Abstract
We sought to determine whether an intact bone marrow is essential to lung repair following bleomycin-induced lung injury in mice, and the mechanisms of any protective effects conferred by bone marrow–derived mesenchymal stem cell (BMDMSC) transfer. We found that myelosupression increased susceptibility to bleomycin injury and that BMDMSC transfer was protective. Protection was associated with the differentiation of engrafted BMDMSC into specific and distinct lung cell phenotypes, with an increase in circulating levels of G-CSF and GM-CSF (known for their ability to promote the mobilization of endogenous stem cells) and with a decrease in inflammatory cytokines. In vitro, cells from injured, but not from normal, mouse lung produced soluble factors that caused BMDMSC to proliferate and migrate toward the injured lung. We conclude that bone marrow stem cells are important in the repair of bleomycin-injured lung and that transfer of mesenchymal stem cells protects against the injury. BMDMSC localize to the injured lung and assume lung cell phenotypes, but protection from injury and fibrosis also involves suppression of inflammation and triggering production of reparative growth factors.
Keywords: stem cells, lung injury, bleomycin
An emerging concept of tissue repair holds that target organ injury is “sensed” by bone marrow stem cells that migrate to the site of damage and undergo differentiation promoting structural and functional repair (1). Pluripotent adult stem cells, normally resident in the bone marrow, are divided into at least two distinct populations: hematopoietic stem cells (HSC) and bone marrow–derived mesenchymal stem cells (BMDMSC). HSC are nonadherent cells negative for lineage-specific markers (2). BMDMSC are a group of plastic adherent cells, negative for CD45 and CD11b, that have been shown capable of differentiating into a variety of cell types, including endothelial, epithelial, and neuronal cells as well as adipocytes, depending on the culture conditions (3). When BMDMSC are infused into mice they can be found in the liver, muscle, heart, intestine, and lung, with phenotypic characteristics of cells in the organ where they reside (4). In the lung, BMDMSC have been detected as type I and type II alveolar epithelial cells, endothelial cells, fibroblasts, and bronchial epithelial cells (5).
It seems certain that BMDMSC have the capacity to localize to injured lung and differentiate into specific cell types (6). However, the origin and nature of the factors that determine localization and differentiation are yet to be determined, and the quantitative importance of this process in repairing injured lung is not yet clear. In animal experiments in which lung repair appears to be augmented by stem cell transplantation, the magnitude of the effect on repair appears out of proportion to the numbers of donor-derived parenchymal cells engrafting in the lungs (7).
In mice either myelosuppressed with a single intraperitoneal dose of busulfan or not, we administered bleomycin intratracheally in a dose that is reported to be non-lethal (8). In some animals from both groups, we administered BMDMSC from a green fluorescent protein (GFP)- donor 6 h after bleomycin. All of the normal but only one-third of the myelosuppressed animals survived to Day 14 after bleomycin; administration of BMDMSC after bleomycin resulted in 100% survival. Transplanted stem cells localized to the injured lung and assumed parenchymal cell phenotypes. Stem cell transplant prevented increased lung expression of immune-related cytokines and caused circulating levels of growth factors that can mobilize endogenous stem cells from bone marrow to increase. Cells from injured lungs produced humoral factors that both stimulated proliferation of mesenchymal stem cells and caused such cells to migrate toward the lung.
We conclude that marrow-derived stem cells are important to recovery of the lungs from injury and that administration of BMDMSC can hasten lung repair. The ability of stem cell transplantation to decrease persistent bleomycin-induced lung injury and fibrosis is a result not only of supplying stem cells to the lung, but includes attenuation of lung inflammation and increased production of growth factors that may mobilize endogenous stem cells. Lung repair includes specific signals from injured lung that cause mesenchymal cells to proliferate and migrate to sites of injury.
MATERIALS AND METHODS
Animal Maintenance
C57BL/6 mice (6–8 wk old; Jackson Laboratories, Bar Harbor, ME) were used in all experiments. They were randomized into various groups, weighed, and blood samples were collected. Animals were maintained in the animal care facility at Emory University. Approval of the experimental protocol by the IACUC was obtained before conducting the experiments.
Bleomycin Administration
Mice were anesthetized by isofluorane inhalation, the trachea exposed using sterile technique, and 4 U/kg bleomycin (Sigma, St. Louis, MO) in 100 μl of phosphate-buffered saline (PBS) were injected into the tracheal lumen. Results from our group and other groups have demonstrated that this dose causes fibrosis with a low mortality (9). After inoculation, the incision was closed and the animals were allowed to recover.
Bone Marrow Suppression by Busulfan Administration
Mice were anesthetized by isofluorane inhalation, and 20 mg/kg of busulfan was administrated intraperitoneally (Orphan Medical, Minnetonka, MN) in a single dose of 200 μl. Experiments were begun 24 h later.
Generation and Administration of Mesenchymal Stem Cells
BMDMSC were generated from male donor mice expressing a GFP transgene driven by a β-actin promoter described previously (10). Fresh bone marrow cells were isolated by flushing Dulbecco's modified Eagle's medium (DMEM; ATCC, Manassas, MD) containing 1% penicillin-streptomycin (ATCC) through the medullary cavity of mouse femurs. The cells were washed once with DMEM (ATCC) and plated at 1 × 106 cells per 100-mm cell culture dish (Corning, Corning, NY) in DMEM (ATCC) media containing 10% fetal calf serum (ATCC) supplemented with HEPES (ATCC), nonessential aminoacids (Cellgro, Herndon, VA), and pyruvate (Invitrogen, Carlsbad, CA), and cultured at 37°C in 5% CO2. After 48 h, nonadherent cells were removed, fresh media was added, and the culture was maintained for 7 d. Cells were harvested and using magnetic beads (Miltenyi Biotech, Auburn, CA), macrophages were depleted with anti-CD11b (BD, Palo Alto, CA) antibody, and hematopoietic cells were removed using an anti-CD45 (BD) antibody. Before infusion, cells were washed twice with PBS and resuspended at a concentration of 5 × 106cells/ml. Mice were anesthetized by inhalation of isofluorane and 0.1 ml of cell suspension was infused through a tail vein puncture. The cells were administered 6 h after intratracheal administration of bleomycin.
Histopathology
Frozen lung sections were fixed with 4% paraformadehyde for 30 min and treated with 1% bovine serum albumin plus 0.1% triton for another 30 min. Sections were then blocked with normal donkey serum (Sigma) for 30 min at room temperature. For double labeling, sections were incubated with monoclonal anti-GFP antibody (Molecular Probes, Eugene, OR) and polyclonal goat anti-SPC antibody (Santa Cruz Biotech., Santa Cruz, CA), polyclonal rabbit anti-aquaporin 5 antibody (Chemicon, Temecula, CA), polyclonal rabbit anti-α smooth muscle actin antibody (Spring Bioscience, Fremont, CA), or polyclonal goat anti-vimentin antibody (Santa Cruz Biotech). FITC-conjugated donkey anti-mouse and Rhodamine-conjugated donkey anti-rabbit, or goat IgG (Jackson Immunoresearch laboratories, West Grove, PA) were used as second antibodies. Each one of the experiments included a control for the secondary antibody to demonstrate the specificity of the reaction; these controls were negative. Endothelial cells were detected using Lectin from Ulex europaeus TRITC conjugated (Sigma); sections were counterstained with DAPI (Molecular Probes). Photographs were taken in an Olympus EX41 fluorescence microscope (Olympus America, Melville, NY) using ×100 and ×40 lenses with an Olympus MagnaFire camera. For quantitation of percentage of cells that were GFP+ and double positive for GFP/rhodamine, twenty ×40 magnification fields were randomly selected and the cells counted. Cells that were GFP+, GFP−, and double positive for GFP/rhodamine were enumerated separately. The percentage of GFP+ cells = (number of GFP+ cells)/[(number of GFP+ cells) + (number of GFP− cells)]. The percentage double positive cells for GFP/rhodamine = (number of cells double positive for GFP/rhodamine)/(number of GFP+ cells). Formalin-fixed lung specimens were used for preparing hematoxylin and eosin–stained sections and for immunohistochemical detection of GFP.
Morphometric Analysis
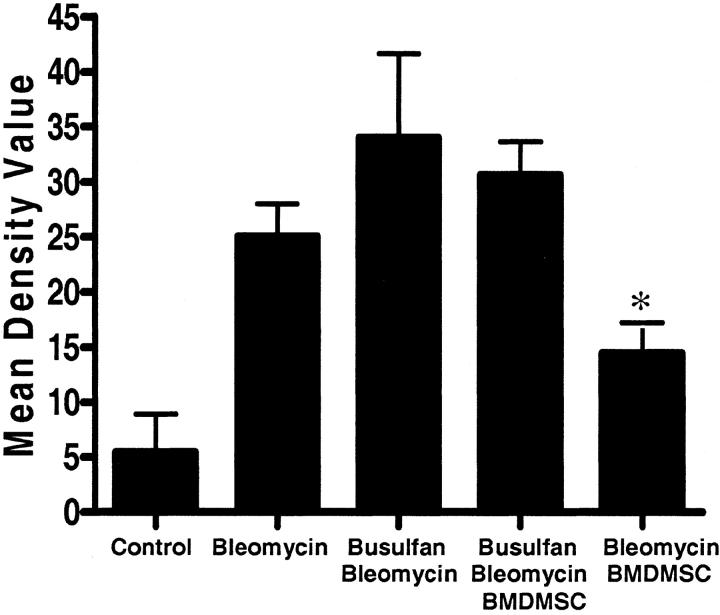
To assess lung tissue damage quantitatively, photographs were taken through an Olympus EX41 fluorescence microscope (Olympus America, Melville, NY) with an Olympus MagnaFire camera and the tissue density, as an indicator of injury, was determined quantitatively by determining density from ten images at ×2 magnification, which includes the total lung section, using Scion Image Software (Frederick, MD). To plot the data, we express the differences by subtracting from each single value the mean from the control group.
Detection of GM-CSF and G-CSF
Concentrations of G-CSF and GM-CSF in blood were determined using a Luminex system (Luminex, Austin, TX) with an anti-mouse kit obtained from Linco (St. Charles, MO). Well filters were prewashed, and 1:1 diluted samples were applied to each well. Specific antibody–coated beads were added to the wells and incubated for 18 h at 4°C temperature. After incubation, the plate was washed twice. Biotinylated antibodies against the growth factors were added and the mixture was incubated for 1 h. Afterwards, the cytokine–antibody complexes were detected by adding streptavidin coupled to PE. The number of positive complexes was determined by reading each sample in a Luminex XYP platform. Data were analyzed using a MasterPlex 1.2 from Mai-Rabio, and data related to concentration expressed in pg/ml.
Co-Culture Experiments
The entire lung was placed in ice-cold PBS in a 100-mm cell culture plate and the tissue mechanically macerated to create a suspension. The cells were pelleted, washed twice with DMEM (ATCC), resuspended in a final volume of 2 ml, and counted. A quantity of 5 × 105 lung cells from mice treated with bleomycin or untreated were placed in a 3-μm pore size membrane (Millipore, Billerica, MA) and inserted into a well of a 6-well plate (Millipore) containing 50% confluent BMDMSC obtained from GFP-expressing mice.
Cytokine Expression in the Lung
Cytokine expression in the lungs obtained 14 d after bleomycin was determined using RT-PCR. Total RNA was extracted using a Qiagen kit according to the manufacturer's recommendations (Qiagen, Valencia, CA). cDNA was generated from 5 μg of total RNA using oligo dT primers and superscript II reverse transcriptase (Invitrogen), and RT-PCR was performed using primers specific for the cytokines of interest. Quantification of the products was measured by the amount cDNA amplified and using amplification reactions of a 200-bp fragment from β-actin cDNA as control. The amount was normalized using β-actin as a standard. The primers used were: interleukin (IL)-1β, 5′-GCAACTGTTCCTGAACTCA and 5′-CTCGGAGCCTGTAGTGCAG; interferon (IFN)-γ, 5′-AACGCTACACACTGCATCT and 5′-GAGCTCATTGAATGCTTGG; IL-2, 5′-AACAGCGCACCCACTTCAA and 5′-TTGAGATGATGCTTTGACA; IL-4: 5′-TAGTTGTCATCCTGCTCTT and 5′-CTACGAGTAATCCATTTGC; β-actin, 5′-ATGGATGACGATATCGCT and 5′-ATGAGGTAGTCTGTCAGGT.
Statistical Methods
For comparisons between groups, paired or unpaired t test and repeated measures ANOVA (three way ANOVA) tests were used (P values < 0.05 were considered significant). We used GraphPad Prism and GraphPad InStat (GraphPad Software, Inc., San Diego, CA) to calculate the statistics.
RESULTS
Animal Survival and Lung Histology
The dose of bleomycin that we used was not lethal in nonmyelosupressed animals. Although different percentages of mortality are reported in the literature, in our hand this dose of bleomycin is not lethal in the strain of mice used (9, 11). Figure 1a shows survival at 14 d after bleomycin in each of our experimental groups. All animals receiving bleomycin alone survived, but only one third of the myelosuppressed animals receiving bleomycin survived to Day 14. Administration of BMDMSC after bleomycin resulted in 100% survival of the busulfan-treated animals as well as the animals without bone marrow suppression.
Figure 1.
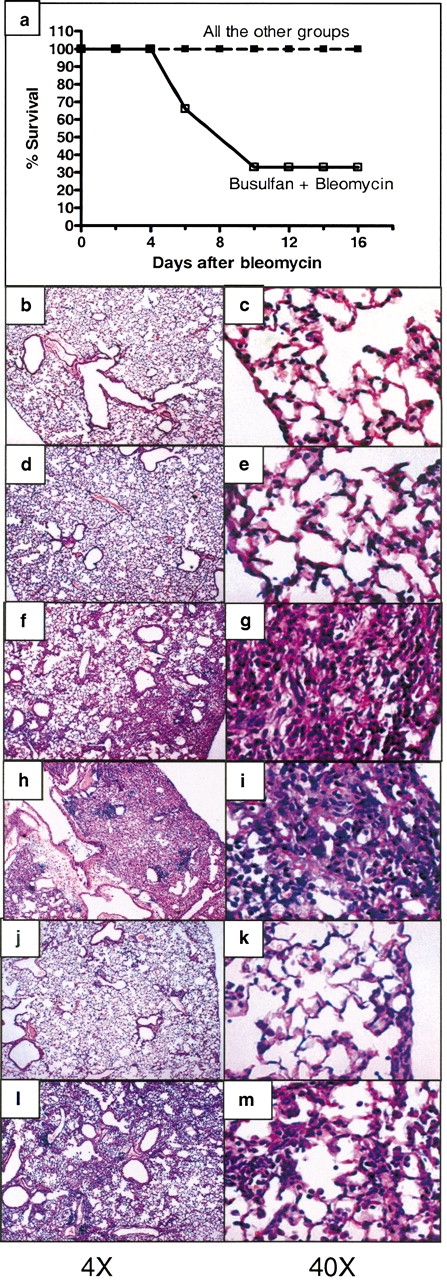
(a) Infusion of BMDMSC prevented mortality in mice myelosuppressed with busulfan before bleomycin. Sixty-six percent of myelosuppressed mice (n = 6) given bleomycin died without BMDMSC transfer. There was no mortality in any of the other experimental groups (n = 5 animals per group). Histologic sections of lungs obtained from: (b, c) normal C57BL/6 mice; (d, e) 14 d after 4 U/kg busulfan (lungs appear normal); (f, g) 14 d after bleomycin (increased cellularity and fibrosis typical of bleomycin injury); (h, i) 14 d after bleomycin following busulfan myelosuppression (apparently more extensive increased cellularity and fibrosis compared with f and g); (j, k) 14 d after bleomycin followed by BMDMSC transfer (minimal alterations in lung architecture compared with f and g); (l, m) 14 d after bleomycin followed by BMDMSC transfer in myelosuppressed mice showing apparent protection (compare with h and i), but more abnormalities than present in the BMDMSC transplanted mice without myelosuppression (compare with j and k).
Representative histologic sections from lungs of animals from each experimental group are shown in Figures 1b–1m. Lungs from animals receiving busulfan but no bleomycin appeared histologically normal. Lungs from animals given bleomycin and no other intervention demonstrated the changes typical of this model 14 d after administering the drug (12). There is marked alteration in lung architecture, with increased cellularity and fibrosis. In animals treated with busulfan before administration of bleomycin, we were able to examine only the lungs of the animals that survived for 14 d (two thirds of the animals died before that time), so that the lung histology in this group is likely less severe than in the group as a whole. In these surviving animals, however, the qualitative effect of bleomycin was similar to that in animals with intact bone marrow, but the severity of the persistent injury appeared to be greater subjectively. Infusion of BMDMSC after bleomycin administration resulted in less persistent injury and fibrosis whether or not the animals were myelosupressed. However, in bone marrow–suppressed animals, there was still evidence of a substantial bleomycin effect, whereas in the bone marrow–sufficient animals receiving BMDMSC transplant, there was minimal evidence of lung injury 14 d after bleomycin. Morphometric analysis of the histologic sections, demonstrated a significant decrease in the bleomycin-induced lung injury in animals receiving a BMDMSC infusion (Figure 2). On average, this measure of injury was worse in the myelosuppressed animals than in nonmyelosuppressed animals given bleomycin, but, again, these are only the animals that survived for 14 d and likely had less injury than the animals that died earlier. When myelosuppressed animals given bleomycin also received BMDMSC, they all survived to 14 d and the quantitative measure of injury was less on average even than in the animals that survived without BMDMSC.
Figure 2.
Infusion of BMDMSC reduces lung damage induce by bleomycin. Morphometric analysis of histologic sections of total left lung was done to determine the percentage of the lung that was affected. Pictures (magnification: ×2) were analyzed using AxioVision 4.2 software (Carl Zeiss, Thornwood, NY). The graphic represents the average from 5–9 histologic lung sections. Bleomycin caused substantial injury and BMDMSC infusion reduced bleomycin-induced injury (*P < 0.05, bleomycin-BMDMSC versus bleomycin, versus busulfan-bleomycin, and versus busulfan-bleomycin-BMDMSC). In myelosuppressed animals, this measure of injury was on average worse than in nonmyelosuppressed animals and on average less severe when animals received BMDMSC. Since only the lungs of the one-third of the myelosuppressed animals that received BMDMSC survived to 14 d were analyzed, the histologic measurements likely underestimate the degree of injury in the entire group.
Donor-Derived Cells in the Lungs after BMDMSC Transplant
To demonstrate localization of the infused BMDMSC to the lung, we identified donor cells by staining the sections with an anti-GFP–specific antibody (Figure 3). Lungs from control animals that did not receive BMDMSC infusions showed minimal background staining. Lungs from animals that received busulfan and BMDMSC transfer without receiving bleomycin also showed minimal deposition of donor cells. Donor-derived cells were detected in the lungs of animals 14 d after bleomycin, and in animals that received busulfan, large numbers of donor cells were present in the lungs at that time. If the therapeutic effect of stem cell transfer were solely due to the delivery of cells that reconstitute lung architecture, then it would not be expected that the animals with less protection (busulfan treated) would have larger numbers of donor-derived cells in the injured tissue. These findings could imply that both endogenous stem cells and exogenously administered stem cells contributed to lung repair when bone marrow reserve was intact so that myelosuppressed animals had more persistent injury and less “therapeutic” effect of BMDMSC transfer.
Figure 3.

Bleomycin injury induces localization of BMDMSC to injured lung. Histologic analysis by indirect immunofluorescence assay (IIFA) with anti-GFP antibodies (green), of the lungs from animals: (a) 14 d after bleomycin without BMDMSC infusion; (b) normal mice infused with BMDMSC (no GFP-positive cells); (c) 14 d after bleomycin followed by BMDMSC (a modest number of green fluorescing cells are present); (d) 14 d after bleomycin followed by BMDMSC in a busulfan myelosuppressed animal (numerous green cells are present); (e) Busulfan treated mice infused with BMDMSC (no localization of donor cells to the lungs); (f) Morphometric analysis of the intensity of GFP staining from the different samples. All microphotographs were taking at ×40 magnification.
We examined tissue sections by immunofluorescent staining for cell type–specific markers so that co-localization of green fluorescence (indicating GFP-positive donor cells) with cell-specific markers would indicate the phenotype of the donor cells. Figure 4 shows a series of fluorescent photomicrographs illustrating fibroblast (vimentin-positive [13], Figures 4a–4d), type I alveolar epithelial (aquaporin [14] antibody–positive, Figures 4e–4f), type II alveolar epithelial (pro-SPC [15] antibody–positive, Figures 4g–4h), or myofibroblast (SMA-1 [13]-positive, Figures 4i–4j) phenotypes. These data suggest that BMDMSC can assume phenotypic characteristics of the major cell types that compose lung parenchyma, including fibroblasts. Before the cells were infused, immunostaining for the cell specific markers was negative (data not shown). Quantitative estimates of donor cell localization in the lungs and the phenotypes of these cells are shown in Figure 4k for animals receiving bleomycin after myelosuppression. In this circumstance, ∼ 29% of the lung cells were donor-derived. Less than 5% of cells were donor-derived in bleomycin-treated animals with intact bone marrow. No donor cells were detected in animals that did not receive bleomycin whether or not they were myelosuppressed. The donor-derived cells before the infusion were negative for all of the lung cell markers that we studied. After transplant, cells were fairly evenly distributed among all cell types for which we tested. It is possible that the donor cell phenotype in these studies included stem cells that fused with resident cells, as has been suggested in other studies (16), rather than differentiation (17). We cannot rule out that possibility, but if that was the case it did not prevent the ability of exogenous BMDMSC to ameliorate the toxic effects of bleomycin.
Figure 4.

Donor BMDMSC localizing to injured lung assume lung cell phenotypes. Sections were analyzed in double-stained IIFA with anti-GFP (green) and antibodies to specific cell type markers (red); co-localization in each case appears yellow (arrows point to double positive cells). (a–d) Anti-vimentin (fibroblast). (a) Normal control; (b) 14 d after bleomycin; (c) 14 d after busulfan followed by BMDMSC (no lung injury); (d) 14 d after bleomycin followed by BMDMSC in a busulfan myelosuppressed animal. (e, f) Anti-aquaporin (type I alveolar epithelium). (e) Fourteen days after busulfan followed by BMDMSC (no lung injury); (f) 14 d after bleomycin followed by BMDMSC in busulfan myelosuppressed animal. (g, h) Anti-pro–surfactant protein C (type II alveolar epithelium). (g) Fourteen days after busulfan followed by BMDMSC (no lung injury); (h) 14 d after bleomycin followed by BMDMSC in busulfan myelosuppressed animal. (i, j) Anti–smooth muscle actin (SMA-1, myofibroblasts). (i) Fourteen days after busulfan followed by BMDMSC (no lung injury); (j) 14 d after bleomycin followed by BMDMSC in busulfan myelosuppressed animal. (k) Percentage of GFP-positive cells that express lung cell phenotype markers in myelosupressed mice treated with bleomycin and infused with BMDMSC. All microphotographs were taken at ×40 magnification.
Circulating G-CSF and GM-CSF
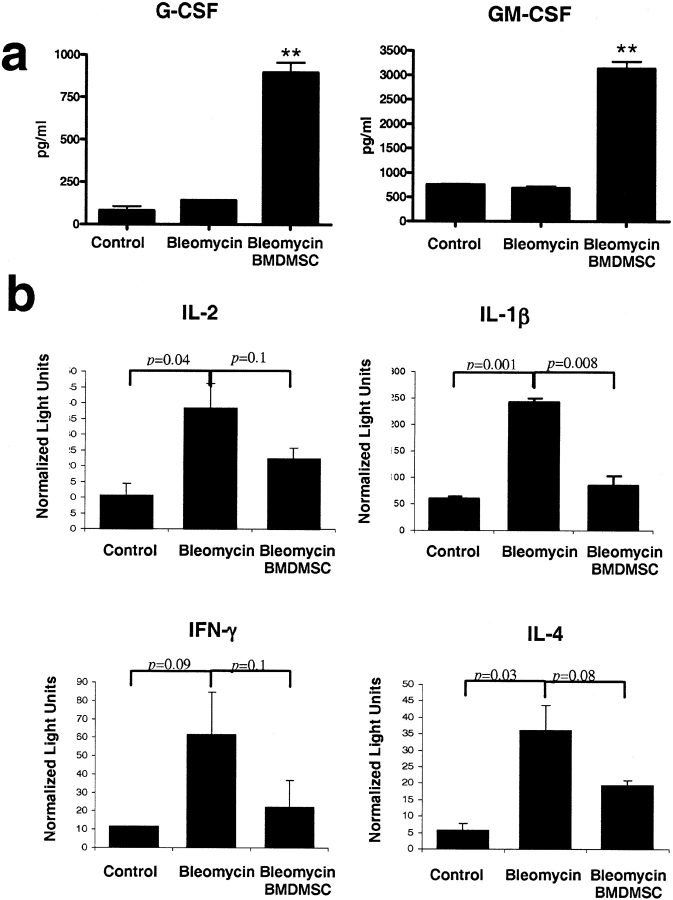
The effects of stem cell transplantation on production of several humoral factors may help to explain the apparent therapeutic effect. We determined the circulating concentrations of G-CSF and GM-CSF at 14 d after bleomycin administration. As shown in Figure 5A, when bleomycin was given, the circulating levels of GM-CSF and G-CSF, both of which can mobilize stem cells from bone marrow pools (18), were not increased 14 d after bleomycin. When animals received stem cells, there was a marked and significant increase in circulating levels of G-CSF and GM-CSF at the same time point. Thus, part of the effect of stem cell transplantation on the bleomycin response may be due to mobilization of endogenous stem cells. This would also be consistent with the greater protection conferred by stem cell transplant in the bone marrow sufficient animals.
Figure 5.
BMDMSC infusion attenuates bleomycin-induced increases in lung expression of immune-related cytokines and increases circulating concentrations of G-CSF and GM-CSF. (a) Serum concentrations of G-CSF and GM-CSF in control animals (n = 4) and 14 d after bleomycin with (n = 4) or without (n = 5) BMDMSC transfer. BMDMSC transfer resulted in significantly elevated serum concentrations of both factors. The differences were significant (P < 0.05) after one-way ANOVA and repeated-measures ANOVA between animals treated with BMDMSC and the other two groups (**). (b) Expression of cytokines in lung tissue determined by real-time PCR in control mice, (n = 3) and mice given bleomycin with (n = 3) or without (n = 3) BMDMSC transfer 14 d after bleomycin. Bleomycin treatment caused prolonged increased expression of these immune-related cytokines and transfer BMDMSC tended to suppress this response.
Expression of Immune Related Cytokines in the Lungs
To determine effects of BMDMSC transfer on the local inflammatory milieu in the lungs, we determined expression of several immune system related cytokines in lung tissue by real-time PCR (Figure 5B). Lung expression of IFN-γ, IL-2, IL-1β, and IL-4 was increased at 14 d after bleomycin and stem cell transplant resulted in return of expression of these cytokines toward normal. This suppression of the prolonged inflammatory response in the lungs (19) may provide an environment more favorable to normal repair.
In Vitro Effects of Cells from Injured Lungs on Mesenchymal Stem Cell Behavior
To determine whether humoral factors that affect stem cell behavior originate in the injured lung, we measured the effects of cell suspensions prepared from lung harvested 14 d after bleomycin administration on the proliferation and migration of GFP-expressing BMDMSC in co-culture experiments. Figure 6A shows fluorescent photomicrographs of the upper surface of the filter separating the lung and stem cells and of the lower chamber that contained only GFP-expressing stem cells after 5 d in culture. When the upper chamber contained cells from injured lung, there was a marked increase in the number of GFP-expressing stem cells in the lower chamber compared with experiments in which cells from uninjured lung were in the upper chamber. When the upper chamber contained cells from uninjured lung, there was no evidence of migration of the GFP-positive stem cells from the lower chamber toward the lung cells. However, when cells from bleomycin-injured lungs were in the upper chamber, numerous GFP-positive stem cells migrated to the upper chamber. The migrating cells also appeared different morphologically, with many large cells and cell clusters. Figure 6B summarizes quantitative measurements of fluorescence in the lower chamber and in the filter as an estimate of the location of the GFP-expressing BMDMSC.
Figure 6.
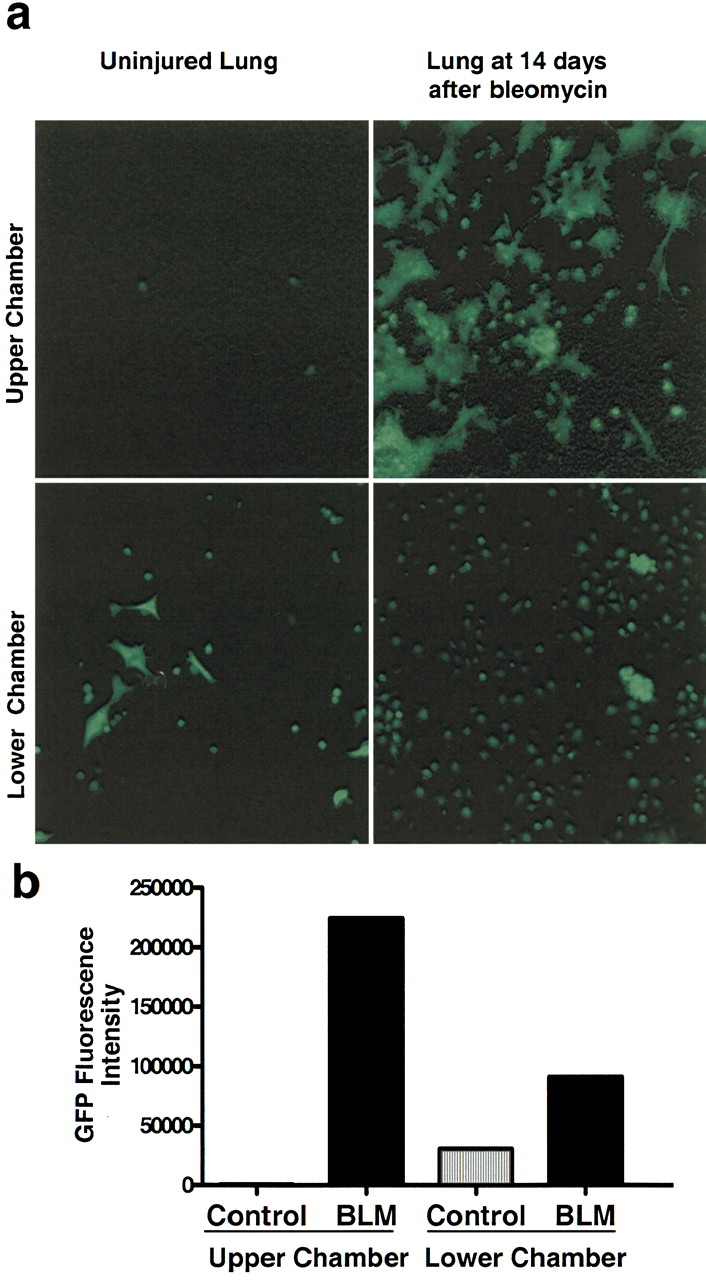
Cells from injured lungs produce humoral factor(s) that cause BMDMSC proliferation and chemotaxis in vitro. BMDMSC obtained from GFP-positive mice were grown for 7 d, and equally distributed in the lower well of a 6-well Transwell plate. Cell preparations from lungs of normal mice or lungs from mice 14 d after bleomycin were placed in the upper well on a 1.5-inch filter with 3-μm pores. The co-cultures were maintained for 5 d and then analyzed for GFP-positive cells. (a) Photomicrographs of the upper and lower chambers of a Transwell in which the same number of BMDMSC were placed in the lower well and the same number of cells from either normal or bleomycin-injured lung was placed in the upper well. In the presence of injured lung, there were many more GFP-positive cells in the lower chamber. With normal lung, there was no evidence of migration of the GFP-positive cells to the upper chamber, but in the presence of injured lung there was marked migration and the cells appeared in clumps. (b) Quantitative assessment of GFP-positive cells in both upper and lower chambers by fluorescence intensity.
DISCUSSION
One theory of tissue repair holds that organ injury is “sensed” by distant stem cells that migrate to the site of damage and differentiate into organ-specific cells, promoting structural and functional repair (1, 20). Several recent studies derive from that general concept. For example, stem cells obtained from bone marrow are reported to be capable of homing selectively to infarcted myocardium and differentiating into cardiac muscle, endothelial cells, and vascular smooth muscle cells (21), decreasing infarct size and improving cardiac function. Mobilization of endogenous progenitor cells by administration of G-CSF and GM-CSF in mice is reported to cause tissue regeneration in an area of experimental myocardial infarct (21) with new myocytes and arterioles and capillaries connected to the circulation of the unaffected ventricle.
Bleomycin is one of the most extensively studied and reproducible models for lung fibrosis in mice. When bleomycin is given into the airway, it produces lung epithelial injury, followed by an inflammatory response over several days that is followed by lung fibrosis that eventually resolves (8). Ortiz and colleagues delivered purified BMDMSC from bleomycin-resistant mice to susceptible mice after bleomycin administration (5). They found that the donor cells homed to the injured lung and adopted epithelial phenotypes, including that of type II alveolar epithelial cells. Because the numbers of donor-derived cells engrafting the lung did not appear sufficient to account for the therapeutic response, they suggest that donor stem cells may have other local effects. The quantitative importance of this process is not known, and the source of signals that are responsible for mobilization and homing of endogenous stem cells remains to be defined. Effects of stem cell transplant other than provision of pluripotent cells to the area of injury that may contribute to the apparent therapeutic effect also remain to be determined.
In our studies, mice given bleomycin survived and showed the histologic findings of increased cellularity and fibrosis in the lungs that are typical of this model (8). To suppress bone marrow reserve, we administered busulfan in a single dose. Reports in the literature indicate that 24 h after a single administration of the dose of busulfan that we used, bone marrow responses are suppressed (22, 23). Busulfan can injure the lungs directly in some circumstances, but the single dose we used is reported to suppress bone marrow without toxicity (24) and we saw no histologic evidence of lung injury after busulfan alone. In addition, when BMDMSC were given after busulfan alone they did not localize to the lungs, suggesting that the lungs were not injured. It remains possible, however that subclinical injury of the lung by busulfan contributed to the greater injury produced by bleomycin in those animals When bleomycin was administered 24 h after busulfan, the injury was exaggerated to the degree that two-thirds of the animals did not survive for 14 d, and the histologic appearance of the lungs of animals that did survive to that time tended to be worse than the animals that were not myelosuppressed. We interpret these data to indicate that an intact bone marrow serves to limit the severity of the response to bleomycin in the lungs.
We found that animals receiving mesenchymal stem cell transplantation after bleomycin had substantial numbers of donor-derived cells in their lungs at 14 d after bleomycin. The number of donor-derived cells was much greater in the lungs of animals that had bone marrow suppression with busulfan before bleomycin. If the effect of stem cell transplant were solely due to the delivery of progenitor cells that restore lung architecture, then it would not be expected that the animals with less protection (busulfan treated) would have larger numbers of donor-derived cells in the injured tissue. We interpret these findings to mean that both endogenous stem cells and exogenously administered stem cells contributed to lung repair when bone marrow reserve was intact so that the magnitude of the rescue from bleomycin injury was greater in the animals not treated with busulfan. Donor-derived cells in the lungs showed phenotypic characteristics of several lung cell types, including type I and type II pneumocytes, fibroblasts, and endothelial cells, documenting the plasticity of mesenchymal stem cells in this setting (25). It is possible that the donor cell phenotype in these studies could have included stem cells that fused with resident cells, as has been suggested in other studies (16), rather than differentiation, but if that was the case it did not prevent the ability of exogenous BMDMSC to ameliorate the toxic effects of bleomycin.
The effects of stem cell transplantation on production of several humoral factors may help to explain the apparent therapeutic effect. When bleomycin was given, the circulating levels of GM-CSF and G-CSF, both of which can mobilize stem cells from bone marrow pools (26), were similar to control values after 14 d. When animals received stem cells, circulating levels of G-CSF and GM-CSF were higher at the same time point. Thus, part of the effect of stem cell transplantation on the bleomycin response may be due to humorally mediated mobilization of endogenous stem cells. This would also be consistent with the greater protection conferred by stem cell transplant in bone marrow–sufficient animals.
Using RT-PCR, we determined expression of cytokines in the lungs at 14 d after bleomycin. The pattern of effects on expression of IFN-γ, IL-2, IL-1β, and IL-4 was similar in all the groups. Lung expression of these cytokines was increased at 14 d after bleomycin. Stem cell transplant after bleomycin resulted in return of expression of these cytokines toward normal. This suppression of the prolonged local inflammatory response may provide an environment more favorable to the normal repair process.
To determine whether humoral factors that affect stem cell behavior originate in the injured lung, we studied interactions of cell suspensions from lung tissue with BMDMSC in co-cultures in vitro. We found that when stem cells were co-cultured with cells from lungs obtained 14 d after bleomycin, there was a marked proliferation of the stem cells and the cells migrated toward the lung cell suspensions. Neither of these effects was observed with cell suspensions from the lungs of animals that had not received bleomycin. This generation of humoral mediators by injured lung that stimulate stem cell proliferation could cause local proliferation of stem cells mobilized from the bone marrow (or delivered as a stem cell transplant), or could be a signaling mechanism to the bone marrow to expand the pool of progenitor cells in response to tissue injury. The production of substances chemotactic for stem cells by injured lung may help to explain the selective homing of these progenitor cells to areas of tissue injury. Further studies are necessary to determine the specific cell source of these mediators, the nature of the soluble mediators, and effects of interactions of injured lung and stem cells on their differentiation.
We conclude that recovery from bleomycin-induced lung injury requires an intact bone marrow pool of progenitor cells. Delivery of mesenchymal stem cells greatly limits the degree of prolonged injury and fibrosis caused by bleomycin, but persistence of some injury in bone marrow–suppressed animals suggests that both endogenous and exogenous stem cells contribute to the repair process. Transplanted mesenchymal stem cells localize to the injured lung and acquire lung parenchymal cell characteristics, but the numbers of donor-derived cells may not be sufficient to account for the therapeutic effect. Both increased mobilization of endogenous progenitor cells and alterations in the local cytokine milieu in favor of repair may contribute to the protective effects of stem cell transplantation. Homing of stem cells to injured lung is at least partly a result of production of humoral mediators by injured but not by normal lung that are chemotactic for stem cells. Injured lung also produces soluble factors that stimulate stem cell proliferation, which could serve either as a message for expansion of endogenous stem cell pools or as a local stimulus for expansion of populations of stem cells in the lungs.
Acknowledgments
The authors thank Susanne Roser and John LaVoy for their technical assistance.
This research was supported by grant number 5 P01 HL0669496-02 from the National Heart Lung and Blood Institute and the McKelvey Center for Lung Transplantation at Emory University.
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Abkowitz JL, Robinson AE, Kale S, Long MW, Chen J. Mobilization of hematopoietic stem cells during homeostasis and after cytokine exposure. Blood 2003;102:1249–1253. [DOI] [PubMed] [Google Scholar]
- 2.Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood 2003;102:3483–3493. [DOI] [PubMed] [Google Scholar]
- 3.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997;276:71–74. [DOI] [PubMed] [Google Scholar]
- 4.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 2002;105:93–98. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fine A. Marrow cells as progenitors of lung tissue. Blood Cells Mol Dis 2004;32:95–96. [DOI] [PubMed] [Google Scholar]
- 7.Kotton DN, Summer R, Fine A. Lung stem cells: new paradigms. Exp Hematol 2004;32:340–343. [DOI] [PubMed] [Google Scholar]
- 8.Bowden DH. Unraveling pulmonary fibrosis: the bleomycin model. Lab Invest 1984;50:487–488. [PubMed] [Google Scholar]
- 9.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol 1998;275:L365–L371. [DOI] [PubMed] [Google Scholar]
- 10.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. “Green mice” as a source of ubiquitous green cells. FEBS Lett 1997;407:313–319. [DOI] [PubMed] [Google Scholar]
- 11.Piguet PF, Collart MA, Grau GE, Kapanci Y, Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med 1989;170:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olman MA, Simmons WL, Pollman DJ, Loftis AY, Bini A, Miller EJ, Fuller GM, Rivera KE. Polymerization of fibrinogen in murine bleomycin-induced lung injury. Am J Physiol 1996;271:L519–L526. [DOI] [PubMed] [Google Scholar]
- 13.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol 2003;29:213–224. [DOI] [PubMed] [Google Scholar]
- 14.Kotton DN, Fine A. Derivation of lung epithelium from bone marrow cells. Cytotherapy 2003;5:169–173. [DOI] [PubMed] [Google Scholar]
- 15.Bishop AE. Pulmonary epithelial stem cells. Cell Prolif 2004;37:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell 2004;116:639–648. [DOI] [PubMed] [Google Scholar]
- 17.Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, Peister A, Wang MY, Prockop DJ. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA 2003;100:2397–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho HJ, Kim HS, Lee MM, Kim DH, Yang HJ, Hur J, Hwang KK, Oh S, Choi YJ, Chae IH, et al. Mobilized endothelial progenitor cells by granulocyte-macrophage colony-stimulating factor accelerate reendothelialization and reduce vascular inflammation after intravascular radiation. Circulation 2003;108:2918–2925. [DOI] [PubMed] [Google Scholar]
- 19.Zhu J, Kaplan AM, Goud SN. Immunologic alterations in bleomycin-treated mice: role of pulmonary fibrosis in the modulation of immune responses. Am J Respir Crit Care Med 1996;153:1924–1930. [DOI] [PubMed] [Google Scholar]
- 20.Hille A, Rave-Frank M, Pradier O, Damm C, Dorr W, Jackel MC, Christiansen H, Hess CF, Schmidberger H. Effect of keratinocyte growth factor on the proliferation, clonogenic capacity and colony size of human epithelial tumour cells in vitro. Int J Radiat Biol 2003;79:119–128. [PubMed] [Google Scholar]
- 21.Orlic D, Kajstura J, Chimenti S, Bodine DM, Leri A, Anversa P. Bone marrow stem cells regenerate infarcted myocardium. Pediatr Transplant 2003;7:86–88. [DOI] [PubMed] [Google Scholar]
- 22.Kean LS, Manci EA, Perry J, Balkan C, Coley S, Holtzclaw D, Adams AB, Larsen CP, Hsu LL, Archer DR. Chimerism and cure: hematologic and pathologic correction of murine sickle cell disease. Blood 2003;102:4582–4593. [DOI] [PubMed] [Google Scholar]
- 23.Guo Z, Wang J, Dong Y, Adams AB, Shirasugi N, Kim O, Hart J, Newton-West M, Pearson TC, Larsen CP, et al. Long-term survival of intestinal allografts induced by costimulation blockade, busulfan and donor bone marrow infusion. Am J Transplant 2003;3:1091–1098. [DOI] [PubMed] [Google Scholar]
- 24.Gibson FM, Andrews CM, Diamanti P, Rizzo S, Macharia G, Gordon-Smith EC, Williams T, Turton J. A new model of busulphan-induced chronic bone marrow aplasia in the female BALB/c mouse. Int J Exp Pathol 2003;84:31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 26.Hirohata S, Yanagida T, Tomita T, Yoshikawa H, Ochi T. Bone marrow CD34+ progenitor cells stimulated with stem cell factor and GM-CSF have the capacity to activate IgD- B cells through direct cellular interaction. J Leukoc Biol 2002;71:987–995. [PubMed] [Google Scholar]