Abstract
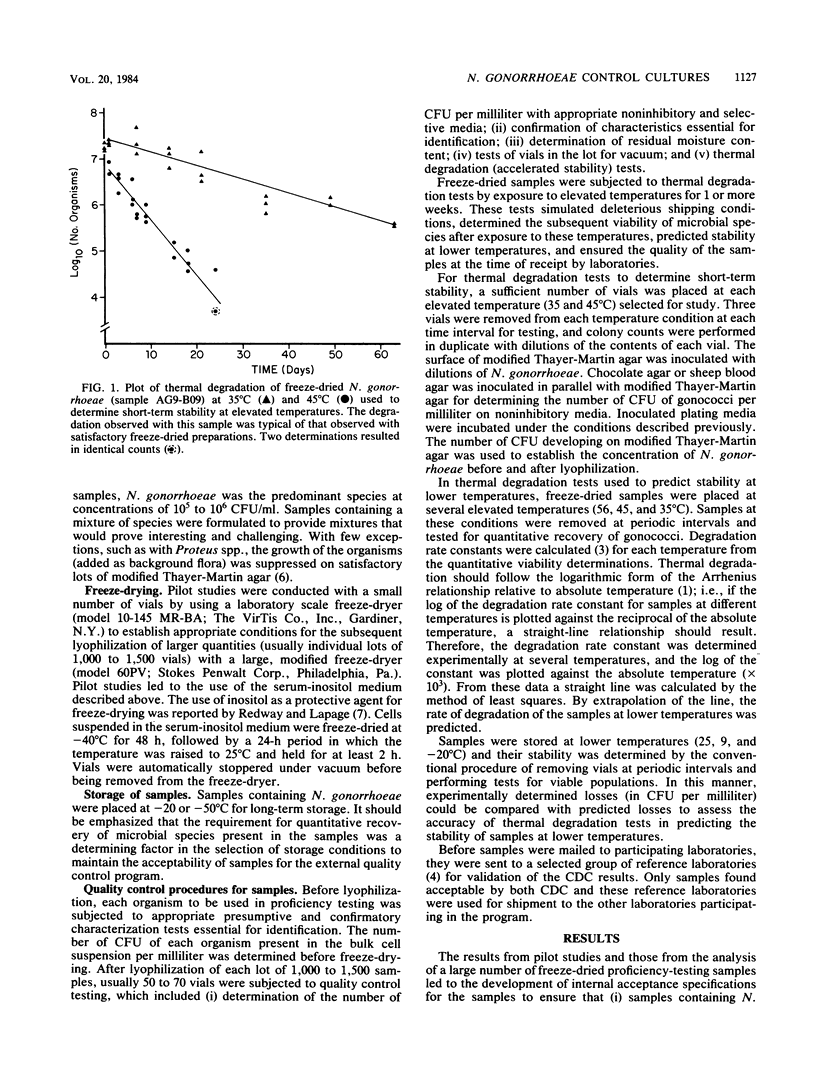
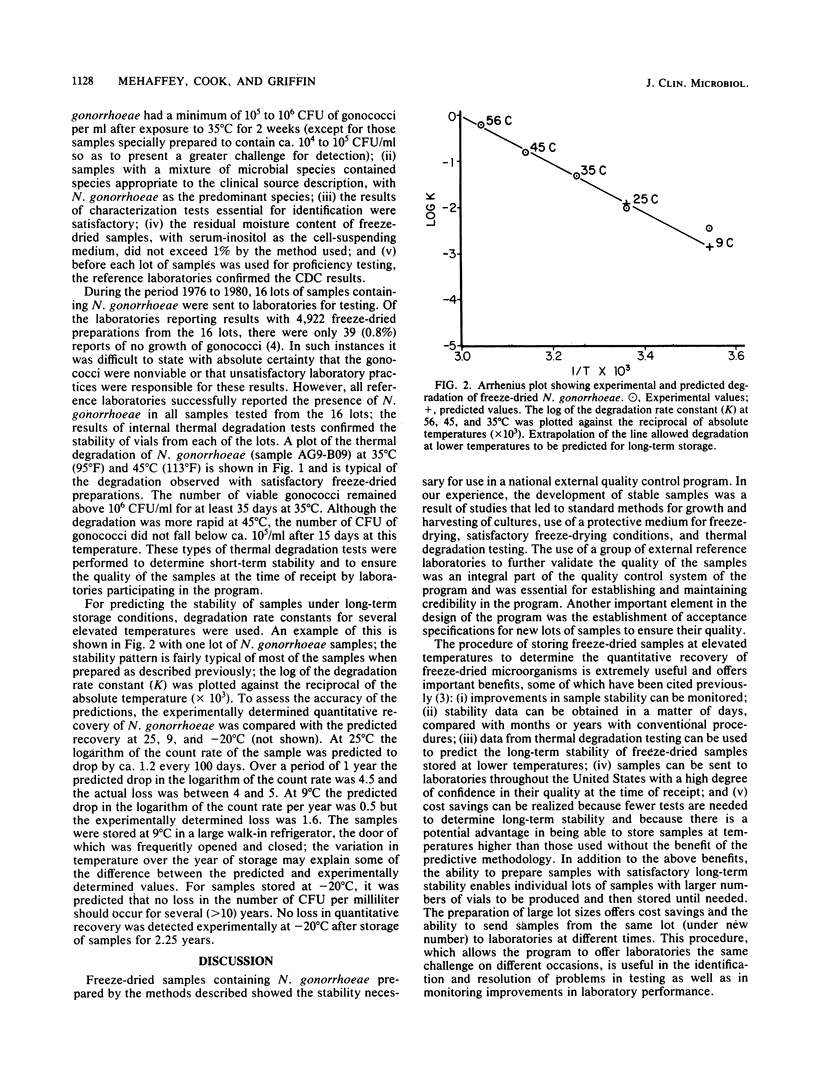
In 1976, the Center for Disease Control initiated an external quality control program for the isolation and identification of Neisseria gonorrhoeae. This program required microbial samples of sufficient stability for shipment to laboratories throughout the United States. The Centers for Disease Control undertook studies to determine the most appropriate media for the propagation of strains for freeze-drying, the cell-suspending media that would afford protection during and after freeze-drying, the most favorable growth conditions, the proper times and methods for harvesting cells, the appropriate lyophilization conditions, the critical residual moisture content, and the stability of samples. These studies resulted in the development of methods for preparing and testing freeze-dried samples suitable for shipment.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Damjanović V., Radulović D. Predicting the stability of freeze-dried Lacto-bacillus bifidus by the accelerated storage test. Cryobiology. 1968 Sep-Oct;5(2):101–104. doi: 10.1016/s0011-2240(68)80150-6. [DOI] [PubMed] [Google Scholar]
- GREIFF D., RIGHTSEL W. A. AN ACCELERATED STORAGE TEST FOR PREDICTING THE STABILITY OF SUSPENSIONS OF MEASLES VIRUS DRIED BY SUBLIMATION IN VACUO. J Immunol. 1965 Mar;94:395–400. [PubMed] [Google Scholar]
- Griffin C. W., 3rd, Mehaffey M. A., Cook E. C. Five years of experience with a national external quality control program for the culture and identification of Neisseria gonorrhoeae. J Clin Microbiol. 1983 Nov;18(5):1150–1159. doi: 10.1128/jcm.18.5.1150-1159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin C. W., Cook E. C., Mehaffey M. A. Predicting the stability of freeze-dried Fusobacterium mortiferum proficiency testing samples by accelerated storage tests. Cryobiology. 1981 Aug;18(4):420–425. doi: 10.1016/0011-2240(81)90117-6. [DOI] [PubMed] [Google Scholar]
- Jacobs N. F., Jr, Kraus S. J. Comparison of hemoglobin-free culture media and thayer-martin medium for the primary isolation of Neisseria gonorrhoeae. J Clin Microbiol. 1975 May;1(5):401–404. doi: 10.1128/jcm.1.5.401-404.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. E., Armstrong J. H., Smith P. B. New system for cultivation of Neisseria gonorrhoeae. Appl Microbiol. 1974 Apr;27(4):802–805. doi: 10.1128/am.27.4.802-805.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redway K. F., Lapage S. P. Effect of carbohydrates and related compounds on the long-term preservation of freeze-dried bacteria. Cryobiology. 1974 Feb;11(1):73–79. doi: 10.1016/0011-2240(74)90040-6. [DOI] [PubMed] [Google Scholar]


