Abstract
Ribosomal S6 kinase 1 (S6K1) is a downstream component of the mammalian target of rapamycin (mTOR) signaling pathway and plays a regulatory role in translation initiation, protein synthesis, and muscle hypertrophy. AMP-activated protein kinase (AMPK) is a cellular energy sensor, a negative regulator of mTOR, and an inhibitor of protein synthesis. The purpose of this study was to determine whether the hypertrophy/cell growth-associated mTOR pathway was down-regulated during muscle atrophy associated with chronic paraplegia. Soleus muscle was collected from male Sprague-Dawley rats 10 wk following complete T4-T5 spinal cord transection (paraplegic) and from sham-operated (control) rats. We utilized immunoprecipitation and Western blotting techniques to measure upstream [AMPK, Akt/protein kinase B (PKB)] and downstream components of the mTOR signaling pathway [mTOR, S6K1, SKAR, 4E-binding protein 1 (4E-BP1), and eukaryotic initiation factor (eIF) 4G and 2α]. Paraplegia was associated with significant soleus muscle atrophy (174 ± 8 vs. 240 ± 13 mg; P < 0.05). There was a reduction in phosphorylation of mTOR, S6K1, and eIF4G (P < 0.05) with no change in Akt/PKB or 4E-BP1 (P > 0.05). Total protein abundance of mTOR, S6K1, eIF2α, and Akt/PKB was decreased, and increased for SKAR (P < 0.05), whereas 4E-BP1 and eIF4G did not change (P > 0.05). S6K1 activity was significantly reduced in the paraplegic group (P < 0.05); however, AMPKα2 activity was not altered (3.5 ± 0.4 vs. 3.7 ± 0.5 pmol·mg-1 ·min-1, control vs. paraplegic rats). We conclude that paraplegia-induced muscle atrophy in rats is associated with a general downregulation of the mTOR signaling pathway. Therefore, in addition to upregulation of atrophy signaling during muscle wasting, downregulation of muscle cell growth/hypertrophy-associated signaling appears to be an important component of long-term muscle loss.
Keywords: muscle wasting, spinal cord injury, rehabilitation, AMP-activated protein kinase, Akt
SKELETAL MUSCLE DISPLAYS AN incredible ability to respond to alterations in daily loading. For example, muscle atrophy occurs at an elevated rate in the initial few months following disuse in a variety of animal models such as spinal cord transection, hindlimb suspension, and spaceflight (3, 11, 14, 16, 21, 25, 43). The resulting muscle loss from these unweighting or denervation models appears to affect type I skeletal muscle fibers, such as those predominantly found in the soleus, to a greater extent (1). Both type I and type II muscle fiber size decrease following spinal cord injury (32, 33, 35). However, type II fibers are affected first followed by type I fiber atrophy, which becomes predominant during the later stages of paraplegia (35). Of the total soleus muscle weight ultimately lost due to atrophy, the majority (60%) is lost within the first 10 days (16), suggesting that alterations in protein synthesis and breakdown occur rapidly. Indeed, a decrease in protein synthesis has been measured after only 5 h of hindlimb suspension in rats (37) while in humans immobilized for only 48 h, mRNA for components of the ubiquitin-proteosome pathway were significantly upregulated (40).
Muscle protein synthesis is primarily regulated at the level of mRNA translation (27, 28). Alterations in skeletal muscle mass are controlled by the regulation of complex cell signaling pathways that govern muscle protein synthesis, breakdown, and cell proliferation (20). Skeletal muscle ribosomal S6 kinase 1 (S6K1) is a downstream component of the mammalian target of rapamycin (mTOR) signaling pathway and is a key regulator of translation initiation and protein synthesis (27, 28). Phosphorylation of S6K1 is associated with increased muscle mass following hypertrophic stimuli (2), whereas muscle atrophy is associated with reduced S6K1 phosphorylation and overall protein abundance (4).
SKAR is a downstream binding partner and substrate of S6K1 and has been implicated in preliminary findings as a regulator of cell size (34). It has been shown that observed muscular atrophy in immobilization models is the result of decreases in muscle fiber diameter (i.e., cell size) and not due to a decrease in the number of muscle fibers (18). Because SKAR has been linked with S6K1 in regulation of cell size (34), this was a logical step in the pathway to examine. Phosphorylation of another downstream target of mTOR, 4E binding protein 1 (4E-BP1), is a key event in translation initiation, releasing eukaryotic initiation factor 4E (eIF4E) to complex with eukaryotic initiation factor 4G (eIF4G) in the beginning steps of translation (20). On the other hand, activation of skeletal muscle AMP-activated protein kinase (AMPK) during conditions of energetic stress is as an upstream negative regulator of mTOR signaling and protein synthesis (5, 6). Although there has been a considerable amount of work done assessing the role of muscle protein breakdown during muscle atrophy (26), less is known about the role of cell signaling pathways that regulate protein synthesis. In fact, it is not been determined whether mTOR or AMPK signaling is altered during paraplegia-induced muscle atrophy.
Before World War II, 80% of individuals with spinal cord injury died within 3 yr of the injury primarily due to kidney and pulmonary infections (12). However, with the advent of antibiotic drugs and advancements in acute care and rehabilitation, the life expectancy of individuals with spinal cord injury has increased to near that for able-bodied individuals (13). Thus the advantages of using a chronic muscle atrophy model (as done in the present study) may be useful in identifying the mechanisms of muscle protein synthesis and breakdown in the overall regulation of muscle mass. This could be clinically important in the rehabilitation of individuals with spinal cord injury because it may lead to the design of nutritional and/or contractile interventions targeting mTOR, muscle protein synthesis, and cell growth. Therefore, the purpose of this study was to determine the effect of chronic paraplegia-induced muscle atrophy on components of the skeletal muscle mTOR signaling pathway.
MATERIALS AND METHODS
Study design
All surgical procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Wayne State University School of Medicine (Detroit, MI). Sixteen male Sprague-Dawley rats (24 ± 0.2 wk of age at death) were separated into two groups: complete spinal cord transection between thoracic level 4 and 5(T4-T5, paraplegic, n = 8) or sham spinal cord transection (control, n = 8). Following 10 wk of spinal cord transected-induced paralysis or sham-operated free living, the rats were killed and the soleus muscle was isolated, freeze clamped, immediately frozen in liquid nitrogen, and stored at -80°C until analyzed. Soleus muscles were homogenized (1:9 wt/vol) in a buffer containing 50 mM Tris·HCl, 250 mM mannitol, 50 mM NaF, 5 mM Na pyrophosphate, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, pH 7.4, 1 mM DTT, 1 mM benzamadine, 0.1 mM PMSF, and 5 μg/ml of soybean trypsin inhibitor were added just before use. Total protein concentrations were determined for all supernatants with the Bradford assay using a Smartspec+ spectrophotometer (Bio-Rad) on the day of homogenization.
Induced paraplegia
All surgical procedures were performed using aseptic techniques. Rats were anesthetized with pentobarbital sodium (45 mg/kg ip), and supplemental doses (10-20 mg/kg ip) were administered if rats regained the blink reflex or responded during the surgical procedure. After anesthesia, rats were positioned prone over a thoracic roll resulting in a moderately flexed spine. The T4 and T5 vertebrae were exposed via a midline dorsal incision. The underlying spinal cord between T4 and T5 was completely transected through the intervertebral space as previously described (7, 8). The control animals (sham operated) had the spinal cord exposed in an identical procedure; however, the spinal cord was not transected. This approach allowed for minimal impact on the stability of the vertebral column. During the first week of recovery, all rats were handled several times daily. During the handling periods, visual inspection and physical manipulations were performed to prevent pressure ulcers. Additionally, bladder voiding was accomplished by manual compression after which all animals were weighed. After the first week of recovery, handling was reduced to one time each day, and bladders no longer required manual compression for voiding. At day 7, rats underwent a motor activity score using previously described criteria (41). Briefly, the motor activity score was determined by placing the animal on a table and observing hindlimb movement for 1 min. Scores ranged from 0 to 5 with a 5 indicating normal walking and a 0 indicating zero weight bearing or spontaneous movement specific to the hindlimbs. All paralyzed rats had a score of 0.
SDS-PAGE and immunoblotting
Aliquots from homogenates (described above), except for 4E-BP1, were boiled at 100°C for 3 min in 2× sample buffer (SB) containing 125 mM Tris, pH 6.8, 25% glycerol, 2.5% SDS, 2.5% β-mercaptoethanol, and 0.002% bromophenol blue. Aliquots used to detect 4E-BP1 were initially boiled at 100°C for 10 min, spun for 30 min at 10,000 rpm before combining the supernatants with 2× SB. Samples containing 50 μg of total protein per lane were loaded in duplicate and separated by SDS-PAGE for 60 min at 150 V using 7.5% gels on Criterion electrophoresis cell, except 4E-BP1, which was run on a 15% gel. Following SDS PAGE, proteins were transferred to polyvinylidene diflouride membranes (PVDF) (Hybond-P, Amersham Biosciences, Piscataway, NJ) at 50 V for 1 h. Once transferred, PVDF membranes were placed in blocking buffer [5% nonfat dry milk (NFDM) in TBST (Tris-buffered saline and 0.1% Tween 20)] for 1 h. Blots were then serially washed two times in deionized water and two more times in TBST and incubated with primary antibody in 5% NFDM in TBST overnight at 4°C with constant agitation. Blots were washed in TBST twice and incubated with secondary antibody for 1 h in 5% NFDM in TBST at room temperature with constant agitation. Blots were washed for 15 min followed by three additional washes lasting 5 min with TBST. Blots were then incubated for 5 min with enhanced chemiluminescence reagent (ECL plus Western Blotting Detection System, Amersham Biosciences, Piscataway, NJ) to detect horseradish peroxidase activity. Optical density measurements were obtained with a charge-coupled device camera mounted in a ChemiDoc XRS imaging system (Bio-Rad, Hercules, CA). Once the appropriate image was captured, Densitometric analysis was performed using Quantity One 1-D analysis Software (version 4.5.2, Bio-Rad). Data are expressed as raw value of the band minus a representative background sample from the membrane, divided by an internal loading control (50 μg/lane) loaded on every gel to ensure comparability across membranes.
AMPK activity assay
After homogenization, total protein concentrations were determined using the Bradford assay (Bio-Rad, Smartspec plus spectrophotometer) as described above. The immunoprecipitation method and the enzyme activity measurements were completed as previously described (23). Slight adaptations to this method included performing the immunoprecipitation overnight and the AMPK assay on resuspended immunoprecipitates in the medium (44). Tissue samples were homogenized, immunoprecipitated, and assayed as previously described (15). Activity is expressed as picomoles of phosphate incorporated per milligram of muscle protein subjected to immunoprecipitation per minute (pmol·mg-1 ·min-1).
S6K1 activity assay
The S6K1 activity assay was prepared as the AMPK activity described above with the following changes. The immunoprecipitation method and the enzyme activity measurements were completed as previously described with slight modifications (39). Supernatant was collected after centrifugation at 3,500 g for 10 min at 4°C. Immunoprecipitation was achieved by incubating 40 μl of supernatant with protein A-Sepharose beads (Sigma, St. Louis, MO) complexed with antibodies against rabbit anti-p70S6K kinase (C-18) (Santa Cruz, Santa Cruz, CA) for 2 h on a roller mixer at 4°C. The suspension was washed twice in a buffer containing 50 mM Tris·HCl, 50 mM β-glycerophosphate, 10 mM NaF, 2 mM Na3VO4, 1 mM EDTA, 1 mM EGTA, and 50 mM β-mercaptoethanol, pH 8.0, and washed once in a kinase buffer containing 50 mM MOPS, 25 mM β-glycerophosphate, 2 mM EDTA, 5 mM EGTA, 20 mM MgCl2, 1 mM DTT, 0. 2 mM Na3VO4, and 10 mM NaF. Immunoprecipitated p70S6K was then suspended in 30 μl of HEPES-Brij buffer containing 25 mM HEPES, 0.02% Brij, and 1 mM DTT, pH 7.0. S6K1 activity was measured by adding 15 μl of the working assay cocktail containing 0.1 mM ATP added to kinase buffer and 0.1 mM S6K1 substrate corresponding to amino acids 231-239 of human 40S ribosomal protein S6 (sequence RRRLSSLRA; Santa Cruz Biotechnologies, Santa Cruz, CA) and 10 mCi/ml [γ-32P]ATP (MP Biomedicals, Aurora, OH). The mixture was incubated for 10 min in a water bath ~30°C with 60 rpm of constant agitation to facilitate mixing. At the end of the incubation period, a 15-μl aliquot was spotted onto a 1-cm2 P81 Whatman filter paper (Whatman International, Maidstone, UK) and immediately placed in 200 ml of 1% phosphoric acid to stop the kinase reaction. Each filter paper was washed six times in 1% phosphoric acid for 5 min. After air drying, they were added to 3 ml of Ecolite liquid scintillation fluid (MP Biomedicals, Aurora, OH) and counted for 10 min in a LS 6500 multipurpose scintillation counter (Beckman Coulter, Fullerton, CA). Activity is expressed in picomoles of phosphate incorporated per milligram of muscle protein subjected to immunoprecipitation per minute (pmol·mg-1 ·min-1). Each sample was run in duplicate and background activity (HEPES-Brij buffer + working assay cocktail) was subtracted.
Antibodies
The primary antibodies used were all purchased from Cell Signaling (Beverly, MA): phospho-mTOR (Ser2448, 1:1,000); phospho-p70 S6K1 (Thr389, 1:500); phospho-Akt/PKB (Ser473, 1:500); phospho-4E-BP1 (Thr37/46); phospho-eIF4G (Ser1108); total-mTOR (1:1,000); total-p70 S6K1 (1:500); total-Akt/PKB (1:1,000); total-4E-BP1 (1:1,000); total-eIF4G (1:1,000) and total SKAR (1:1,000). The antibodies for eIF2 were purchased from BioSource (Camarillo, CA): phospho-eIF2α (Ser52, 1:1,000) and total eIF2α (1:1,000). Anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody was purchased from Amersham Bioscience (1:2,000).
Statistical analysis
All values are expressed as means ± SE. All comparisons between control and paraplegic rats were performed with an independent t-test using SPSS statistical software version 14.0. (SPSS, Chicago, IL). Significance was predetermined at P < 0.05.
RESULTS
Whole body and soleus muscle weight
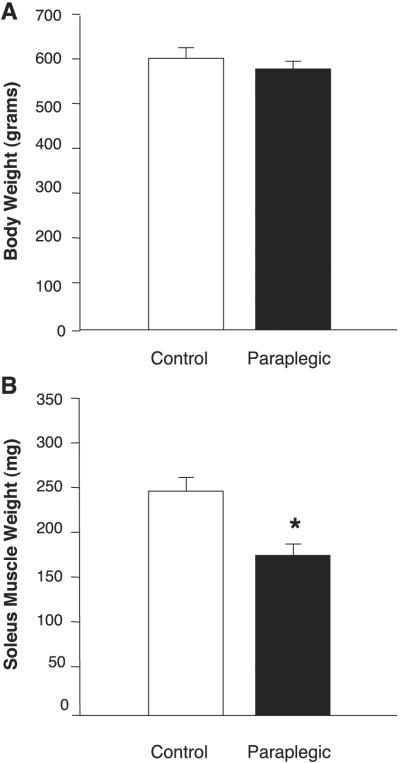
Whole body weight was not different between control and paraplegic rats (608 ± 9 vs. 571 ± 2 g, respectively; P > 0.05; Fig. 1A). Soleus muscle weight, however, was 28% lower in the paraplegic rats compared with control rats (174 ± 8 vs. 240 ± 13 mg, respectively; P < 0.05; Fig. 1B).
Fig. 1.
Rat whole body and soleus wet muscle weight. Body weight is expressed in grams (A) and soleus weight in milligrams (B). Body weight was not different between the control group (open bars) and the paraplegic group (solid bars) but soleus weight was reduced by 28% (B). Values are means ± SE; n = 8 per group. *Significantly different from control, P < 0.05.
Western blot analyses
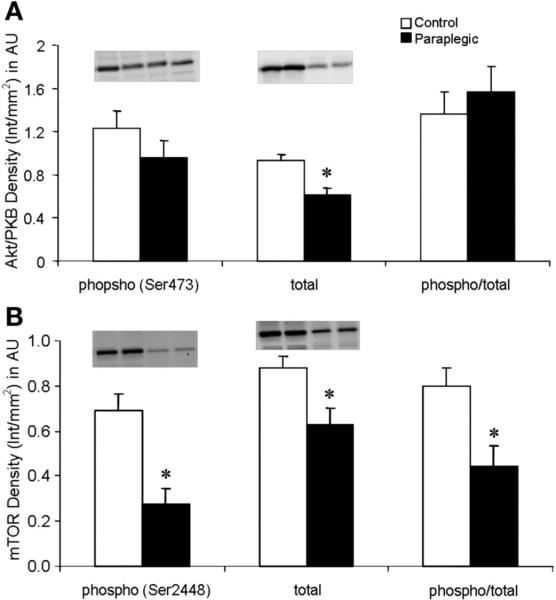
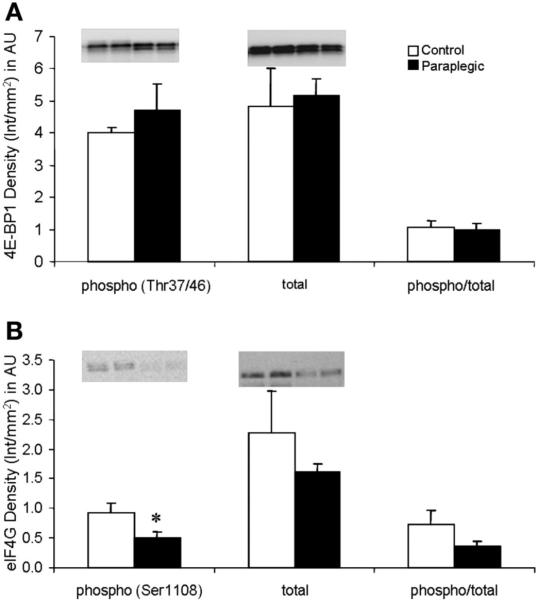
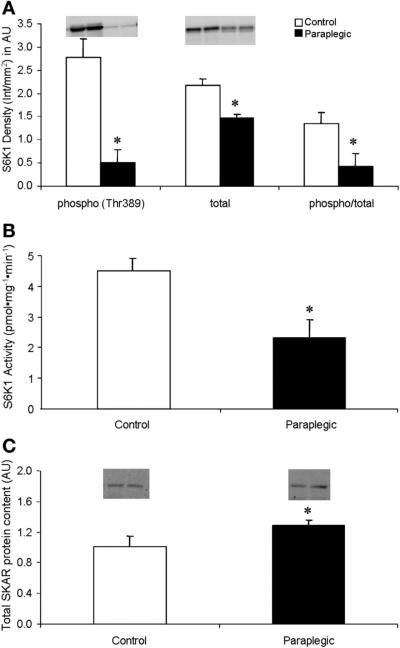
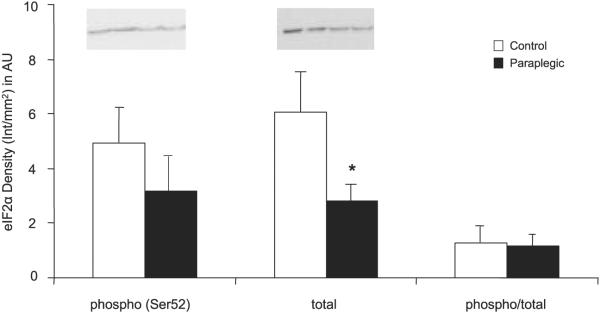
There was no change in the phosphorylation status of Akt/PKB (Ser473); however, there was a significant decrease in total protein content of Akt/PKB in paraplegic vs. control rats (P < 0.05; Fig. 2A). There were significant decreases in the paraplegic rats in the phosphorylation status of mTOR (Ser2448) as well as the total protein content of mTOR (P < 0.05; Fig. 2B). The phosphorylation status of eIF4G (Ser1108) was significantly reduced in the Paraplegic rats (P < 0.05) with no change in phosphorylation of 4E-BP1 (Thr37/46) or total protein content of either eIF4G or 4E-BP1 (P > 0.05; Fig. 3, A and B). Phosphorylation (Thr389) and total protein content of S6K1 were reduced in paraplegic rats (P < 0.05, Fig. 4A), mirroring a significant decrease in paraplegic soleus muscle S6K1 activity by 50% (2.3 ± 0.6 vs. 4.5 ± 0.3 pmol·mg-1 ·min-1 respectively; P < 0.05, Fig. 4B). Total protein content of SKAR was reduced in paraplegic rats (P < 0.05; Fig. 4C). Phosphorylation (Ser52) and phospho/total protein content of eIF2α were not different between groups (P > 0.05; Fig. 5). However, total protein content of eIF2α was reduced in the paraplegic rats (P < 0.05; Fig. 5).
Fig. 2.
Soleus muscle phosphorylation status (Ser473) (phospho) and total Akt/protein kinase B (PKB) protein abundance (A), and phosphorylation status (Ser2448) and total mTOR protein abundance (B). Data are expressed relative to an internal loading control (Int) and as means ± SE; n = 8 per group. Inset, representative Western blot for duplicate samples for control and paraplegic rats. AU, arbitrary units. *Significantly different from control, P < 0.05.
Fig. 3.
Soleus muscle phosphorylation status (Thr37/46) and total 4E-binding protein 1 (4E-BP1) protein abundance (A), and phosphorylation status (Ser1108) and total eukaryotic initiation factor-4G (eIF4G) protein abundance (B). Data are expressed relative to an internal loading control and as means ± SE; n = 8 except total and phospho/total eIF4G, n = 6. Inset, representative Western blot for duplicate samples for control and paraplegic rats. *Significantly different from control, P < 0.05.
Fig. 4.
Soleus muscle phosphorylation status (Thr389) and total Ribosomal S6 kinase 1 (S6K1) protein abundance (A) and total SKAR protein abundance (C). Data are expressed relative to an internal loading control and as means ± SE; n = 8 per group. Inset, representative Western Blot for duplicate samples for control and paraplegic rats. Soleus muscle S6K1 activity (B): data are expressed as means ± SE, n = 4 per group. *Significantly different from control, P < 0.05.
Fig. 5.
Soleus muscle phosphorylation status (Ser52) and total eukaryotic initiation factor 2α (eIF2α) protein abundance. Data are expressed relative to an internal loading control and as means ± SE; n = 6 per group. Inset, representative Western blot for duplicate samples for control and paraplegic rats. *Significantly different from control, P < 0.05.
AMPKα2 activity
Soleus muscle AMPKα2 activity was not different between control and paraplegic rats, (3.5 ± 0.4 vs. 3.7 ± 0.5 pmol·mg-1 ·min-1; respectively, P > 0.05; Fig. 6).
Fig. 6.
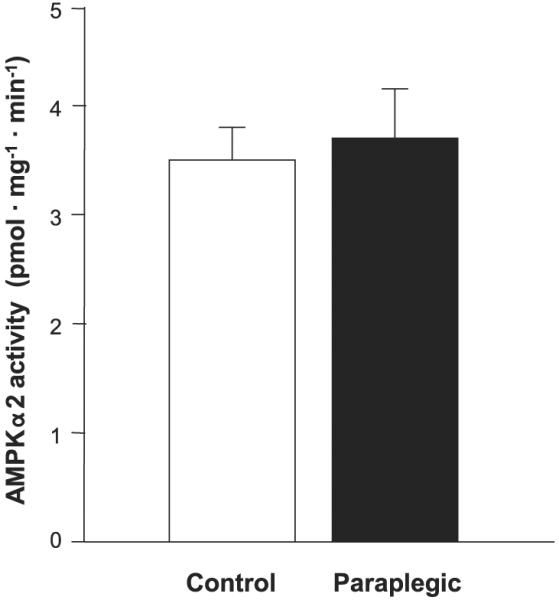
Soleus muscle AMP-activated protein kinaseα2 (AMPKα2) activity. AMPK activity was not different between groups. Open bar, control rats; filled bar, paraplegic rats. Values are means ± SE; n = 8 per group.
DISCUSSION
The literature concerning the acute effects (up to 15 days) of differing atrophy models on protein synthesis, morphology, gene expression and certain signaling events is extensive. However, it is important to note that paraplegia, a chronic condition, should be studied as such. The primary finding from our study was that there are numerous proteins in the mTOR/S6K1 signaling pathway (mTOR, S6K1, eIF4G, Akt/PKB) that are significantly downregulated following muscle atrophy induced by chronic paraplegia in rats. Furthermore, we found that AMPKα2 activity is unchanged in soleus muscle, suggesting that AMPK is not involved in the downregulation of mTOR signaling in long-term paraplegic muscle. However, S6K1 activity significantly decreased in the paraplegic group, reflecting what was measured in the immunoblots for S6K1 and suggesting that this kinase plays an important role in the regulation of initiation of protein synthesis and cell size. The abrupt reductions in the rate of soleus muscle protein synthesis during hindlimb unweighting are followed, albeit at a slower rate, by an increase in muscle protein breakdown resulting in a reduction of muscle mass (37). However, similar alterations in muscle morphology have also been identified in human paraplegic muscle (10). Muscle protein synthesis is primarily regulated through a complex signaling pathway involving the protein kinase mTOR (17) and is controlled by growth factors and nutrients such as insulin and amino acids (29, 42). The activation of mTOR enhances mRNA translation initiation and elongation resulting in an increase in muscle protein synthesis and growth (42). Accordingly, mTOR signaling represents an important potential regulator of muscle growth and recovery following muscle stress or injury such as that brought on by paraplegia.
The purpose of this study was to determine the effects of 10 wk of paraplegia on components of the mTOR signaling pathway. Specifically, we were interested in the “potential” for muscle growth as indicated by the signaling status of mTOR (phosphorylation and total protein content) as well as proteins associated with this important kinase. Ten weeks of paraplegia resulted in a 28% reduction in soleus muscle wet weight with no change in body mass. Accompanying the loss of soleus muscle mass was a significant reduction of both phosphorylated and total mTOR and S6K1 compared with sham-operated controls. These findings are novel as acute studies have not attempted to measure mTOR, and report varying results on the status of S6K1 in different atrophy models (4, 21, 25). Reduction in both the phosphorylation and total amount of mTOR and S6K1, along with a decreased activity of S6K1, suggest that the mTOR/S6K1 pathway may be an important regulator of muscle growth in this chronic model. This is supported by studies showing inhibition of mTOR by the drug rapamycin blocks downstream targets S6K1 and 4E-BP1, and almost completely inhibits hypertrophy without causing atrophy (4). The combination of these results would suggest that both hypertrophy and atrophy pathways are at work in the chronic state of paraplegia and that the atrophied cells are not able to rebuild muscle, in part, due to downregulation of components of the mTOR/S6K1 pathway. It is also likely that regulators of both hypertrophy and atrophy are downregulated in our chronic paraplegia model, which is significantly different from other growth or atrophy models.
Many other proteins in the mTOR pathway were affected in the paraplegic group compared with the controls. Though there were no changes in the phosphorylation of Akt/PKB between groups, paraplegic rats had a significantly reduced amount of total Akt/PKB. Initially, a reduction in total protein may be thought to explain the reduced mTOR activation, because Akt/PKB is an upstream activator of mTOR. However, in examining the phosphorylation data, it is clear that activation of Akt/PKB is similar between groups, so this is not a likely explanation. Further downstream of mTOR, we saw a decrease in the phosphorylation of eIF4G in paraplegic rats. eIF4G acts as a scaffold protein for the assembly of eIF4E and eIF4A to form the eIF4F complex that mediates translation initiation (19). Decreased phosphorylation seems to indicate a reduced ability to initiate translation in the paraplegic rats and could partially explain the atrophy in these rats.
A recently identified protein, SKAR (S6K1 Aly/REF-like target), has been found to be a novel binding protein and substrate of S6K1 that is insulin sensitive and specifically affects cell size (34). SKAR protein is phosphorylated by activated S6K1 and inhibiting both SKAR and S6K1 results in cells that are smaller (34). SKAR protein content in the soleus muscles from paraplegic rats was significantly and paradoxically elevated despite reduced total S6K1 protein content and activity. SKAR protein is a substrate of S6K1 and may potentially influence muscle cell growth (34). Presumably the elevated SKAR protein content is an adaptive response by the muscle in an attempt to prime the muscle for regrowth if the appropriate and necessary conditions are established. However, this is mere speculation because the physiological function of SKAR is currently unknown and future research is needed to determine why SKAR total protein content would be upregulated in soleus muscle following 10 wk of paraplegia.
We would also like to point out that the mTOR signaling pathway is most likely not the sole contributor to the reduction in muscle mass and/or changes in translation initiation. The eukaryotic initiation factor 2 (eIF2) pathway also has a regulatory role for translation initiation and protein synthesis in skeletal muscle. When eIF2α is phosphorylated, eIF2B is inhibited from producing a complex with GTP and initiator methionyl tRNA. This mechanism prevents the complete assembly of the 48S preinitiation complex and, ultimately, mRNA translation (31). Therefore, the phosphorylation status of eIF2α Ser52 provides a marker as to the role of the eIF2 pathway in the control of protein synthesis. In our study, we found the eIF2α total protein content was reduced in paraplegia; however, eIF2α Ser52 phosphorylation was unaffected. It appears that eIF2 regulation may not be affected to the same extent as the mTOR pathway following chronic paraplegia.
AMPK activity, a potential upstream negative regulator of mTOR signaling, was not different between paraplegic and sham-operated control rats. Originally, we hypothesized that AMPK activity would be elevated following 10 wk of paraplegia partially explaining the reduced muscle protein synthesis known to occur (37) with muscle atrophy and the proposed negative regulation on mTOR (5, 6). Moreover, AMPK activation has been suggested to activate p38 MAPK (45), which has recently been shown to promote GLUT4 translocation (36) and glucose uptake into insulin-resistant denervated muscles (22). AMPK has also been shown to phosphorylate a novel protein called AS160 (Akt substrate molecular weight 160), which is involved in the upregulation of GLUT4 translocation to the sarcolemma (30, 38). Our data do not support a physiological role for AMPK in either the inhibition of mTOR signaling and protein synthesis or glucose uptake following long-term paraplegia. However, the duration of our study (10 wk) may have been too long to have identified changes in AMPK activity. For example, a recent study reported an increase in AMPK activity at 4 and 8 wk of hindlimb suspension in rats (24). The majority of the loss of muscle mass almost certainly occurred sooner, within the first two wk of paraplegia, as previously demonstrated (16). Therefore, we cannot rule out the possibility that AMPK may have been playing a key role in regulating mTOR signaling, protein synthesis, and glucose uptake in soleus muscle during the initial stages following the spinal cord transection procedure.
In addition to the general downregulation of the mTOR pathway, we found a decrease in S6K1 total protein content following 10 wk of paraplegia (P = 0.059). Our data extend upon the findings of Haddad et al. (21), who demonstrated that up to 15 days of spinal transection did not have significant effects on markers of cell signaling (4E-BP1, S6K1 and eIF2α), suggesting that in the paraplegic rat changes in a few of the proteins downstream of mTOR may require more time for the differences to emerge. Our results are in agreement with others that have shown the acute effects of 14 days of hindlimb suspension resulted in a significant decrease in total S6K1 and accompanied a 25-55% reduction in soleus muscle weight (4). S6K1 protein content and activity is important because it regulates translation of mRNAs that encode proteins necessary for elongation factors and ribosomal subunits (28). Moreover, in a subset of our rats, we measured a significant decrease in the activity of S6K1 (-48%). As with total mTOR content, these findings expand on the acute affects of muscle atrophy on downstream components of the mTOR signaling pathway to include chronic (10 wk) changes in the paraplegic rat. In any event, 10 wk of paraplegia resulted in a downregulation of the mTOR/S6K1 signaling, which may compromise the ability of the soleus muscle to respond to anabolic stimuli. Future studies are required to determine whether nutritional or contractile interventions in paraplegic muscle can attenuate or reverse the reduction in mTOR signaling.
Our findings are in agreement with the acute muscle atrophy models showing a reduction in mTOR signaling (4, 25). Our results expand on the reported alterations known to occur with an acute (14 day) response to muscle unloading, denervation and spinal cord isolation and are an initial characterization of a prolonged/chronic adaptation to reduced mechanical loading and/or the elimination of neural input. However, it is important to note that peripheral nerves are intact in the paraplegic rat (the paraplegic rat is not denervated but rather decentralized) and thus the muscle response to denervation and paraplegia may be different. Future studies are warranted to compare the differences between disuse models of muscle atrophy (e.g., hindlimb unloading, denervation, spaceflight, bed rest) and paraplegia (e.g., spinal cord transection/injury). Furthermore, we chose to examine a slow twitch muscle (soleus) in the present study because of the well-known atrophy this muscle experiences during unweighting atrophy models (1) and the observation that type I muscle fibers atrophy predominantly during the later stages of spinal cord injury (35). However, it should be acknowledged that both type I and type II skeletal muscle fibers atrophy to a similar extent following paralysis/spinal cord injury (9, 32, 33, 35), and therefore our results should be taken in the context of having only examined a primarily slow-twitch muscle.
We conclude that 10 wk of paraplegia-induced muscle atrophy (28% reduction in soleus muscle mass) is coupled with a downregulation of a key signaling pathway associated with muscle cell growth (i.e., mTOR/S6K1). Further investigation is necessary to determine the potential for upregulating the mTOR signaling pathway to promote muscle cell growth in paraplegic muscle and other muscle-wasting conditions. Therefore, potential interventions such as the use of highly anabolic nutritional supplements/meal (e.g., essential amino acid mixtures enriched in leucine) and/or muscle contractions (e.g., electrical stimulation and/or assistive exercise therapies) can be designed to activate the mTOR/S6K1 pathway, which may be useful for counteracting muscle atrophy in individuals with spinal cord injury.
Acknowledgments
GRANTS This study was supported by National Heart, Lung, and Blood Institute Grant R01 HL-074122, and Institute for Arthritis and Musculoskeletal and Skin Diseases Grant R01 AR-049877. H. C. Dreyer was supported by National Institute on Disability and Rehabilitation Research, Department of Education, Grant H133P040003.
REFERENCES
- 1.Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol. 2003;95:2185–2201. doi: 10.1152/japplphysiol.00346.2003. [DOI] [PubMed] [Google Scholar]
- 2.Baar K, Esser K. Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol. 1999;276:C120–C127. doi: 10.1152/ajpcell.1999.276.1.C120. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin KM, Herrick RE, Ilyina-Kakueva E, Oganov VS. Effects of zero gravity on myofibril content and isomyosin distribution in rodent skeletal muscle. FASEB J. 1990;4:79–83. doi: 10.1096/fasebj.4.1.2136840. [DOI] [PubMed] [Google Scholar]
- 4.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 5.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277:23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- 6.Cheng SW, Fryer LG, Carling D, Shepherd PR. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem. 2004;279:15719–15722. doi: 10.1074/jbc.C300534200. [DOI] [PubMed] [Google Scholar]
- 7.Collins HL, DiCarlo SE. Acute exercise reduces the response to colon distension in T(5) spinal rats. Am J Physiol Heart Circ Physiol. 2002;282:H1566–H1570. doi: 10.1152/ajpheart.00733.2001. [DOI] [PubMed] [Google Scholar]
- 8.Collins HL, DiCarlo SE. TENS attenuates response to colon distension in paraplegic and quadriplegic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1734–H1739. doi: 10.1152/ajpheart.00253.2002. [DOI] [PubMed] [Google Scholar]
- 9.Cormery B, Pons F, Marini JF, Gardiner PF. Myosin heavy chains in fibers of TTX-paralyzed rat soleus and medial gastrocnemius muscles. J Appl Physiol. 2000;88:66–76. doi: 10.1152/jappl.2000.88.1.66. [DOI] [PubMed] [Google Scholar]
- 10.Crameri RM, Weston AR, Rutkowski S, Middleton JW, Davis GM, Sutton JR. Effects of electrical stimulation leg training during the acute phase of spinal cord injury: a pilot study. Eur J Appl Physiol. 2000;83:409–415. doi: 10.1007/s004210000263. [DOI] [PubMed] [Google Scholar]
- 11.Desplanches D, Mayet MH, Ilyina-Kakueva EI, Sempore B, Flandrois R. Skeletal muscle adaptation in rats flown on Cosmos 1667. J Appl Physiol. 1990;68:48–52. doi: 10.1152/jappl.1990.68.1.48. [DOI] [PubMed] [Google Scholar]
- 12.DeVivo MJ, Kartus PL, Stover SL, Rutt RD, Fine PR. Cause of death for patients with spinal cord injuries. Arch Intern Med. 1989;149:1761–1766. [PubMed] [Google Scholar]
- 13.DeVivo MJ, Shewchuk RM, Stover SL, Black KJ, Go BK. A cross-sectional study of the relationship between age and current health status for persons with spinal cord injuries. Paraplegia. 1992;30:820–827. doi: 10.1038/sc.1992.158. [DOI] [PubMed] [Google Scholar]
- 14.Diffee GM, Caiozzo VJ, Herrick RE, Baldwin KM. Contractile and biochemical properties of rat soleus and plantaris after hindlimb suspension. Am J Physiol Cell Physiol. 1991;260:C528–C534. doi: 10.1152/ajpcell.1991.260.3.C528. [DOI] [PubMed] [Google Scholar]
- 15.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupont-Versteegden EE, Houle JD, Gurley CM, Peterson CA. Early changes in muscle fiber size and gene expression in response to spinal cord transection and exercise. Am J Physiol Cell Physiol. 1998;275:C1124–C1133. doi: 10.1152/ajpcell.1998.275.4.C1124. [DOI] [PubMed] [Google Scholar]
- 17.Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- 18.Gibson JN, Halliday D, Morrison WL, Stoward PJ, Hornsby GA, Watt PW, Murdoch G, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci (Lond) 1987;72:503–509. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- 19.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 20.Glass DJ. Signalling pathways that mediate skeletal muscle hypertrophy and atrophy. Nat Cell Biol. 2003;5:87–90. doi: 10.1038/ncb0203-87. [DOI] [PubMed] [Google Scholar]
- 21.Haddad F, Roy RR, Zhong H, Edgerton VR, Baldwin KM. Atrophy responses to muscle inactivity. II. Molecular markers of protein deficits. J Appl Physiol. 2003;95:791–802. doi: 10.1152/japplphysiol.01113.2002. [DOI] [PubMed] [Google Scholar]
- 22.Handberg A, Megeney LA, McCullagh KJ, Kayser L, Han XX, Bonen A. Reciprocal GLUT-1 and GLUT-4 expression and glucose transport in denervated muscles. Am J Physiol Endocrinol Metab. 1996;271:E50–E57. doi: 10.1152/ajpendo.1996.271.1.E50. [DOI] [PubMed] [Google Scholar]
- 23.Hardie DG, Salt IP, Davies SP. Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol Biol. 2000;99:63–74. doi: 10.1385/1-59259-054-3:63. [DOI] [PubMed] [Google Scholar]
- 24.Hilder TL, Baer LA, Fuller PM, Fuller CA, Grindeland RE, Wade CE, Graves LM. Insulin-independent pathways mediating glucose uptake in hindlimb-suspended skeletal muscle. J Appl Physiol. 2005;99:2181–2188. doi: 10.1152/japplphysiol.00743.2005. [DOI] [PubMed] [Google Scholar]
- 25.Hornberger TA, Hunter RB, Kandarian SC, Esser KA. Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats. Am J Physiol Cell Physiol. 2001;281:C179–C187. doi: 10.1152/ajpcell.2001.281.1.C179. [DOI] [PubMed] [Google Scholar]
- 26.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–165. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 27.Kapp LD, Lorsch JR. The molecular mechanics of eukaryotic translation. Annu Rev Biochem. 2004;73:657–704. doi: 10.1146/annurev.biochem.73.030403.080419. [DOI] [PubMed] [Google Scholar]
- 28.Kimball SR. Interaction between the AMP-activated protein kinase and mTOR signaling pathways. Med Sci Sports Exerc. 2006;38:1958–1964. doi: 10.1249/01.mss.0000233796.16411.13. [DOI] [PubMed] [Google Scholar]
- 29.Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- 30.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 31.Kubica N, Jefferson LS, Kimball SR. Eukaryotic initiation factor 2B and its role in alterations in mRNA translation that occur under a number of pathophysiological and physiological conditions. Prog Nucleic Acid Res Mol Biol. 2006;81:271–296. doi: 10.1016/S0079-6603(06)81007-X. [DOI] [PubMed] [Google Scholar]
- 32.Malisoux L, Jamart C, Delplace K, Nielens H, Francaux M, Theisen D. Effect of long-term muscle paralysis on human single fiber mechanics. J Appl Physiol. 2007;102:340–349. doi: 10.1152/japplphysiol.00609.2006. [DOI] [PubMed] [Google Scholar]
- 33.Mayer RF, Burke RE, Toop J, Walmsley B, Hodgson JA. The effect of spinal cord transection on motor units in cat medial gastrocnemius muscles. Muscle Nerve. 1984;7:23–31. doi: 10.1002/mus.880070105. [DOI] [PubMed] [Google Scholar]
- 34.Richardson CJ, Broenstrup M, Fingar DC, Julich K, Ballif BA, Gygi S, Blenis J. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr Biol. 2004;14:1540–1549. doi: 10.1016/j.cub.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 35.Scelsi R, Marchetti C, Poggi P, Lotta S, Lommi G. Muscle fiber type morphology and distribution in paraplegic patients with traumatic cord lesion. Histochemical and ultrastructural aspects of rectus femoris muscle. Acta Neuropathol (Berl) 1982;57:243–248. doi: 10.1007/BF00692178. [DOI] [PubMed] [Google Scholar]
- 36.Somwar R, Kim DY, Sweeney G, Huang C, Niu W, Lador C, Ramlal T, Klip A. GLUT4 translocation precedes the stimulation of glucose uptake by insulin in muscle cells: potential activation of GLUT4 via p38 mitogen-activated protein kinase. Biochem J. 2001;359:639–649. doi: 10.1042/0264-6021:3590639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thomason DB, Biggs RB, Booth FW. Protein metabolism and β-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Physiol Regul Integr Comp Physiol. 1989;257:R300–R305. doi: 10.1152/ajpregu.1989.257.2.R300. [DOI] [PubMed] [Google Scholar]
- 38.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes. 2006;55:2051–2058. doi: 10.2337/db06-0175. [DOI] [PubMed] [Google Scholar]
- 39.Tremblay F, Krebs M, Dombrowski L, Brehm A, Bernroider E, Roth E, Nowotny P, Waldhausl W, Marette A, Roden M. Overactivation of S6 kinase 1 as a cause of human insulin resistance during increased amino acid availability. Diabetes. 2005;54:2674–2684. doi: 10.2337/diabetes.54.9.2674. [DOI] [PubMed] [Google Scholar]
- 40.Urso ML, Scrimgeour AG, Chen YW, Thompson PD, Clarkson PM. Analysis of human skeletal muscle after 48 h immobilization reveals alterations in mRNA and protein for extracellular matrix components. J Appl Physiol. 2006;101:1136–1148. doi: 10.1152/japplphysiol.00180.2006. [DOI] [PubMed] [Google Scholar]
- 41.von Euler M, Akesson E, Samuelsson EB, Seiger A, Sundstrom E. Motor performance score: a new algorithm for accurate behavioral testing of spinal cord injury in rats. Exp Neurol. 1996;137:242–254. doi: 10.1006/exnr.1996.0023. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 2006;21:362–369. doi: 10.1152/physiol.00024.2006. [DOI] [PubMed] [Google Scholar]
- 43.West SP, Roy RR, Edgerton VR. Fiber type and fiber size of cat ankle, knee, and hip extensors and flexors following low thoracic spinal cord transection at an early age. Exp Neurol. 1986;91:174–182. doi: 10.1016/0014-4886(86)90035-x. [DOI] [PubMed] [Google Scholar]
- 44.Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- 45.Xi X, Han J, Zhang JZ. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J Biol Chem. 2001;276:41029–41034. doi: 10.1074/jbc.M102824200. [DOI] [PubMed] [Google Scholar]