Abstract
Pulmonary lymphangioleiomyomatosis (LAM) and renal angiomyolipoma (AML) are proliferative lesions that occur in sporadic patients, and at much higher frequency in patients with tuberous sclerosis (TSC). The TSC1 and TSC2 genes play a critical role in their pathogenesis. Here we report a marked decrease in interferon (IFN)-γ expression in both sporadic and TSC-associated AML and LAM. A marked increase in Stat1 expression and phosphorylation at Ser 727, and in phospho-Tyr705-Stat3 levels, was also seen in both AML and LAM tissues. Our results demonstrate that the IFN-γ-Jak-Stat pathway is perturbed in TSC-related and sporadic LAM and AML, and suggest that IFN-γ has potential therapeutic benefit for treatment of those lesions.
Keywords: angiomyolipoma, interferon-γ, lymphangioleiomyomatosis, Stats, tuberous sclerosis
Pulmonary lymphangioleiomyomatosis (LAM) is a non-neoplastic disorder that affects women during their reproductive years or while receiving estrogen replacement therapy (1). The clinical symptoms include recurrent spontaneous pneumothorax, chylothorax, hemoptysis, and progressive respiratory failure. Histologically, LAM is characterized by smooth muscle cell (SMC) proliferation within the lung parenchyma and formation of cysts that are diffusely distributed throughout the lung (1). Up to 63% of women with LAM also have renal angiomyolipoma (AML) (2, 3). AML are benign tumors consisting of SMCs, fibrous and adipose tissues, and abnormally formed vascular channels (4). Clinically, most AML are asymptomatic, but they can cause life-threatening hemorrhage and chronic renal failure. AML are more common in women than in men, with a lifetime incidence of 1 in 330 and 1 in 5,000, respectively (5).
Both AML and pulmonary LAM occur either sporadically or in association with tuberous sclerosis (TSC). TSC is characterized by benign tumors in several organs, and is inherited as an autosomal dominant disorder as a result of mutations in either TSC1 or TSC2 (4). Both sporadic and TSC-associated LAM and AML follow the Knudson model of tumor suppressor gene function in that bi-allelic inactivation of either TSC1 or TSC2 is seen (6, 7). In patients with TSC, the frequency of AML and LAM is age-dependent, reaching 80% and 5%, respectively, in adult women (4, 8). However, subclinical evidence of LAM is seen by chest CT scans in ∼ 50% of adult TSC women (9, 10).
Clinical observations on the increased frequency and severity of both AML and LAM in females have suggested that female sex hormones have a critical role in their pathogenesis. Although there are anecdotal reports of responses of LAM to antiestrogen treatments (3), there is no standard treatment for LAM and AML. A major role of the TSC gene products is in the regulation of mTOR, such that loss of either TSC gene results in unregulated mTOR activation, with activation and phosphorylation of S6K and S6, in cultured cell lines, patient TSC lesions, and Tsc mouse model tumors (11–15). This observation has led to initial trials of rapamycin (mTOR inhibitor) treatment in patients with TSC and LAM, and these studies are ongoing.
We have also observed dramatic changes in the interferon (IFN)-γ-Jak1-Stat signaling pathway in TSC cell lines and tumors. IFN-γ expression is reduced, whereas Stat1 expression and phosphorylation at Ser 727, as well as phosphorylation of Stat3 at Tyr705, are all increased in both Tsc1 null and Tsc2 null cells and in mouse model tumors (16). Treatment of Tsc1 or Tsc2 null cells with IFN-γ induced apoptosis at a faster rate than that seen in controls, with reduction in pStat3 Tyr705 levels, and major increases in pStat1 Tyr701, bax, caspase 1, and caspase 9 levels (16). A combination of IFN-γ and rapamycin was synergistic in induction of apoptosis in Tsc1 or Tsc2 null cells, as pStat3 Tyr705 phosphorylation was completely abolished and the other effects of IFN-γ were maintained or enhanced (16).
Therefore, we examined the IFN-γ-Jak-Stat1 pathway in both sporadic and TSC-associated LAM and renal AML.
MATERIALS AND METHODS
Sporadic LAM (three cases) and AML (one case) paraffin-embedded sections were a gift from Dr. Thomas Colby (Mayo Clinic, Scottsdale, AZ), and Department of Pathology (Brigham and Women's Hospital, Boston, MA), respectively. TSC2-associated LAM (two cases) and AML (four cases) and control normal lung and kidney frozen and paraffin-embedded tissues were obtained from the Brain and Tissue Bank (University of Maryland, MD).
Antibodies against the following proteins were obtained from the following sources: Tuberin, IFNγ, IFNγ-Rα, IFNγ-Rβ, Jak1, Jak2, ERK, and actin from Santa Cruz Biotechnology (Santa Cruz, CA); pS6(Ser235/236), Stat1, pStat1 Tyr701, and pStat3 Tyr705 from Cell Signaling Technology (Beverly, MA); pStat1 Ser727 from Upstate Signaling (Lake Placid, NY); and Stat3 from BD Biosciences (Palo Alto, CA).
Immunohistochemistry was performed on 5-μm paraffin-embedded tissue sections, after being deparaffinized in xylene, and rehydrated in an ethanol/water series. Sections were stained by the peroxidase method (rabbit and goat Immuno-Cruz staining kits; Santa Cruz Biotechnology) using primary antibodies against pS6-Ser235/236 and IFN-γ, and counterstained with hematoxylin. Negative control immunohistochemical procedures were conducted on adjacent tissue sections, including replacement of the primary antibody with normal IgG.
Immunoblot analysis was performed on extracts from LAM and AML from patients with TSC, and on control normal lung and kidney tissue samples. Proteins were separated by SDS-PAGE, transferred to nylon membranes, and probed and visualized using enhanced chemiluminescence, as described (16).
RESULTS
We used immunoperoxidase and immunoblotting techniques to investigate IFN-γ expression in sporadic and TSC2-associated LAM and AML. IFN-γ expression was completely absent in both the sporadic and TSC-associated LAM and AML compared with normal control tissues, as assessed by immunohistochemistry (Figures 1A–1C) and immunoblotting (Figure 2, bottom lane). Levels of phospho-S6 were high in both AML and LAM (Figures 1D and 1E), similar to previous observations (12, 13).
Figure 1.
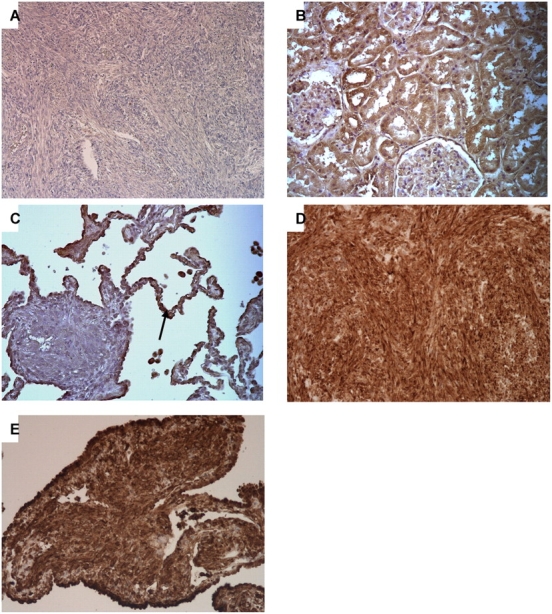
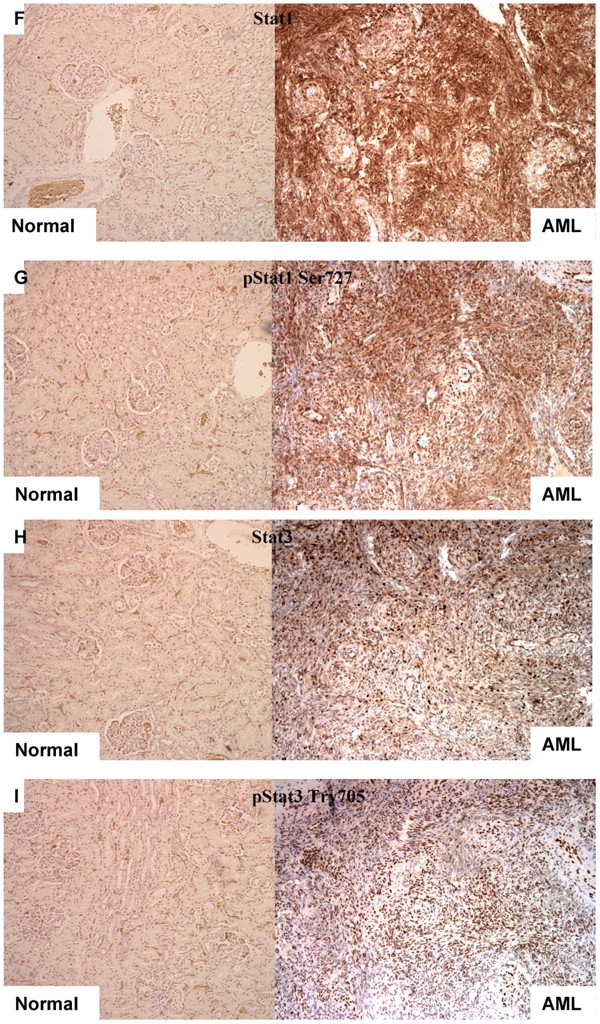
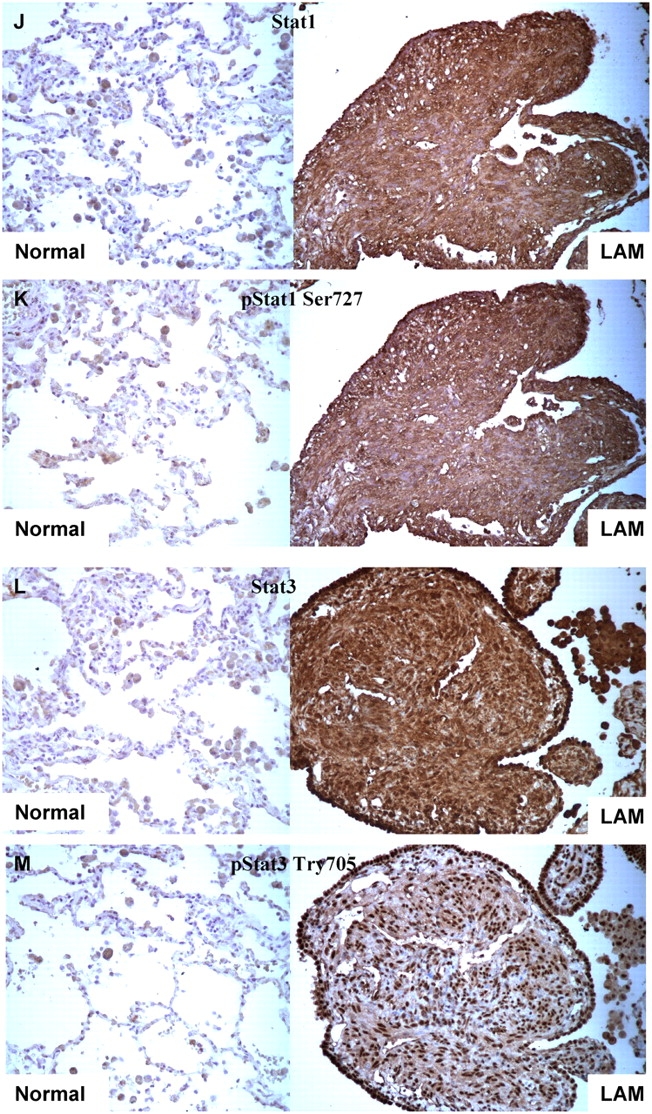
Immunohistochemistry analysis of pS6, IFN-γ, Stat1, and Stat3 in sporadic AML and LAM tissues. (A–C) IFN-γ staining shows no expression in sporadic AML (A) and a LAM nodule in contrast to normal lung (C, arrow) and kidney tissue (B). (D and E) pS6 staining is strong in sporadic AML and LAM, respectively. (F–M) Expression of Stat1 (F, J), pStat1 S727 (G, K), Stat3 (H, L), and pStat3 Y705 (I, M) in sporadic AML and LAM, respectively, compared with normal lung and kidney tissues. Note nuclear localization of pStat3-Y705.
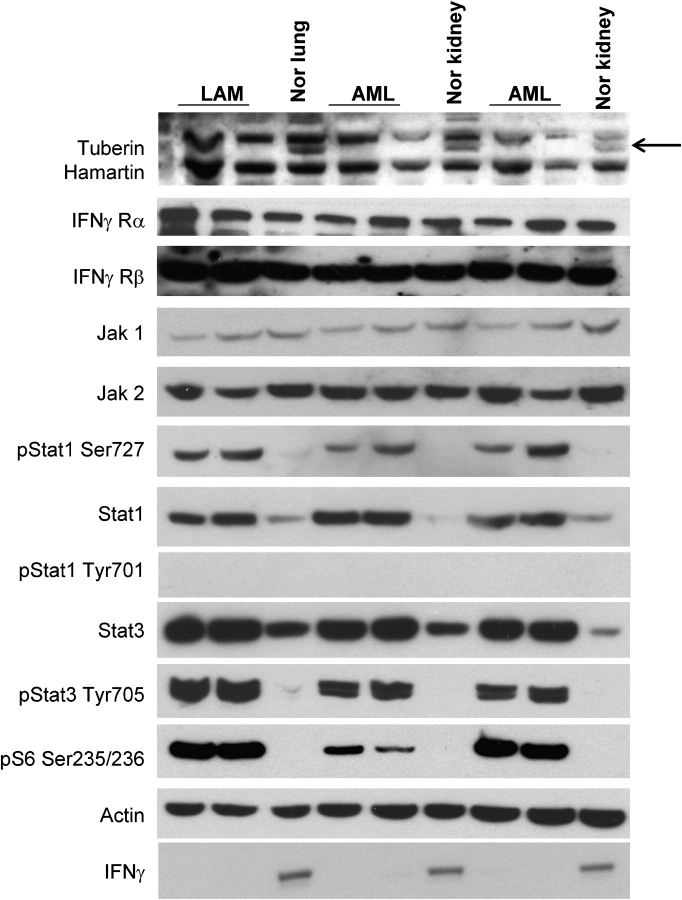
Figure 2.
IFN-γ-Jak-Stat pathway in TSC2-associated LAM and AML. Immunoblot analysis shows the absence of tuberin expression in two LAM cases and four AML cases in contrast to control lung and kidney. Arrow indicates tuberin band. IFN-γ Rα, IFN-γ Rβ, Jak1, and Jak2 levels are similar in LAM, AMLs, and control tissues. Stat1 and Stat3 expression is increased in both AML and LAM samples, whereas pStat1-Ser727 and pStat3-Tyr705 levels are markedly increased in LAM and AMLs. pStat1 Tyr701 is absent in LAM, AML and normal tissues. pS6 expression in LAM and AML tissues indicates the activation of mTOR in these lesions. Actin is a loading control. The expression of IFN-γ expression is absent in both LAM and AML lesions but not in normal kidney and lung (bottom lane).
IFN-γ exerts its effects on cells by interacting with its receptors (IFN-γ Rα, and IFN-γ Rβ), which induce activation of the receptor-associated Janus kinases Jak1 and Jak2, leading to phosphorylation of Stat1 on tyrosine 701 (17). Stat1 is also phosphorylated on serine 727 in response to IFN-γ in many cell types, and this enhances its transcriptional activity (17). To explore Stat activation in AML and LAM, we performed both immunohistochemistry and immunoblotting (Figures 1 and 2).
Immunoblot analysis of TSC2-associated LAM and AML showed that these lesions did not express tuberin (TSC2 gene product), whereas the expression levels of IFN-γRα, IFN-γRβ, Jak1, and Jak2 were similar in AMLs and LAM and control normal kidney and lung (Figure 2). In contrast, both Stat1 and pStat1 Ser727 (but not pStat1 Tyr701) levels were significantly increased in both sporadic (Figures 1F, 1G, 1J, and 1K) and TSC-associated LAM and AML compared with normal kidneys and lungs (Figure 2).
Because Stat3 has effects in many cell types that counterbalance those of Stat1, and we had previously seen Stat3 phosphorylation in Tsc2 null neuroepithelial precursor cells (18) and Tsc null MEFs (16), we also examined Stat3 expression and activation in LAM and AML. We found that Stat3 levels were higher and pStat3-Tyr705 levels were markedly increased in LAM and AML tissues compared with control lung and kidney by both immunohistochemistry and immunoblotting (Figures 1H, 1I, 1L, 1M, and 2).
These results on patient specimens are entirely similar to what we have seen previously in the analysis of murine fibroblasts and neuroepithelial cells lacking Tsc1 or Tsc2, as well as tumors derived from Tsc mouse models (16, 18). In vitro, the elevated pStat1-S727 and pStat3-Tyr705 levels were rapidly reduced by rapamycin treatment, suggesting that mTOR activation is responsible, directly or indirectly (16). Moreover, we observed that IFN-γ alone and combined IFN-γ–rapamycin treatment induced apoptotic cell death in cells lacking Tsc1 or Tsc2, with the two drugs showing enhanced effect (16). We have also seen a 74% reduction in kidney cystadenomas in Tsc2+/− mice treated with CCI-779 (rapamycin analog) for 3 mo and a 61% reduction in these tumors when treated with IFN-γ for 3 mo, compared with untreated controls (19).
Thus, the current observations extend previous observations in Tsc mouse model systems to clinical samples of LAM and AML, both sporadic and TSC-related (16). Therefore, they suggest that IFN-γ may be a beneficial treatment for both LAM and AML. In addition, there may be significant synergy to the combination of rapamycin and IFN-γ. As both of these agents are available in the clinic, translation of a synergistic effect to a clinical treatment paradigm is possible within the foreseeable future. Appropriate clinical trials will be required to assess this possibility.
Acknowledgments
The authors thank John Godleski and Chris Fletcher (Brigham and Women's Hospital, Boston, MA) for assistance with interpretation of immunohistochemistry, and Thomas Colby for provision of LAM paraffin sections.
This work was supported by the LAM Foundation, the Rothberg Fund, and NIH NINDS NS31535.
Conflict of Interest Statement: Neither author has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hancock E, Osborne J. Lymphangioleiomyomatosis: a review of the literature. Respir Med 2002;96:1–6. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein SM, Newell JD Jr, Adamczyk D, Mortenson RL, King TE Jr, Lynch DA. How common are renal angiomyolipomas in patients with pulmonary lymphangiomyomatosis? Am J Respir Crit Care Med 1995;152:2138–2143. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan EJ. Lymphangioleiomyomatosis. Chest 1998;114:1689–1703. [DOI] [PubMed] [Google Scholar]
- 4.Gomez M, Sampson J, Whitemore V. The tuberous sclerosis complex, 3rd ed. New York: Oxford University Press; 1999.
- 5.Hajdu SI, Foote FW Jr. Angiomyolipoma of the kidney: report of 27 cases and review of the literature. J Urol 1969;102:396–401. [DOI] [PubMed] [Google Scholar]
- 6.Cheadle JP, Reeve MP, Sampson JR, Kwiatkowski DJ. Molecular genetic advances in tuberous sclerosis. Hum Genet 2000;107:97–114. [DOI] [PubMed] [Google Scholar]
- 7.Niida Y, Stemmer-Rachamimov AO, Logrip M, Tapon D, Perez R, Kwiatkowski DJ, Sims K, MacCollin M, Louis DN, Ramesh V. Survey of somatic mutations in tuberous sclerosis complex (TSC) hamartomas suggests different genetic mechanisms for pathogenesis of TSC lesions. Am J Hum Genet 2001;69:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro M, Shepherd C, Gomez M, Lie J, Ryu J. Pulmonary Tuberous Sclerosis. Chest 1995;107:189–195. [DOI] [PubMed] [Google Scholar]
- 9.Franz DN, Brody A, Meyer C, Leonard J, Chuck G, Dabora S, Sethuraman G, Colby TV, Kwiatkowski DJ, McCormack FX. Mutational and radiographic analysis of pulmonary disease consistent with lymphangioleiomyomatosis and micronodular pneumocyte hyperplasia in women with tuberous sclerosis. Am J Respir Crit Care Med 2001;164:661–668. [DOI] [PubMed] [Google Scholar]
- 10.Moss J, Avila NA, Barnes PM, Litzenberger RA, Bechtle J, Brooks PG, Hedin CJ, Hunsberger S, Kristof AS. Prevalence and clinical characteristics of lymphangioleiomyomatosis (LAM) in patients with tuberous sclerosis complex. Am J Respir Crit Care Med 2001;164:669–671. [DOI] [PubMed] [Google Scholar]
- 11.Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet 2002;11:525–534. [DOI] [PubMed] [Google Scholar]
- 12.El-Hashemite N, Zhang H, Henske EP, Kwiatkowski DJ. Mutation in TSC2 and activation of mammalian target of rapamycin signalling pathway in renal angiomyolipoma. Lancet 2003;36:1348–1349. [DOI] [PubMed] [Google Scholar]
- 13.Goncharova EA, Goncharov DA, Eszterhas A, Hunter DS, Glassberg MK, Yeung RS, Walker CL, Noonan D, Kwiatkowski DJ, Chou MM, et al. Tuberin regulates p70 S6 kinase activation and ribosomal protein S6 phosphorylation: A role for the TSC2 tumor suppressor gene in pulmonary lymphangioleiomyomatosis (LAM). J Biol Chem 2002;277:30958–30967. [DOI] [PubMed] [Google Scholar]
- 14.Tee AR, Fingar DC, Manning BD, Kwiatkowski DJ, Cantley LC, Blenis J. Tuberous sclerosis complex-1 and -2 gene products function together to inhibit mammalian target of rapamycin (mTOR)-mediated downstream signaling. Proc Natl Acad Sci USA 2002;99:13571–13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenerson HL, Aicher LD, True LD, Yeung RS. Activated mammalian target of rapamycin pathway in the pathogenesis of tuberous sclerosis complex renal tumors. Cancer Res 2002;62:5645–5650. [PubMed] [Google Scholar]
- 16.El-Hashemite N, Zhang H, Walker V, Hoffmeister KM, Kwiatkowski DJ. Perturbed IFN-gamma-Jak-signal transducers and activators of transcription signaling in tuberous sclerosis mouse models: synergistic effects of rapamycin-IFN-gamma treatment. Cancer Res 2004;64:3436–3443. [DOI] [PubMed] [Google Scholar]
- 17.Ramana CV, Gil MP, Schreiber RD, Stark GR. Stat1-dependent and -independent pathways in IFN-gamma-dependent signaling. Trends Immunol 2002;23:96–101. [DOI] [PubMed] [Google Scholar]
- 18.Onda H, Crino PB, Zhang H, Murphey RD, Rastelli L, Gould Rothberg BE, Kwiatkowski DJ. Tsc2 null murine neuroepithelial cells are a model for human tuber giant cells, and show activation of an mTOR pathway. Mol Cell Neurosci 2002;21:561–574. [DOI] [PubMed] [Google Scholar]
- 19.Lee L, Sudentas P, Donohue B, Asrican K, Worku A, Walker V, Sun Y, Schmidt K, Albert MS, El Hashemite N, et al. Efficacy of a rapamycin analog (CCI-779) and IFN-g in tuberous sclerosis mouse models. Genes Chromosomes Cancer 2005;42:213–227. [DOI] [PubMed] [Google Scholar]