Abstract
Background
X-linked severe combined immunodeficiency (XSCID) results from defects in the common cytokine receptor γ chain (γc) required for signaling by receptors for interleukin (IL)-2, -4, -7, -9, -15, and -21 (1). Following haploidentical bone marrow transplant without myelo-conditioning for XSCID, most patients achieve partial reconstitution(2) often limited to T lymphocytes. Many partially corrected patients manifest extreme short stature (<5th percentile). Previous reports have implicated γc in growth hormone (GH) receptor signaling, thus severe growth failure in XSCID may be related to the underlying γc defect.
Objective
To evaluate the GH/insulin-like growth factor (IGF-1) axes in 3 children with XSCID and partial immune reconstitution with profound growth failure.
Methods
The IGF-1 generation test was performed by administering recombinant GH subcutaneously for 5 days, and measuring serum levels for IGF-1 before GH injection, and on days 5 and 8.
Results
Study of the somatotropic axis revealed profoundly diminished IGF-1 production following rGH challenge in all 3 patients.
Conclusion
The data indicate that the GH/IGF-1 axes in these partially corrected XSCID patients with severe short stature is profoundly impaired, and supports previous studies suggesting that the underlying γc defect may contribute to the severe growth failure in XSCID. This supports a role for defective γc in extreme short stature of XSCID, and raises the possibility of recombinant IGF-1 treatment to bypass this defect.
Keywords: X-linked severe combined immunodeficiency, growth hormone, IGF-1, γc, short stature
Introduction
X-linked severe combined immunodeficiency (XSCID) due to the common cytokine receptor gamma chain γc defect is characterized by few or absent T lymphocytes and natural killer (NK) cells, and normal numbers of non-functional B lymphocytes (1). Due to their profound immune deficiency, most patients with XSCID do not survive beyond infancy unless they receive allogeneic hematopoietic stem cell transplant (HSCT), or more recently, ex vivo autologous stem cell gene therapy (3). Although best results occur with an HLA-matched sibling donor, >75% of patients lack a matched sibling, and therefore receive an HLA-haploidentical graft from a parent. In the past decade, an unique strategy using T-cell depleted HSC for transplants without prior myelo-conditioning has been the standard of care, for most patients with XSCID. Recipients of a haploidentical graft have a 60%–70% survival, though patients transplanted before three months of age do better. Many survivors of haploidentical transplant achieve only partial immune reconstitution; retaining donor engraftment only in the T lymphocyte compartment; and requiring chronic intravenous immune globulin (IVIG) and prophylactic antibiotics (2, 4, 5).
Many of the long term survivors of the T cell depleted, haploidentical transplants (6) now approach adolescence with obvious growth failure. Often this is manifested by severe short stature that generally places these individuals well below the 5th percentile for age. Many of them may experience ongoing medical problems that include recurrent sinopulmonary infections, autoimmune and alloimmune diseases. However, in some cases, the severity of the short stature appears out of proportion to the clinical history of infection or nutritional status. This raises the possibility that short stature observed in these children might be in part a manifestation of the molecular mechanism of the disease due to the underlying γc defect rather than just secondary to infections or gastrointestinal dysfunction.
The common gamma chain subunit (γc) is required for signaling by receptors for multiple cytokines, namely interleukin (IL)-2, -4, -7, -9, -15, and -21 (7). Furthermore, a recent report also suggested a role for γc in cellular response to growth hormone (GH) (8). Clinically, GH responses can be assessed by the insulin-like growth factor (IGF)-1 generation test used for evaluation of children with short stature (9). IGF-1 is an essential downstream signaling hormone produced in response to stimulation of GH receptor (GHR). IGF-1 acts on chondrocytes at the epiphyseal plates effecting growth in height (10), anabolic responses, and protein synthesis (11). We present in this report three XSCID boys (ages 12–15 years) with extreme short stature (<5th percentile) who all demonstrate abnormal IGF-1 production responses to high dose GH. While chronic illnesses and poor nutrition can certainly result in impaired growth, the abnormal IGF1 production in response to GH challenge provides direct clinical support for the physiologic role of γc in GH signaling. We hypothesize that the underlying γc defect may be in part responsible for the profoundly short stature observed in many pre-adolescent and adolescent patients with XSCID.
Patient Reports
Case #1
Patient #1 is a 14-year old with variant XSCID resulting in production of trace amounts of γc). Despite four attempts with a T cell depleted, haploidentical BMT, he failed to engraft. His medical problems after transplant included recurrent respiratory infections, skin rashes, alopecia, esophageal strictures, and chronic diarrhea. He enrolled at age 11 in our protocol approved by our IRB (02-I-0057) for ex vivo gene therapy, receiving a single dose of γc-retrovirus transduced, autologous, mobilized peripheral blood CD34+ stem cells (12). Sustained low level genetic marking (4–5% of T cells) was achieved, but was not associated with expansion of corrected cells nor with measurable improvement in ex vivo measures of T cell function (12). Chimerism studies both before and after gene therapy have demonstrated no detectable donor cells and only host derived T- B- and myeloid cells. While there was some objective clinical improvement in eczema, alopecia and diarrhea, he continues to require intravenous immunoglobulins (IVIG), prophylactic antibiotics (trimethoprim/sulfamethoxazole, fluconazole, acyclovir), and low dose prednisone (2mg/day; 0.1mg/kg) for control of diarrhea. His growth rate did not improve following gene therapy, with current height 124.6 cm and weight 21.7 kg significantly below the 5th percentile for his chronological age of 14 yrs 5 months (Figure 1), and Tanner stage 1. His bone age is 7 years, and has a predicted mid-parental height of 183.1cm. His baseline GH level was 5.8 ng/µL (normal 0.0–5.0ng/µL). At a time when he was clinically stable and free from infections, an abbreviated IGF-1 generation test was performed. High dose recombinant growth hormone (rGH) (0.05mg/kg/day) was administered subcutaneously for 5 days, and serum levels for IGF-1 measured on days 0 (baseline), 5, and 8. A poor IGF-1 response was demonstrated (Table 1, Figure 2).
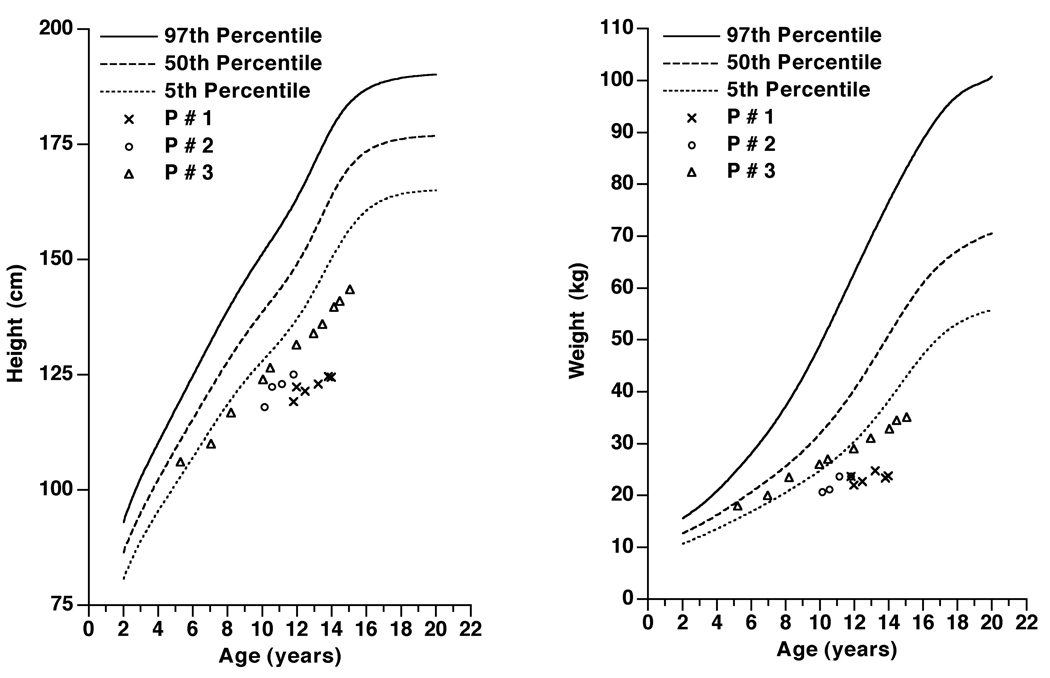
Figure 1.
Serial measurements of the height and weight of Patient #1 (crosses), #2 (circles) and #3 (triangles) are plotted on graphs a and b respectively. Measurements for the percentiles are derived from Centers for Disease Control and Prevention27. Proportionate short stature was observed in all 3 patients.
Table 1.
IGF-1 levels (ng/mL) at day 0 (baseline), 5 and 8 of the IGF-1 generation test for the 3 patients, as well as previously published data from normal controls for age <10 years and 10–18 year old (11). CA represents chronological age.
| Day 0 | Day 5 | Day 8 | |
|---|---|---|---|
| P#1 (CA=15 yr) | 7 | 36 | 25 |
| P#2 (CA=12 yr) | 1 | 73 | 93 |
| P#3 (CA=15 yr) | 117 | 320 | 188 |
|
Normal (<10 yr) (mean) |
250 | 460 | 429 |
|
Normal (10–18 yr) (mean) |
391 | 603 | 638 |
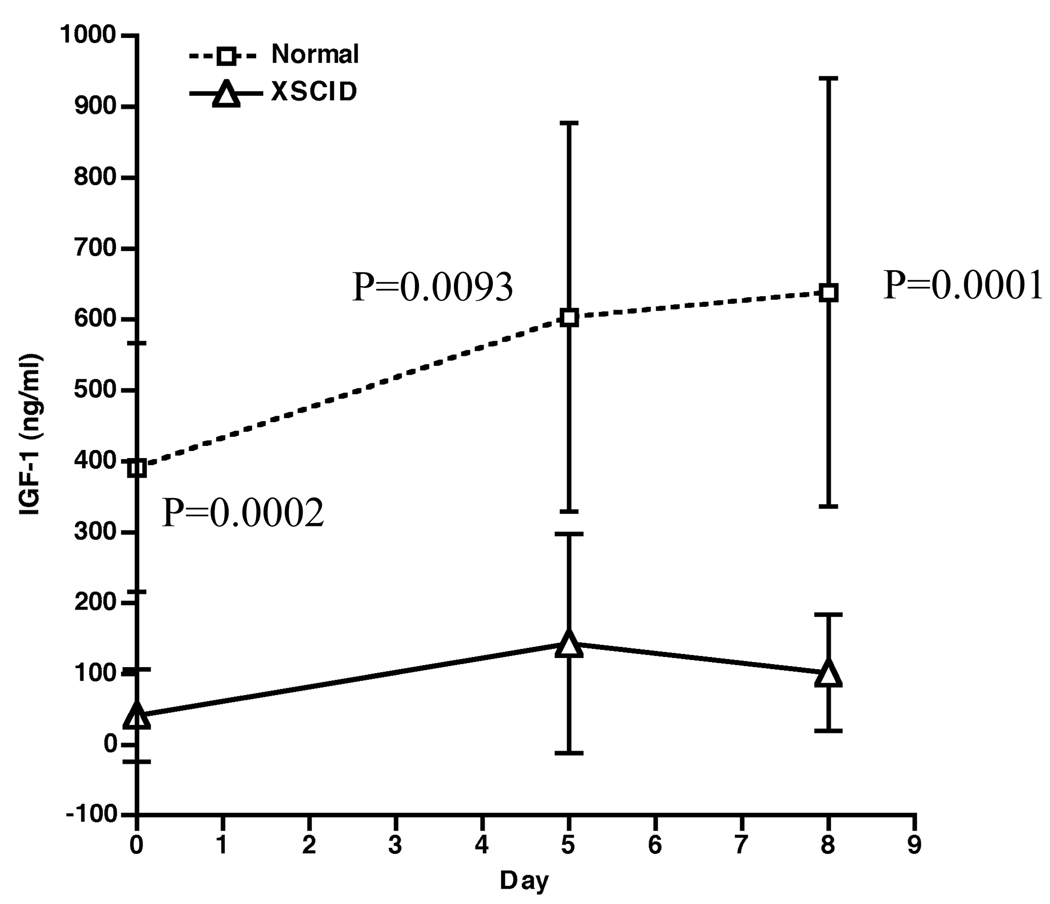
Figure 2.
IGF-1 generation tests in three partially corrected XSCID patients. Depicted are the means ± SD of plasma IGF-1 levels after 5 daily rGH (0.05mg/kg) injections for the XSCID patients (open triangles) and age-matched normal males (open squares). Of note, the normals received 7 days of rGH. The difference between the XSCID patients and age-matched normal males was compared using the two-sample t test with unequal variances and the resulting p-values are shown.
Patient #2 is a 12-year old with XSCID who received two haploidentical transplants achieving sustained donor engraftment of subnormal numbers and function of T lymphocytes. Following transplant, he experienced recurrent sinopulmonary infections, alopecia, eczema and chronic diarrhea. Supplemental gastrostomy feeds were used in an attempt to increase growth. At 10 years of age, gene therapy was performed under the same protocol as Patient #1. Gene marking occurred in ≥95% of his own T cells with significant increase in number and function of his T cells (12). Chimerism demonstrates 88% host T lymphocytes and 12% donor T lymphocyte, but only host derived B lymphocytes and myeloid cells. He continues to be supplemented with IVIG and takes prophylactic antibiotics (trimethoprim/sulfamethoxazole) without any corticosteroids. Clinically, he has been free from infections and the need for gastrostomy supplementation, but has gained very little height. His height was 126.5 cm and weight 24.0 kg, both <5th percentile at a chronological age of 12 yrs 2 months (Figure 1), with a predicted mid-parental height of 174.2cm. He is also prepubertal, with a bone age of 10 years. In this patient also, a very poor IGF-1 response was demonstrated (Table 1, Figure 2) following rGH challenge. His baseline GH level was 3.8 ng/µL (normal 0.0–5.0ng/µL).
Patient #3 is a 15-year old boy with XSCID who was transplanted at 9 months of age without myelo-conditioning. Following BMT, he developed graft versus host disease involving his gastrointestinal system, followed by hemolytic anemia, both of which resolved on oral prednisone therapy. Due to a recent increased IVIG requirement and abdominal symptoms, an endoscopy was performed and gastrointestinal biopsy revealed villous atrophy. This finding, in addition to his HLA DQ2 and DQ8 genotype prompted a diagnosis of celiac disease. This is currently controlled on a gluten-free diet. He is clinically well, with chronic sinusitis being the main ongoing medical problem. Other than gamma globulin supplement, he also receives trimethorpim/sulfamethoxazole for prophylaxis, but has not been on regular corticosteoids. Chimerism studies demonstrate he is engrafted only in the T lymphocyte compartment (100% donor T cells), but only host derived B lymphoctyes and myeloid cells are detectable. At age 15, he had Tanner stage 3, testicular volume 6mls bilaterally,, bone age of 11 years, and both height and weight of <5th percentiles (134 cm, 37.4kg), thus prompting an evaluation of his somatotrophic axis. His predicted mid-parental height is 176.7cm, and baseline GH level was 1.4ng/µl (normal 0.0–5.0ng/µL). While he did produce some IGF-1 in response to high dose rGH, his baseline IGF-1 level was below lower limit of normal, and the level rapidly dropped to below lower normal range by day 8 (Figure 2).
Assays
The specimens were centrifuged within one hour of collection from outpatient phlebotomy. The IGF1 level was measured by immunoradiometric assay kits (Immulite2500, Siemens Medical Solutions Diagnostics) by the Department of Laboratory Medicine, Warren Grant Magnuson Clinical Center, NIH).
IGF-I Generation Tests
The IGF-1 levels at baseline, days 5 and 8 from our XSCID patients were compared to published data from age-appropriate normal males (Table 1) (9). The number of subjects enrolled in the normative data set was 9 and 13 for the <10yr and 10–18yr groups respectively. We have included both sets of normative data, to permit assessment of our three subjects, two of whom were prepubertal at the time of evaluation. Of note, the high dose rGH 0.05mg/kg/day was given until day 5 for our patient group, compared to 7 days of rGH in the published data, although the normative data set includes IGF-I determinations on day 5 and day 8 of GH treatment. A comparison was made using the two-sample t test with unequal variances for each day (baseline, day 5 and day 8) with age-matched normal group (Figure 2). The IGF-1 levels were significantly lower in each of the 3 days tested (baseline, day 5 and day 8 with p =0.0002, 0.0093, and 0.0001 respectively) (Figure 3). Furthermore, the IGF-1 levels in our patients are low even when compared with the normative levels from the <10 year old group.
Discussion
We report extreme poor growth in some partially corrected XSCID patients, with height significantly below the 5th percentile for age. Poor growth in this group of patients is generally assumed to be attributable to chronic illness alone. While the adverse effect of chronic illness and malnutrition on growth is undeniable, our data from the IGF1 generation tests in these partially corrected XSCID patients may support a role for γc in GH signaling and downstream IGF1 production. It is our observation that the severity of short stature in many cases appears disproportionate to their infection history or nutritional status. Treatment with rGH in XSCID patients may only produce minimal clinical responses, thus requiring high dose rGH (case not presented) (13, 14). We present in this report deficient IGF-1 generation in clinical testing in response to high dose rGH challenge test in these partially corrected XSCID patients who remain immune deficient. Standardization of IG-1 generation tests is a challenge, and despite multiple protocols for IG-1 generation have been used, there is little published standardized normals. We chose for this study, a 5-day GH challenge test, with serum IGF-1 levels measured up to day 8. The IGF-1 levels produced in response to high dose rGH in our patients were significantly lower than age-matched controls (9) on days 0, 5 and 8 respectively (Figure 2). In contrast to our IGF-1 generation test protocol, the high dose rGH at 0.05mg/kg/day was given for 7 days in the study for the normal controls. Since both bone age and pubertal development are delayed in the patients described in this report, the normative data for boys under 10 years and the 10–18year groups are both presented in Table 1 (9). Of note, the IGF-1 levels from our three patients are low compared to either normative data set, thus should compensate for any difference due to pubertal delay in the patients.
Stimulation of γc signals through Janus kinase 3 (JAK3), and downstream signal transducer and activator of transcription (STAT5) tyrosine phosphorylation (7, 15), while GH signals through JAK2 and STAT5 (16, 17). Thus, γc and GHR share a common downstream signaling element. Mutations in STAT5b result in profound GH unresponsiveness with impaired IGF-1 production, resulting in growth failure (18). There is circumstantial evidence for potential cross-talk of γc with GHR as suggested by demonstration of stimulation induced co-localization of γc with GHR at the cell membrane (8). In the same study defective phosphorylation of STAT5 in response to GH observed ex vivo in XSCID patient-derived EBV-transformed B cells was restored by transfection of these B cells with wild-type γc (8). Abnormal IGF-1 levels in XSCID and normal growth was restored following complete immune correction through successful conventional myeloablative allogeneic transplant (19, 20). Unlike the situation with non-conditioning haploidentical transplants which may only engraft T cells, conventional transplants with conditioning engraft the myeloid cell compartment (5, 6), potentially resulting in donor cell osteoblasts, chondrocytes, liver macrophages and many other tissues, which may restore GH responsiveness in these critical organs.
Thus, we propose that in XSCID children who are partially corrected by haploidentical transplant, short stature may at least in part, be attributable to the underlying γc genetic defect that persists in the critical target tissues that remain host in origin. This is not to minimize the potential role that persistence of immune deficiency with associated recurrent infections and nutritional defects can have on growth (21). However, it appears that severe short stature is observed in this condition even in patients whose infections are mostly controlled and have adequate nutritional status. An ideal control would be IGF-1 responses to GH in patients with SCID due to different underlying molecular defects such as JAK3 deficiency. However, these diseases are rare, and allogeneic BMT without myelo-conditioning resulting in unique partial T-lymphoid immune reconstitution occurs most frequently in XSCID patients. The degree of growth failure associated with XSCID, however, appears more severe than has been observed in two other immune deficiencies associated with recurrent infections, namely in adenosine deaminase deficiency-SCID (personal communication), and chronic granulomatous disease (22). The appreciation for a clinical role of the γc defect as a contributor to growth failure in children with XSCID has important clinical implications; recombinant IGF-1 is now a commercially available FDA approved drug, and may potentially bypass defective γc mediated GH hypo-responsiveness (23).
Acknowledgment
We thank Drs Courtney Lappas and Robert Sokolic for helpful comments and information regarding patients with ADA-SCID. We also thank Dr Chiung-Yu Huang, National Institutes of Allergy and Infectious Diseases, NIH, who assisted with the statistical analysis of the clinical tests.
Financial support
This project was supported by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases, and, in part, by NICHD, NIH intramural project Z01-HD-000642-04 to Dr. C. A. Stratakis. We have no other financial relationships to disclose.
Abbreviations
- GH
growth hormone
- GHR
growth hormone receptor
- IGF-1
Insulin-like growth factor 1
- Jak
Janus kinase
- STAT
signal transducer and activator of transcription
- TCR
T cell receptor
- XSCID
X-linked severe combined immunodeficiency
References
- 1.Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130(3):378–387. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 2.Buckley RH, Schiff SE, Schiff RI, Markert L, Williams LW, Roberts JL, et al. Hematopoietic stem-cell transplantation for the treatment of severe combined immunodeficiency. N Engl J Med. 1999;340(7):508–516. doi: 10.1056/NEJM199902183400703. [DOI] [PubMed] [Google Scholar]
- 3.Hacein-Bey-Abina S, Le Deist F, Carlier F, Bouneaud C, Hue C, De Villartay JP, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346(16):1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- 4.Bertrand Y, Landais P, Friedrich W, Gerritsen B, Morgan G, Fasth A, et al. Influence of severe combined immunodeficiency phenotype on the outcome of HLA non-identical, T-cell-depleted bone marrow transplantation: a retrospective European survey from the European group for bone marrow transplantation and the european society for immunodeficiency. J Pediatr. 1999;134(6):740–748. doi: 10.1016/s0022-3476(99)70291-x. [DOI] [PubMed] [Google Scholar]
- 5.Haddad E, Landais P, Friedrich W, Gerritsen B, Cavazzana-Calvo M, Morgan G, et al. Long-term immune reconstitution and outcome after HLA-nonidentical T-cell-depleted bone marrow transplantation for severe combined immunodeficiency: a European retrospective study of 116 patients. Blood. 1998;91(10):3646–3653. [PubMed] [Google Scholar]
- 6.de la Morena MT, Wayne AS, Day NK, Haag MM, Hinds-Frey KR, Nelson RP, et al. Recipient T-cell immune reconstitution in X-linked SCID after haploidentical maternal bone marrow transplant. Ann N Y Acad Sci. 1995;770:376–377. doi: 10.1111/j.1749-6632.1995.tb31074.x. [DOI] [PubMed] [Google Scholar]
- 7.Leonard WJ, Shores EW, Love PE. Role of the common cytokine receptor gamma chain in cytokine signaling and lymphoid development. Immunol Rev. 1995;148:97–114. doi: 10.1111/j.1600-065x.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 8.Adriani M, Garbi C, Amodio G, Russo I, Giovannini M, Amorosi S, et al. Functional Interaction of Common {gamma}-Chain and Growth Hormone Receptor Signaling Apparatus. J Immunol. 2006;177(10):6889–6895. doi: 10.4049/jimmunol.177.10.6889. [DOI] [PubMed] [Google Scholar]
- 9.Buckway CK, Guevara-Aguirre J, Pratt KL, Burren CP, Rosenfeld RG. The IGF-I generation test revisited: a marker of GH sensitivity. J Clin Endocrinol Metab. 2001;86(11):5176–5183. doi: 10.1210/jcem.86.11.8019. [DOI] [PubMed] [Google Scholar]
- 10.Kelly PA, Finidori J, Moulin S, Kedzia C, Binart N. Growth hormone receptor signalling and actions in bone growth. Horm Res. 2001;55 Suppl 2:14–17. doi: 10.1159/000063467. [DOI] [PubMed] [Google Scholar]
- 11.Svanberg E, Powell-Braxton L, Ohlsson C, Zachrisson H, Lundholm K. The role of the growth hormone/insulin-like growth factor I axis in stimulation of protein synthesis in skeletal muscles following oral refeeding. Endocrinology. 1998;139(12):4906–4910. doi: 10.1210/endo.139.12.6362. [DOI] [PubMed] [Google Scholar]
- 12.Chinen J, Davis J, De Ravin SS, Hay BN, Hsu AP, Linton GF, et al. Gene therapy improves immune function in pre-adolescents with X-linked severe combined immunodeficiency. Blood. 2007;110(1):67–73. doi: 10.1182/blood-2006-11-058933. Epub 2007 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johannsson G. Individualized dose titration of growth hormone (GH) during GH replacement in hypopituitary adults. Clin. Endocrinol. 1997;47(5):571–581. doi: 10.1046/j.1365-2265.1997.3271123.x. [DOI] [PubMed] [Google Scholar]
- 14.Mauras N Cooperative Study Group. High dose recombinant human growth hormone (GH) treatment of GH-deficient patients in puberty increases near-final height: a randomized, multicenter trial, Genentech, Inc. J Clin endocrinol. Metab. 2000;85(10):3653–3660. doi: 10.1210/jcem.85.10.6906. [DOI] [PubMed] [Google Scholar]
- 15.Horvath CM. STAT proteins and transcriptional responses to extracellular signals. Trends Biochem Sci. 2000;25(10):496–502. doi: 10.1016/s0968-0004(00)01624-8. [DOI] [PubMed] [Google Scholar]
- 16.Postel-Vinay MC, Kelly PA. Growth hormone receptor signalling. Baillieres Clin Endocrinol Metab. 1996;10(3):323–336. doi: 10.1016/s0950-351x(96)80455-1. [DOI] [PubMed] [Google Scholar]
- 17.Winston LA, Hunter T. JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J Biol Chem. 1995;270(52):30837–30840. doi: 10.1074/jbc.270.52.30837. [DOI] [PubMed] [Google Scholar]
- 18.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, et al. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med. 2003;349(12):1139–1147. doi: 10.1056/NEJMoa022926. [DOI] [PubMed] [Google Scholar]
- 19.Ursini MV, Gaetaniello L, Ambrosio R, Matrecano E, Apicella AJ, Salerno MC, et al. Atypical X-linked SCID phenotype associated with growth hormone hyporesponsiveness. Clin Exp Immunol. 2002;129(3):502–509. doi: 10.1046/j.1365-2249.2002.01823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salerno M, Busiello R, Esposito V, Cosentini E, Adriani M, Selleri C, et al. Allogeneic bone marrow transplantation restores IGF-I production and linear growth in a gamma-SCID patient with abnormal growth hormone receptor signaling. Bone Marrow Transplant. 2004;33(7):773–775. doi: 10.1038/sj.bmt.1704421. [DOI] [PubMed] [Google Scholar]
- 21.Lang CH, Hong-Brown L, Frost RA. Cytokine inhibition of JAK-STAT signaling: a new mechanism of growth hormone resistance. Pediatr Nephrol. 2005;20(3):306–312. doi: 10.1007/s00467-004-1607-9. [DOI] [PubMed] [Google Scholar]
- 22.Buescher ES, Gallin JI. Stature and weight in chronic granulomatous disease. J Pediatr. 1984 Jun;104(6):911–913. doi: 10.1016/s0022-3476(84)80497-7. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfeld RG. Molecular mechanisms of IGF-I deficiency. Horm Res. 2006;65 Suppl 1:15–20. doi: 10.1159/000090642. [DOI] [PubMed] [Google Scholar]
- 24. http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.htm.