walking score (continuous) across the 6 examinations, controlling for time-varying factors: demographic factors [including age (continuous), education (high school or >high school), marital status (married or marriage-like relation or single)], lifestyle factors (including nonwalking physical activity, continuous EU/d), daily calorie intake (continuous), smoking status (current, former, or never smoker), and time-invariant, baseline factors [including baseline race (black or white), sex (women or men), and study center (Birmingham, Chicago, Minneapolis, or Oakland, referent). Interactions between baseline weight and walking score, walking score and sex, walking score and race, and walking score and age were tested by including the appropriate cross-product terms in the model and assessing likelihood ratio tests (P ≤ 0.01). Final models were stratified by sex and included an interaction term for walking score and baseline weight. Variables were retained in models if backward elimination resulted in a >10% change in the estimated effect measures or if variables were conceptually relevant (eg, control for clinic site). Given the interaction terms and complexity of interpretation of the repeated-measures conditional regression model results, we present predictions based on model coefficients from the estimation equation, which estimate predicted cumulative 15-y weight changes, adjusted for model covariates.
Using longitudinal, repeated-measures conditional regression modeling, we also predicted a categorical 15-y weight change. This model assessed the relation between categories of walking score across the 6 examinations (1–24 EU, 24–48 EU, 49–143 EU, and 144 EU relative to 0 EU) and 3 categories of 15-y annualized weight change (kg/y) based on changes between each examination period (ti−ti-1) (weight maintenance ±1 kg/y and >1 kg/y weight loss relative to >1 kg/y weight gain), with control for demographic and lifestyle factors as the longitudinal, repeated-measures conditional regression model. Tests for interaction (cross product term effect measures and likelihood ratio tests) were undertaken. On the basis of this model, interaction terms were not warranted and thus were not retained in the final model.
RESULTS
Descriptive characteristics
At baseline, the sample was approximately equally balanced with respect to race and sex (Table 1). Over the 15-y period, there was an increase in mean body weight and body mass index (BMI; in kg/m2), with each examination period having a higher mean than the preceding period (P ≤ 0.05). Annual weight change (kg/y) was statistically different from zero (P ≤ 0.05).
TABLE 1.
Characteristics of the analysis sample from the Coronary Artery Risk Development in Young Adults (CARDIA) Study (n = 23,633 observations across 4995 individuals; 1985–1986 to 2000–2001)1
| Year 0: 1985–1986 (n = 4949) | Year 2: 1987–1988 (n = 4351) | Year 5: 1990–1991 (n = 3934) | Year 7: 1992–1993 (n = 3691) | Year 10: 1995–1996 (n = 3443) | Year 15: 2000–2001 (n = 3265) | |
| Body weight | ||||||
| Weight (kg)2 | 71.0 ± 16.33 | 72.9 ± 17.04 | 75.9 ± 18.24 | 78.2 ± 19.24 | 80.4 ± 20.04 | 83.9 ± 21.24 |
| Annual weight change (kg) | — | 0.97 ± 2.535 | 0.91 ± 2.135 | 1.02 ± 2.695 | 0.75 ± 2.025 | 0.70 ± 1.435 |
| BMI (kg/m2)2 | 24.5 ± 5.0 | 25.2 ± 5.44 | 26.1 ± 5.84 | 26.8 ± 6.24 | 27.5 ± 6.54 | 28.7 ± 6.84 |
| Physical activity6 | ||||||
| Walking score (EU)7 | 36.0 (4–108) | 28.0 (0–88)8 | 24.0 (0–80)8 | 28.0 (4–72) | 24.0 (0–72) | 32.0 (4–80)8 |
| Nonwalking physical activity score (EU)7 | 300.0 (147–512) | 272.0 (128–474)8 | 262.5 (120–480) | 222.0 (92–422)8 | 224.0 (80–420) | 234.0 (93–428) |
| Race (%) | ||||||
| Black | 49.8 | 47.7 | 46.4 | 46.4 | 46.2 | 45.2 |
| Sex (%) | ||||||
| Women | 55.7 | 54.8 | 55.6 | 55.2 | 55.0 | 55.6 |
| Age (y) | 24.9 ± 3.6 | 27.0 ± 3.6 | 30.1 ± 3.6 | 32.1 ± 3.6 | 35.1 ± 3.6 | 40.2 ± 3.6 |
| Marriage status (%) | ||||||
| Married9 | 22.6 | 30.5 | 40.5 | 44.1 | 50.5 | 60.6 |
| Education (%) | ||||||
| ≤High school | 39.1 | 33.6 | 30.8 | 28.3 | 27.9 | 22.2 |
| Smoking status (%) | ||||||
| Current | 30.2 | 29.4 | 28.0 | 26.3 | 24.5 | 21.2 |
| Former | 13.4 | 13.6 | 14.0 | 15.9 | 16.7 | 18.3 |
| Never | 56.4 | 57.0 | 58.0 | 57.8 | 58.8 | 60.5 |
| Energy intake (kcal/d)10 | 2834 ± 1348 | 2819 ± 1341 | 2805 ± 1334 | 2792 ± 1264 | 2788 ± 1249 | 2779 ± 1237 |
Full sample included 5115 subjects at baseline. Exclusion criteria: women pregnant at time of examination and subjects missing adjacent weight measures to derive weight change. EU, exercise units calculated on the basis of frequency and intensity of activity. Statistical testing applied when the P for trend was <0.01 by examination year. Testing only applied for outcome (body weight) and main exposure (physical activity) data. Differences between control variables were not tested.
Significant main effect of time, P < 0.00001 (one-factor ANOVA).
Mean ± SD (all such values).
Significantly different from previous examination year (eg, year 2 vs year 0), P < 0.01 (t tests with Bonferroni correction).
Significantly different from zero, P < 0.05 (t test with Bonferroni correction).
Values are medians (interquartile ranges) presented because of skewness of the physical activity score.
P for trend < 0.0001.
Significantly different from previous examination year (eg, year 2 vs year 0), P < 0.01 (Wilcoxon's signed-rank tests with Bonferroni correction).
Married or living with someone in a married-like relation.
Intake at examination years 0–5 is based on baseline intake; intake at examination years 7–15 is based on intake at year 7.
Because of the skewed distribution, we presented medians and interquartile ranges for walking and physical activity scores, although mean differences were tested. The median walking score generally declined over time, with significant decreases between examination years 0 and 2 and years 2 and 5 (P ≤ 0.05) and fairly high within-subject variation (intraclass correlation: 0.27; 95% CI: 0.26, 0.29). However, the nonwalking physical activity score decreased significantly between examination years 0 and 2 and years 5 and 7 (P ≤ 0.01). Walking and nonwalking activities were positively associated at all examination years (P = 0.0001). In a crude regression model, on average across all examination years, a 1-unit increase in the walking score predicted a 0.91-unit (95% CI: 0.85, 0.98) increase in total nonwalking physical activity.
Across all years, women had significantly higher mean walking scores than did men (Table 2). The full sample had an annualized weight gain of ≈1 kg/y in the first 3 examinations of follow-up, with a somewhat reduced mean weight gain in the past 2 observation periods. Women had a higher mean annualized weight gain than did men at year 10 only.
TABLE 2.
Walking scores, walking score changes, BMI, weight, and weight change by sex from the Coronary Artery Risk Development in Young Adults (CARDIA) Study (n = 23,633 observations across 4995 individuals; 1985–1986 to 2000–2001)1
| Year 0: 1985–1986 (n = 4949) | Year 2: 1987–1988 (n = 4351) | Year 5: 1990–1991 (n = 3934) | Year 7: 1992–1993 (n = 3605) | Year 10: 1995–1996 (n = 3443) | Year 15: 2000–2001 (n = 3265) | |
| Walking score (EU)2 | ||||||
| Women3 | 57.3 ± 1.04 | 51.7 ± 1.14 | 51.2 ± 1.14 | 49.8 ± 1.14 | 50.0 ± 1.24 | 50.5 ± 1.24 |
| Men3 | 53.4 ± 1.2 | 46.9 ± 1.2 | 43.5 ± 1.2 | 44.2 ± 1.2 | 43.2 ± 1.3 | 47.5 ± 1.4 |
| Walking score change (EU) | ||||||
| Women | — | −6.8 ± 1.3 | −1.5 ± 1.3 | −1.1 ± 1.3 | 0.3 ± 1.3 | 1.2 ± 1.4 |
| Men | — | −7.7 ± 1.4 | −4.5 ± 1.4 | 0.25 ± 1.4 | −0.6 ± 1.4 | 4.4 ± 1.5 |
| BMI (kg/m2) | ||||||
| Women5 | 24.6 ± 0.1 | 25.2 ± 0.1 | 26.3 ± 0.1 | 27.0 ± 0.24 | 27.9 ± 0.24 | 29.1 ± 0.24 |
| Men5 | 24.4 ± 0.1 | 25.1 ± 0.1 | 25.9 ± 0.1 | 26.5 ± 0.1 | 27.2 ± 0.1 | 28.3 ± 0.1 |
| Weight (kg) | ||||||
| Women5 | 66.2 ± 0.34 | 67.8 ± 0.44 | 70.9 ± 0.44 | 73.4 ± 0.44 | 75.7 ± 0.54 | 79.1 ± 0.54 |
| Men5 | 77.0 ± 0.3 | 78.9 ± 0.3 | 82.0 ± 0.4 | 84.0 ± 0.4 | 86.0 ± 0.4 | 89.8 ± 0.5 |
| Annualized weight change (kg/y) | ||||||
| Women | — | 0.9 ± 0.05 | 0.9 ± 0.05 | 1.1 ± 0.06 | 0.8 ± 0.054 | 0.7 ± 0.04 |
| Men | — | 1.0 ± 0.05 | 1.0 ± 0.04 | 0.9 ± 0.06 | 0.7 ± 0.05 | 0.7 ± 0.04 |
All values are mean ± SE. EU, exercise units calculated on the basis of frequency and intensity of activity.
Sex-by-time interaction for walking score, P = 0.02 (likelihood ratio test).
Within sex, P for trend < 0.05.
Significantly different from men, P < 0.05 (t test).
Main effect of time within sex, P < 0.00001 (one-factor ANOVA).
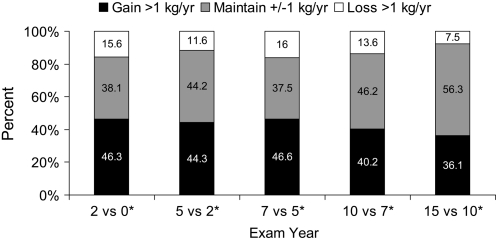
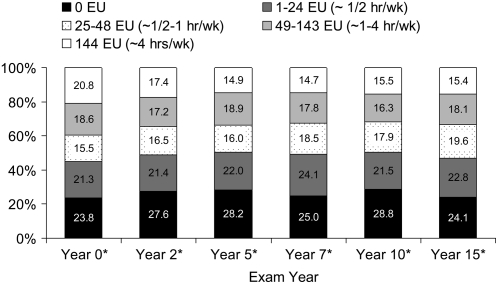
A small proportion of the sample had an average weight loss >1 kg/y across time (Figure 1). The proportion of the sample that gained weight decreased over time (P for trend < 0.0001), and the categories for weight maintenance increased over time (P for trend < 0.0001). The proportion of the sample within categories of walking score fluctuated slightly across examination years, with a general reduction in the proportion of those who walked at the highest level that was observed mostly in the earlier examination years (P for trend < 0.0001) (Figure 2). Across the 15-y period, just under 50% of the sample was in the lower 2 categories of walking (≤0.5 h/wk); with 23–29% reported no walking per week.
FIGURE 1.
Percentage of sample to gain, maintain, or lose weight across 15 y of the Coronary Artery Risk Development in Young Adults (CARDIA) Study (n = 23,633 observations across 4995 individuals; 1985–1986 to 2000–2001). *P for trend < 0.0001 by examination year.
FIGURE 2.
Percentage of sample to fall within the different walking-score categories, ranging from 0 (no walking) exercise units (EU) to 144 EU (≥4 h of walking/wk over 12 mo at 3 metabolic equivalents) across 15 y of the Coronary Artery Risk Development in Young Adults (CARDIA) Study (n = 23,633 observations across 4995 individuals; 1985–1986 to 2000–2001). *P for trend < 0.0001 by examination year. An EU of 72 approximates 2 h of walking/wk, and an EU of 36 approximates 1 h of walking/wk.
Longitudinal models
Using the longitudinal, repeated-measures conditional regression model predicting annualized weight change, we found a substantial association of walking with weight change, after accounting for nonwalking physical activity, calorie intake, and other relevant covariates. We found a significant interaction between baseline weight and walking score and between walking score and sex. Associations were tested by using the 25th, 50th, and 75th percentiles of baseline weight given linear fit and to illustrate effects. These associations were greatest for those with the highest baseline weight and were strongest among women. There were no statistically significant associations at the 25th percentile of baseline weight for men (β = −0.07 kg/y; 95% CI: −0.23, 0.09; P = 0.4) or women (β = −0.12 kg/y; 95% CI: −0.29, 0.05; P = 0.0001). However, at the 50th percentile of baseline weight, each 0.5 h of walking/d was associated with a reduction in weight gain in men (β = −0.15 kg/y; 95% CI: −0.29, −0.02; P = 0.03), particularly in women (β = −0.29 kg/y; 95% CI: −0.43, −0.14; P = 0.0001). At the 75th percentile of baseline weight (BMI equivalent to overweight), 0.5 h of walking/d was associated with even greater reductions in weight gain in men (β = −0.25 kg/y; 95% CI: −0.40, −0.10; P = 0.001), particularly in women (β = −0.53 kg/y; 95% CI: −0.68, −0.38; P < 0.001).
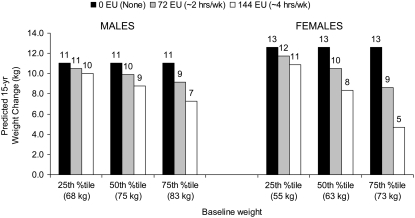
To facilitate interpretation of the walking and baseline weight interaction term from the repeated-measures conditional regression model, we present predictions generated from the model coefficients (Figure 3), interpreted as the average association of a change in walking score with total annualized weight change over 15 y for different levels of baseline weight. In the figure, total 15-y weight change for women at the highest baseline weight (≥75th percentile) with no walking (walking score: 0 EU) was predicted to be 13 kg, whereas it was only 5 kg (or 8 kg less weight gain over the 15 y) for those with the highest 15-y walking score (144 EU). In contrast, for men, the greatest difference in predicted weight gain was 4 kg for the comparison between those at the highest baseline weight (walking score: 144 EU) of walking relative to those with no walking (walking score: 0 EU), regardless of baseline weight. The inverse association between walking and weight gain was evident across all baseline weight categories for women and men.
FIGURE 3.
Predicted cumulative weight change in men and women based on coefficients from the longitudinal, repeated-measures conditional regression model across 15 y of the Coronary Artery Risk Development in Young Adults (CARDIA) Study (n = 23,633 observations across 4995 individuals; 1985–1986 to 2000–2001). Sex-stratified, longitudinal, repeated-measures conditional regression model predicted annualized weight changes with control for total physical activity score (nonwalking), age, marital status, education (referent or ≥high school), smoking (referent or never smoked), and calorie intake; the interaction term was walking by baseline weight. Men: walking score of 144 (referent, 0), 25th percentile baseline weight for men (β = −0.07 kg/y; 95% CI: −0.23, 0.09; P = 0.4), 50th percentile baseline weight (β = −0.15 kg/y; 95% CI: −0.29, −0.02; P = 0.03), and 75th percentile baseline weight, BMI equivalent to overweight (β = −0.25 kg/y; 95% CI: −0.40, −0.10; P = 0.001). Women: 25th percentile baseline weight for women (β = −0.12 kg/y; 95% CI: −0.29, 0.05; P = 0.2), 50th percentile baseline weight (β = −0.28 kg/y; 95% CI: −0.43, −0.14; P = 0.001), and 75th percentile baseline weight, BMI equivalent to overweight (β = −0.53 kg/y; 95% CI: −0.68, −0.38; P < 0.001). Values may appear inconsistent because of rounding. Bars for 0 exercise units (EU) are identical across sex and baseline weight because of regression parameterization.
A substantial proportion of the participants gained weight over the 15-y period (36–47% of the sample across each examination year; Figure 1). The likelihood of 15-y weight loss (and weight maintenance) compared with weight gain by longitudinal walking level, with control for key covariates with no need for interaction terms, is shown in Table 3. The likelihood of weight loss and maintenance was relatively higher at higher walking levels. The greatest associations were evident at the highest walking level, ie, a 44% increased odds of weight loss compared with gain relative to those who did not walk at all and a 19% increased odds of weight maintenance compared with gain. We also found associations between smoking (negative), marriage (positive), and weight gain.
TABLE 3.
Predictors of weight change across 15 y of the Coronary Artery Risk Development in Young Adults (CARDIA) Study (n = 23,633 observations across 4995 individuals; 1985–1986 to 2000–2001) from a longitudinal, repeated-measures conditional regression model1
| Weight loss vs gain |
Weight maintenance vs gain |
|||||
| Predictors | OR | 95% CI | P value | OR | 95% CI | P value |
| Walking score: 0 EU | Ref | — | — | Ref | — | — |
| Walking score: 1–24 EU | 1.10 | 0.92, 1.32 | 0.3 | 0.96 | 0.85, 1.08 | 0.5 |
| Walking score: 25–48 EU | 1.26 | 1.04, 1.53 | 0.02 | 1.09 | 0.95, 1.24 | 0.2 |
| Walking score: 49–143 EU | 1.33 | 1.08, 1.63 | 0.006 | 1.07 | 0.93, 1.22 | 0.4 |
| Walking score: 144 EU | 1.44 | 1.16, 1.78 | 0.001 | 1.19 | 1.03, 1.38 | 0.02 |
| Total PA without walking: 50 EU2 | 1.07 | 1.05, 1.09 | 0.0001 | 1.02 | 1.01, 1.03 | 0.001 |
| Age, y | 1.00 | 0.98, 1.01 | 0.6 | 1.06 | 1.05, 1.07 | 0.0001 |
| Married or married-like relation | 0.67 | 0.56, 0.80 | 0.0001 | 0.81 | 0.72, 0.91 | 0.0001 |
| ≤High school education | 1.22 | 0.96, 1.54 | 0.1 | 0.98 | 0.82, 1.16 | 0.8 |
| Current smoker | 2.24 | 1.54, 3.25 | 0.0001 | 1.63 | 1.27, 2.09 | 0.0001 |
| Former smoker | 1.02 | 0.72, 1.45 | 0.9 | 0.90 | 0.72, 1.12 | 0.3 |
| Energy, 500 kcal | 0.97 | 0.93, 1.01 | 0.2 | 0.99 | 0.96, 1.02 | 0.5 |
Coefficients presented as adjusted odds ratios (weight loss vs weight gain; weight maintenance vs weight gain). Longitudinal, repeated-measures conditional regression modeling predicting weight loss (>1 kg/y) and weight maintenance (within ±1 kg/y relative to weight gain >1 kg/y on the basis of changes between each examination period (ti−ti-1), with control for the covariates listed above. These models, conditioned on the subject, do not estimate parameters for variables that are constant within subject (ie, race, sex, baseline weight, and study center). Ref, referent category; EU, exercise units calculated on the basis of frequency and intensity of activity.
To maximize interpretation and because the exercise units (EU) of the analysis were small, the odds ratios are presented at 50 EU (equivalent to ≈1.5 h/wk).
DISCUSSION
We found a statistically robust relation between temporal change in walking and long-term weight change. Furthermore, an increase in walking over the early to middle adult years was associated with less weight gain over time and an increased likelihood of weight loss and maintenance compared with weight gain. The strongest association was found in heavier women (with baseline weight at the 75th percentile or 73.0 kg). For these women, consistent walking of ≥4 h/wk (144 EU) was associated with less weight gain (≈0.5 kg/y), or 8 kg less over the 15-y period relative to women with a walking score of 0 EU, whereas walking 2 h/wk (walking score: 72 EU) for women at the 75th percentile of baseline weight was associated with less weight gain (0.3 kg/y), or 4 kg less over the 15-y period relative to women with a walking score of 0 EU. Essentially, these results translate to a reduced weight gain of ≈0.5 kg/y with each 0.5 h of walking per week for women who were heavier at baseline.
It was our aim to explore the association between walking and weight change, holding other forms of physical activity constant. Thus, in this study, we were explicitly not interested in the physiologic effects of walking compared with other forms of physical activity. Nonetheless, using an interaction between walking and nonwalking physical activity (data not shown), we found that the negative association between walking and annual weight change was stronger for participants with low than for women with high nonwalking physical activity. Furthermore, we were most interested in determining policy and intervention practicality of consistent (as opposed to inconsistent) walking over time.
Our findings are in line with research on other forms of physical activity spanning shorter time frames (28). For example, others found evidence suggesting that physical activity may attenuate weight gain (29–32). However, these findings are based on shorter time periods of follow-up and fewer repeated measures (generally just 2 repeated visits). Other research shows a negative association between walking and adiposity (14, 15). However, additional research suggests that the relation between adiposity and vigorous activity depends on BMI. For example, among women, for each mile of running per week, the decrease in BMI was 9-fold greater at the 95th than at the 5th percentile of BMI (33, 34). A cross-sectional analysis on walking suggests that associations of walking with adiposity may also be greatest among heavy women (35).
Our findings also contribute to the literature on small changes in physical activity and the prevention of weight gain (36, 37). Particularly relevant is the contrast between old-order Amish who walk an average of 18,000 (men) and 14,000 (women) steps/d and who have a very low prevalence of obesity (38) and that of Colorado adults who walk <7000 steps/d on average (with obese adults walking an average of 2000 fewer steps than normal-weight adults) (39). Whereas our findings on the role of walking on weight gain attenuation indicate modest associations, they can have a substantial impact on both the individual and population levels, especially over long periods. Furthermore, adding between 2 and 4 h of walking per week are clearly achievable targets from a public health perspective. Of particular relevance is our finding of a greater association in heavier women at baseline, because heavier weight is often a barrier to physical activity (40). Walking is a particularly good form of activity to target. Furthermore, walking can be integrated beyond leisure into active transportation or commuting (41–44) and overall lifestyle or utilitarian activity (1, 6–8).
The strengths of this study include its use of complete, detailed, longitudinal data over a 15-y time span and standardized repeated measures of physical activity, including estimates of a variety of types of activities made with an instrument with known reliability and validity. We examined the independent associations of walking, controlling for other forms of self-reported physical activity as well as total calorie intake, to assess the independent associations of walking with long-term weight change. Furthermore, longitudinal, repeated-measures conditional regression modeling is the most powerful statistical technique for exploring average associations of walking with average weight gain over time.
Despite these strengths, this study had some limitations. The CARDIA Study data are observational in nature, and our results do not imply causality. We used weight change as our outcome measure and thus were unable to distinguish between weight gain in fat (adverse) and in lean (nonadverse) compartments. The study was further limited by self-reported physical activity data and other lifestyle factors, and we could not completely control for misreporting, which may have resulted in overreporting of walking and nonwalking physical activities. Furthermore, whereas the use of usual patterns is a well-validated approach to collecting time use data and has been extended over the past decade to physical activity measures, it is possible that the yearly recall measure does not capture the variability of walking within the year. Although the CARDIA Study boasts high retention rates over the 15 y of study, some study subjects were not available for adjacent measurements, which precluded our ability to create a measure of weight change for these subjects. It is possible that such data are not missing at random. Similarly, we do not have diet data at every examination, so we cannot discount the possibility that the heavier women were trying to lose weight by walking, changing their diets, or engaging in other weight-loss behaviors, which may be associated with increased actual (or recalled) walking. Individuals who did not lose weight through walking may underreport their walking, whereas those who did lose weight may overreport walking, which may lead to an overestimate of the association between walking and weight change. Analyses of measures of change are also limited by floor and ceiling effects that would tend to attenuate the observed relations.
In summary, this study provides evidence that a higher frequency of walking is accompanied by a reduced weight gain and an increased likelihood of weight loss and weight maintenance over young-to-middle adulthood. That is, each extra 0.5 h/d of walking was associated with an annual reduced weight gain of 1 lb (0.54 kg) or 15 lb over 15 y for women who were heaviest at baseline. The results were similar, but of less magnitude, in men. Importantly, this association held when we separated walking from nonwalking physical activity and held all other energy expenditures constant. Thus, the women who were heaviest at baseline may have received the greatest benefit from walking. Walking is of particular relevance because it is generally an affordable and accessible form of physical activity for most people. The proposition in this study was not that walking is physiologically more beneficial than other forms of physical activity, but rather that walking may be more practical in terms of policy and intervention purposes and that substantial weight control can be attained by walking.
Acknowledgments
We thank Gina Wei (Medical Officer, Epidemiology Branch, Division of Prevention and Population Sciences, NHLBI) for her valuable comments, Frances Dancy (Administrative Assistant, UNC) for administrative assistance, and Shufa Du (Project Manager, UNC) for technical assistance.
The authors' responsibilities were as follows—PG-L, NH, BMP, CEL, and DRJ: conception and design of the study and acquisition of data; PG-L, NH, BMP, CEL, DRJ, SS, and BS: analysis of data; PG-L, NH, BMP, and DRJ: drafting of manuscript; CEL, SS, and BS: revision of manuscript; PG-Larsen, NH, and DRJ: statistical analysis; PG-L, BMP, CEL, DRJ, SS, and BS: funding; PG-L, BMP, and CEL: administrative, technical, or material support; and PG-L: supervision, full access to all of the data in the study, and responsibility for the integrity of the data and the accuracy of the data analysis. All of the authors gave final approval of the version of the manuscript to be published. None of the authors had any conflicts of interest.
The study sponsors had no role in the secondary analysis, the study design, the analysis and interpretation of the data, the writing of the paper, or the decision to submit it for publication. The funders had no role in any aspect of the analysis or in the draft, review, or approval of the manuscript.
REFERENCES
- 1.US Department of Health and Human Services Physical activity and health: a report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, 1996 [Google Scholar]
- 2.US Department of Health and Human Services Healthy people 2010: understanding and improving health. 2nd ed Washington, DC: US Government Printing Office, 2000 [Google Scholar]
- 3.Stephens T, Jacobs D, White C. A descriptive epidemiology of leisure-time physical activity. Public Health Rep 1985;100:147–58 [PMC free article] [PubMed] [Google Scholar]
- 4.Sidney S, Jacobs D, Haskell W, et al. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol 1991;133:1231–45 [DOI] [PubMed] [Google Scholar]
- 5.Simpson ME, Serdula M, Galuska DA, et al. Walking trends among U.S. adults: the Behavioral Risk Factor Surveillance System, 1987–2000. Am J Prev Med 2003;25:95–100 [DOI] [PubMed] [Google Scholar]
- 6.National Institutes of Health NIH consensus statement on physical activity and cardiovascular health. Bethesda, MD: National Institutes of Health, 1995 [Google Scholar]
- 7.Institute of Medicine Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acides (macronutrients). Washington, DC: National Academy Press, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Erlichman J, Kerbey AL, James WP. Physical activity and its impact on health outcomes. Paper 2: Prevention of unhealthy weight gain and obesity by physical activity: an analysis of the evidence. Obes Rev 2002;3:273–87 [DOI] [PubMed] [Google Scholar]
- 9.Saris WH, Blair SN, van Baak MA, et al. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev 2003;4:101–14 [DOI] [PubMed] [Google Scholar]
- 10.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–55 [DOI] [PubMed] [Google Scholar]
- 11.Anderssen N, Jacobs DR, Jr, Sidney S, et al. Change and secular trends in physical activity patterns in young adults: a seven-year longitudinal follow-up in the Coronary Artery Risk Development in Young Adults Study (CARDIA). Am J Epidemiol 1996;143:351–62 [DOI] [PubMed] [Google Scholar]
- 12.Lewis CE, Jacobs DR, Jr, McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol 2000;151:1172–81 [DOI] [PubMed] [Google Scholar]
- 13.Steffen LM, Arnett DK, Blackburn H, et al. Population trends in leisure-time physical activity: Minnesota Heart Survey, 1980–2000. Med Sci Sports Exerc 2006;38:1716–23 [DOI] [PubMed] [Google Scholar]
- 14.Guo SS, Zeller C, Chumlea WC, Siervogel RM. Aging, body composition, and lifestyle: the Fels Longitudinal Study. Am J Clin Nutr 1999;70:405–11 [DOI] [PubMed] [Google Scholar]
- 15.Thompson DL, Rakow J, Perdue SM. Relationship between accumulated walking and body composition in middle-aged women. Med Sci Sports Exerc 2004;36:911–4 [DOI] [PubMed] [Google Scholar]
- 16.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) Study. Control Clin Trials 1987;8:68S–73S [DOI] [PubMed] [Google Scholar]
- 17.Jacobs D, Jr, Ainsworth B, Hartman T, Leon A. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc 1993;25:81–91 [DOI] [PubMed] [Google Scholar]
- 18.Jacobs D, Jr, Hahn L, Haskell W, Pirie P, Sidney S. Validity and reliability of a short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil 1989;9:448–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McDonald A, Van Horn L, Slattery M, et al. The CARDIA dietary history: development, implementation, and evaluation. J Am Diet Assoc 1991;91:1104–12 [PubMed] [Google Scholar]
- 20.Liu K, Slattery M, Jacobs D, Jr, et al. A study of the reliability and comparative validity of the cardia dietary history. Ethn Dis 1994;4:15–27 [PubMed] [Google Scholar]
- 21.Lien N, Lytle LA, Klepp KI. Stability in consumption of fruit, vegetables, and sugary foods in a cohort from age 14 to age 21. Prev Med 2001;33:217–26 [DOI] [PubMed] [Google Scholar]
- 22.Kelder SH, Perry CL, Klepp K-I, Lytle LL. Longitudinal tracking of adolescent smoking, physical activity, and food choice behavior. Am J Public Health 1994;84:1121–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mannino ML, Lee Y, Mitchell DC, Smiciklas-Wright H, Birch LL. The quality of girls' diets declines and tracks across middle childhood. Int J Behav Nutr Phys Act 2004;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.StataCorp Stata release 9, Survey Data Reference Manual. College Station, TX: Stata Corporation, 2005 [Google Scholar]
- 25.Baltagi BH. Econometric analysis of panel data. 2nd ed New York, NY: John Wiley, 2001 [Google Scholar]
- 26.Greene WH. Econometric analysis. 5th ed Upper Saddle River, N.J.: Prentice Hall, 2003 [Google Scholar]
- 27.Hsiao C. Analysis of panel data. 2nd ed New York, NY: Cambridge University Press, 2003 [Google Scholar]
- 28.Jakicic JM, Otto AD. Physical activity considerations for the treatment and prevention of obesity. Am J Clin Nutr 2005;82:226S–9S [DOI] [PubMed] [Google Scholar]
- 29.French S, Jeffery R, Forster J, McGovern P, Kelder S, Baxter J. Predictors of weight change over two years among a population of working adults: the Healthy Worker Project. Int J Obes Relat Metab Disord 1994;18:145–54 [PubMed] [Google Scholar]
- 30.Rissanen AM, Heliovaara M, Knekt P, Reunanen A, Aromaa A. Determinants of weight gain and overweight in adult Finns. Eur J Clin Nutr 1991;45:419–30 [PubMed] [Google Scholar]
- 31.Littman AJ, Kristal AR, White E. Effects of physical activity intensity, frequency, and activity type on 10-y weight change in middle-aged men and women. Int J Obes (Lond) 2005;29:524–33 [DOI] [PubMed] [Google Scholar]
- 32.Di Pietro L, Dziura J, Blair SN. Estimated change in physical activity level (PAL) and prediction of 5-year weight change in men: the Aerobics Center Longitudinal Study. Int J Obes Relat Metab Disord 2004;28:1541–7 [DOI] [PubMed] [Google Scholar]
- 33.Williams PT, Wood PD. The effects of changing exercise levels on weight and age-related weight gain. Int J Obes 2006;30:543–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams PT. Vigorous exercise and the population distribution of body weight. Int J Obes Relat Metab Disord 2004;28:120–8 [DOI] [PubMed] [Google Scholar]
- 35.Williams PT. Nonlinear relationships between weekly walking distance and adiposity in 27,596 women. Med Sci Sports Exerc 2005;37:1893–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–5 [DOI] [PubMed] [Google Scholar]
- 37.Hill JO. Understanding and addressing the epidemic of obesity: an energy balance perspective. Endocr Rev 2006;27:750–61 [DOI] [PubMed] [Google Scholar]
- 38.Bassett DR, Schneider PL, Huntington GE. Physical activity in an old order Amish community. Med Sci Sports Exerc 2004;36:79–85 [DOI] [PubMed] [Google Scholar]
- 39.Wyatt HR, Peters JC, Reed GW, Barry M, Hill JO. A Colorado statewide survey of walking and its relation to excessive weight. Med Sci Sports Exerc 2005;37:724–30 [DOI] [PubMed] [Google Scholar]
- 40.Ball K, Crawford D, Owen N. Too fat to exercise? Obesity as a barrier to physical activity. Aust N Z J Public Health 2000;24:331–3 [DOI] [PubMed] [Google Scholar]
- 41.Saksvig BI, Catellier DJ, Pfeiffer K, et al. Travel by walking before and after school and physical activity among adolescent girls. Arch Pediatr Adolesc Med 2007;161:153–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell AC, Ge K, Popkin BM. The road to obesity or the path to prevention: motorized transportation and obesity in China. Obes Res 2002;10:277–83 [DOI] [PubMed] [Google Scholar]
- 43.Gordon-Larsen P, Nelson MC, Beam K. Associations among active transportation, physical activity, and weight status in young adults. Obes Res 2005;13:868–75 [DOI] [PubMed] [Google Scholar]
- 44.Wagner A, Simon C, Ducimetiere P, et al. Leisure-time physical activity and regular walking or cycling to work are associated with adiposity and 5 y weight gain in middle-aged men: the PRIME Study. Int J Obes Relat Metab Disord 2001;25:940–8 [DOI] [PubMed] [Google Scholar]