Abstract
Eosinophils are an important source of leukotriene (LT)C4, which can be synthesized within lipid bodies—cytoplasmic organelles where eicosanoid formation may take place. Allergy-driven lipid body formation and function have never been investigated. Here, we studied the in vivo induction and role of lipid bodies within eosinophils recruited to sites of allergic inflammation. Using two murine models of allergic inflammation (asthma and pleurisy), we verified that parallel to the eosinophil influx, allergic challenge also induced lipid body formation within recruited eosinophils. Neutralizing antibodies to eotaxin/CCL11, RANTES/CCL5, or CCR3 partially inhibited lipid body formation within recruited eosinophils in the allergic pleurisy model. Likewise, intrapleural administration of RANTES or eotaxin also induced significant influx of eosinophils loaded with lipid bodies. By immunolabeling, we detected the presence of a key enzyme involved in the leukotriene metabolism—5-lipoxygenase—within eosinophil lipid bodies formed in vivo after allergen challenge. Furthermore, specific immunolocalization of newly formed LTC4 demonstrated that lipid bodies were the sites of formation of this eicosanoid within infiltrating eosinophils. Therefore, allergic inflammation triggers in vivo formation of new lipid bodies within infiltrating eosinophils, a phenomenon largely mediated by eotaxin/RANTES acting via CCR3 receptors. Such in vivo allergen-driven lipid bodies function as intracellular compartments of LTC4 synthesis.
Keywords: allergy, CCR3, eosinophils, lipid bodies, LTC4
Eosinophil recruitment and subsequent activation at sites of allergic inflammation are key events in the pathogenesis of asthma and other forms of allergic diseases (1–3), controlling allergic airways remodeling (4, 5). Eosinophils elaborate and release various protein and lipid mediators critical to the development and perpetuation of allergic inflammation. Among eosinophil-derived lipid mediators are platelet-activating factor (PAF), prostaglandins (PGs), and leukotrienes (LTs). Indeed, eosinophils are the major source of cysteinyl leukotrienes (cysLTs), LTC4 and its extracellular derivates LTD4 and LTE4 (6–8). In vivo, these 5-lipoxygenase (LO) pathway-derived eicosanoids cause several proinflammatory effects, such as bronchoconstriction, mucous hypersecretion, increased microvascular permeability, bronchial hyperresponsiveness, and eosinophil infiltration (9, 10). For LTC4 synthesis in eosinophils or in the other cysLT cellular sources (mast cells, alveolar macrophages, and basophils), arachidonic acid released from phospholipids is metabolized by 5-LO in concert with the 5-LO–activating protein (FLAP) to form LTA4, which is conjugated to reduced glutathione to form LTC4 (11, 12). Although the eosinophil enzymatic pathways of leukotriene synthesis have been established, their regulatory mechanisms remain to be fully defined. In vitro studies focusing on the distinct intracellular domains that govern eosinophil LT production have revealed that eosinophil perinuclear membranes and cytoplasmic lipid bodies are sites of localization of key membrane-associated proteins, such as the LT-forming enzymes 5-LO and LTC4 synthase (13, 14). A more direct approach for the study of compartmentalized LTC4 synthesis by human eosinophils, in which newly formed LTC4 was fixed and detected at its sites of synthesis within eosinophils, demonstrated that nonphysiologic calcium ionophore versus physiologic eotaxin elicit LTC4 synthesis within perinuclear membrane versus lipid bodies, respectively (15).
Lipid bodies are non–membrane-bound lipid-rich cytoplasmic organelles surrounded by a monolayer of phospholipids. In human circulating eosinophils, the lipid body numbers are characteristically prominent (for a recent review see Ref. 16). Further, lipid bodies increase in number within eosinophils associated with various forms of eosinophilic inflammation, such as hypereosinophilic syndrome (HES) (17, 18). In vitro, several specific agonists (e.g., PAF, IL-5, eotaxin, and RANTES) can trigger signaling mechanisms that rapidly stimulate the formation of new lipid bodies within human eosinophils (13, 15, 18, 19). Of note, even though these in vitro studies have analyzed lipid body formation and function in human eosinophils, the effects of allergic inflammatory responses in vivo on lipid body genesis and functions have never been investigated.
Employing a murine model of allergic inflammation, the present study shows that allergen challenge can trigger lipid body formation within infiltrating eosinophils by a mechanism dependent on eotaxin- and RANTES-initiated intracellular activities. Moreover, newly formed lipid bodies within recruited eosinophils were the specific cytoplasmic sites of formation of 5-LO–derived LTC4 synthesis.
MATERIALS AND METHODS
Animals
C57Bl/6 mice of 16–20 g from both sexes were used. They were obtained from Oswaldo Cruz Foundation breeding unit (Rio de Janeiro) and maintained with food and water ad libitum in a room with temperature ranging from 22–24°C and a 12-h light/dark cycle in the Department of Physiology and Pharmacology, Fundação Oswaldo Cruz. The Animal Welfare Committee approved the protocols used here.
Reagents
Chicken egg ovalbumin (OVA), lipopolysaccharide (LPS, Escherichia coli serotype 0127:B8), bovine serum albumin (BSA), phosphate-buffered saline (PBS), and 1-ethyl-3-(3-dimethylamino-propyl) carbodiimide (EDAC) were purchased from Sigma Chemical Co. (St. Louis, MO). Neutralizing antibodies to murine eotaxin, RANTES, and CCR3 and recombinant chemokines RANTES and eotaxin were from R&D Systems (Minneapolis, MN). The rabbit polyclonal antiserum anti-LTC4 and 5-LO were from Cayman Chemicals (Ann Arbor, MI). The antibody against adipocyte differentiation-related protein (ADRP) was purchased from RDI (Flanders, NJ). The secondary antibody Cy3-labeled donkey anti-guinea pig IgG was from RDI. The Cy2-labeled donkey anti-rabbit IgG and the irrelevant control rabbit IgG were from Jackson ImmuneResearch (West Grove, PA). Zileuton was from Abbott Laboratories (North Chicago, IL).
Lung Allergic Inflammation in Actively Sensitized Mice
Pulmonary eosinophilia in response to OVA was generated in mice as described (20). Briefly, mice were sensitized with intraperitoneal injection of OVA (10 μg/mouse) and Al(OH)3 (10 mg/ml) in 0.9% NaCl solution (saline; 0.2 ml) on Days 1 and 10. From Days 19–24 after sensitization, mice were challenged daily for 20 min with OVA (5%) in PBS by aerosol. Aerosolized PBS is administered to sensitized mice as a negative control. These procedures were performed in a 30 × 20 × 10 cm acrylic chamber and the aerosol was generated with an ultrasonic nebulizer. Twenty-four hours after the last aerosolization, animals were killed by CO2 administration, and the trachea was surgically exposed and cannulated. The bronchoalveolar lavage (BAL) was collected from the mice by washing the lungs with 1 ml PBS.
Allergic Pleurisy in Actively Sensitized Mice
Active sensitization was achieved by a subcutaneous injection (0.2 ml) of a mixture containing OVA (50 μg) and Al(OH)3 (5 mg) in saline. Allergic challenge was performed 14 d after sensitization by means of an intrathoracic injection of OVA (12 μg/cavity) dissolved in sterile saline. A control group consists of sensitized mice challenged with sterile saline. All intrathoracic injections were performed in a final volume of 0.1 ml. Mice were killed under an excess of CO2 atmosphere 6, 24, 48, and 96 h after allergic challenge. Pleural cavities were then rinsed with 1 ml of PBS containing BSA (0.1%), and the collected pleural effluents had their volumes measured with a graduated syringe.
Leukocyte Counts
Total leukocyte counts were performed using a Neubauer chamber under an optical microscope, after dilution with Turk fluid (2% acetic acid). Differential counts of mononuclear cells, neutrophils, and eosinophils were performed under an oil immersion objective using cytopins (Cytospin 3; Shandon Inc., Pittsburgh, PA) stained by the May-Grunwald-Giemsa method. Counts are reported as number of cells per cavity.
Pleurisy Triggered by Eotaxin or RANTES
Naive mice were stimulated intrathoracically with murine eotaxin (30 pmol/cavity) or murine RANTES (30 pmol/cavity). Eotaxin and RANTES were diluted in sterile saline immediately before use. Control animals were injected with the same volume (0.1 ml) of vehicle. Six hours after stimulation, the animals were killed and pleural fluids analyzed as described above. To ascertain specificity of LTC4 immunolocalization within recruited eosinophils, naive mice were also stimulated intraperitoneally with LPS (500 ng/cavity; 0.1 ml) to attract neutrophils to the pleural cavity (6 h).
Treatments
To evaluate the involvement of CCR3 activation, animals were pretreated with an intraperitoneal injection of anti-eotaxin (10 μg/cavity), anti-RANTES (10 μg/cavity), or anti-CCR3 (10 μg/cavity) 30 min before allergic challenge as previously described (21). Animals pretreated with an irrelevant control antibody showed no alteration of allergic response (data not shown).
To confirm the specifity of LTC4 immunolabeling within infiltrating eosinophils, the animals were pretreated with an intrathoracic injection of zileuton (50 μg/cavity; 0.1 ml)—a specific inhibitor of 5-LO—2 h before challenged animals were killed. Moreover, to ensure that the enzyme stays inhibited during all procedure, the pleural cavities of zileuton-treated animals were rinsed with 500 μl of PBS containing BSA (0.1%) and zileuton (1 μM), and immediately mixed with 500 μl of EDAC (1% in Hanks' balanced salt solution [HBSS]), as further described bellow.
Lipid Body Staining and Enumeration
To enumerate lipid bodies within leukocytes cytoplasm, BAL or pleural fluid cells were cytocentrifuged (450 rpm, 5 min) onto glass slides. Leukocytes, while still moist, were fixed in 3.7% formaldehyde (diluted in Ca2+/Mg2+-free HBSS; pH 7.4), rinsed in 0.1 M cacodylate buffer (pH 7.4), stained with 1.5% OsO4 for 30 min, rinsed in distilled H2O, immersed in 1.0% thiocarbohydrazide for 5 min, rinsed in 0.1 M cacodylate buffer, restained with 1.5% OsO4 for 3 min, rinsed in distilled water, and then dried and mounted (13). Cell morphology was observed and lipid bodies were enumerated by microscopy with an objective lens at ×100 magnification. Twenty-five consecutively scanned eosinophils were evaluated in a blinded fashion by more than one individual and results were expressed as the number of lipid bodies per eosinophil.
Quantification of cysLTs
cysLT concentrations in the BAL fluid were measured by a commercial EIA kit according to the manufacturer's protocol (Cayman Chemicals).
Intracellular Immunodetection of 5-LO and Newly Formed LTC4
To immunolocalize 5-LO and LTC4 at its sites of formation within recruited eosinophils, leukocytes were recovered from the thoracic cavity with 500 μl of HBSS and immediately mixed with 500 μl of water-soluble EDAC (1% in HBSS), used to cross-link eicosanoid carboxyl groups to amines in adjacent proteins (protocols modified from Ref. 15). After 30–40 min incubation at 37°C with EDAC to promote both cell fixation and permeabilization, pleural leukocytes were then washed with HBSS, cytospun onto glass slides, and blocked with HBSS containing 2% normal mouse serum and normal goat serum for 10 min. The cells were then sequentially incubated with either anti–5-LO antibody (1:150 final dilution) or anti-LTC4 antibody (1:5 final dilution) for 45 min. The anti-ADRP (1:300 final dilution) was added for 45 min to distinguish cytoplasmic lipid bodies within infiltrating leukocytes. The cells were washed with HBSS for 10 min (3×) and incubated with Cy2-labeled anti-rabbit IgG (1:500 final dilution) plus Cy3-labeled anti-guinea pig (1:1,000 final dilution) secondary antibodies for 1 h. Slides were then washed with HBSS, and an aqueous mounting medium (Polysciences, Warrington, PA) was applied to each slide before cover-slip attachment. Slides were viewed by both phase-contrast and fluorescent microscopy, and electronic photography was performed by Cool Snap digital camera (Roper Scientific, Ottoburn, Germany) in conjunction with the image program Image Pro Express (Media Cybernetics, Silver Spring, MD).
The specificity of LTC4 immunofluorescent staining within recruited eosinophils was ascertained by three different approaches: (1) a nonimmune rabbit IgG used as an irrelevant control to anti-LTC4 antibody; (2) a pretreatment with the inhibitor of 5-LO zileuton to avoid the synthesis of LTC4; and (3) the analysis of LTC4 staining within leukocytes that are known to not express LTC4 synthase, such as LPS-driven infiltrating neutrophils.
Bone Marrow Granulocyte Purification and Chemotaxis Assay
Medullar granulocytes from naive mice were purified as follows. Briefly, both femurs of mice were removed and the bone marrow was gently eluted with 1 ml of RPMI-1640 (pH 7.2). Using a Ficoll-Histopaque 1077 gradient (Amersham Biosciences, Piscataway, NJ), bone marrow granulocytes were isolated. Granulocyte suspensions (1.5 × 106 cells/100 μl of RPMI-1640 supplemented with 0.5% BSA) were placed at the top chamber of a 6.5-mm diameter, 5-μm-pore polycarbonate Transwell culture insert (Costar, Cambridge, MA) and incubated in triplicate with the indicated concentrations of stimuli in the bottom chamber for 4 h, at 37°C. Migrated cells recovered from the bottom chamber after incubation were counted and cytocentrifuged (450 rpm, 5 min) onto glass slides and stained with osmium as described above. Twenty-five consecutively scanned eosinophils were evaluated and results were expressed as the number of lipid bodies per in vitro migrated eosinophil. For comparison, bone marrow granulocytes were alternatively placed in the bottom chambers and stimulated under same conditions for analysis of formation of new lipid bodies in nonmigrating eosinophils.
Statistical Analysis
Results were expressed as means ± SEM and were analyzed statistically by ANOVA followed by the Newman-Keuls-Student's test with the level of significance set at P ⩽ 0.05.
RESULTS
Analysis of Lipid Formation within Infiltrating Eosinophils during Allergic Inflammation
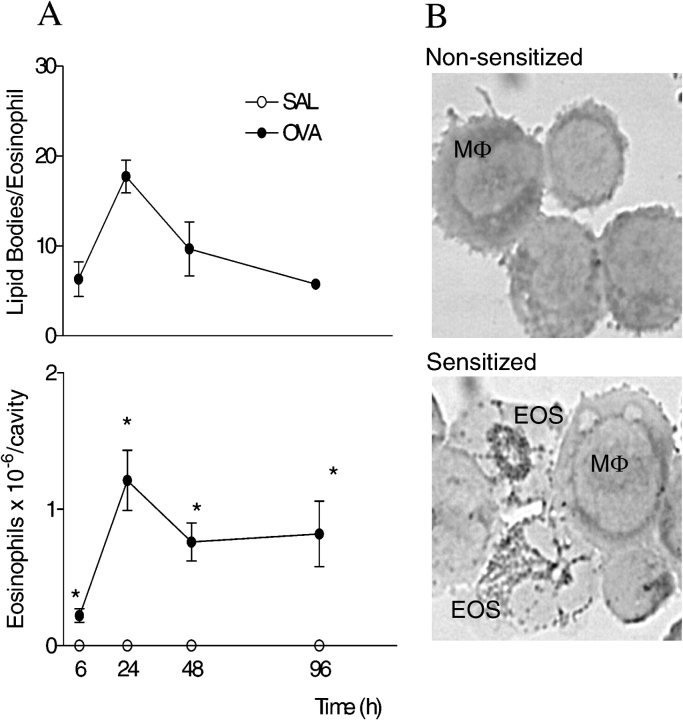
Allergic challenge into actively sensitized mice induced a marked eosinophil recruitment to the BAL (Figure 1A) and pleural cavity (Figure 2, lower panel), in two distinct models of allergic inflammation: lung allergic inflammation (asthma model) and allergic pleurisy. BAL and pleural eosinophilia were associated with a discrete but significant accumulation of mononuclear cells (data not shown). Activation of eosinophils recruited to sites of allergic inflammation is a critical feature in the pathogenesis of allergic diseases. Here we are proposing that increases of cytoplasmic numbers of lipid bodies within infiltrating eosinophils can be used as a parameter of eosinophil activation. The number of cytoplasmic lipid bodies found within recruited eosinophils after allergic challenge was evaluated. Using the asthma model, we verified that BAL eosinophils from allergen-challenged sensitized animals have five times more cytoplasmic lipid bodies than BAL eosinophils from PBS-challenged sensitized animals (Figure 1B). Moreover, in parallel to such dramatic increase in eosinophil lipid body numbers, BAL levels of cysLTs were also increased about five times (Figure 1C). In allergic pleurisy, infiltrating eosinophils were not detectable within 1 h, peaked at 6 h, decreased marginally by 24 h, and were detectable within pleural space up to at least 96 h after allergic challenge (Figure 2, lower panel). The upper panel of Figure 2 shows that new lipid bodies are formed within eosinophils infiltrating the mice pleural cavities. Increases in lipid body numbers were progressive, developing by 6 h, peaking at 24 h after challenge and decreasing thereafter. Inasmuch as eosinophils are not found in pleural cavities of nonsensitized mice, the lipid body numbers of bone marrow eosinophils were enumerated as a control (Table 1). Bone marrow eosinophils from sensitized nonchallenged mice contain ∼ 3 lipid bodies/cell, whereas marrow eosinophils from sensitized and challenged mice contain ∼ 7 lipid bodies/cell. In infiltrating eosinophils, the number of cytoplasmic lipid bodies were much more prominent (∼ 20 lipid bodies/eosinophil within 24 h). Of note, in vivo lipid body formation during the allergic reaction was not limited to eosinophils. As illustrated in Figure 1B, the numbers of lipid bodies also increased within pleural mononuclear cells of allergen-challenged sensitized mice.
Figure 1.
In vivo lipid body formation within recruited eosinophils in a mouse model of asthma. Eosinophil influx (A), eosinophil lipid body formation (B), and production of cysLTs (C) triggered by allergic challenge (aerosolized OVA) of sensitized animals were analyzed within 24 h. Results are expressed as the means ± SEM from at least five animals. *P ⩽ 0.05 as compared with PBS-challenged sensitized mice.
Figure 2.
In vivo lipid body formation within infiltrating eosinophils in a mouse model of allergic pleurisy. In A, results of a kinetic analysis are expressed as the means ± SEM from at least five animals. *P ⩽ 0.05. In B, images depict pleural leukocytes (stained with osmium) recovered from nonsensitized (upper panel) or sensitized mice (lower panel) 24 h after allergic stimulation. EOS = eosinophils; Mφ = macrophages.
TABLE 1.
Number of cytoplasmic lipid bodies within bone marrow eosinophils from allergic pleurisy model
| Mice Condition | Lipid Bodies/Marrow Eosinophil |
|---|---|
| Naive | 3.2 ± 0.2 |
| Sensitized/nonchallenged | 3.9 ± 0.4 |
| Sensitized/challenged | 7.6 ± 0.3* |
Osmium-stained lipid bodies were enumerated within eosinophils recovered from murine bone marrows 24 h after allergic challenge (allergic pleurisy model). Results represent the means ± SEM of at least five animals.
Significantly different from nonchallenged group with P ⩽ 0.05.
Endogenous Eotaxin and RANTES Signal through CCR3 for Allergen-Induced Lipid Body Formation within Recruited Eosinophils
Eotaxin and RANTES are key mediators in the development of allergic eosinophilia (22, 23). In addition, eotaxin- and RANTES-mediated CCR3 activation are potent inducers of lipid bodies in human eosinophils (15). To investigate the in vivo role of CCR3 activation on allergen-induced lipid body formation within recruited eosinophils, we: (1) administered eotaxin or RANTES into pleural cavities of naive mice to analyze the impact of these mediators on lipid body genesis in vivo; and (2) pretreated sensitized mice with neutralizing antibodies to eotaxin, RANTES, or CCR3. Similar to the allergic stimulation, either eotaxin or RANTES induced both eosinophil recruitment and lipid body formation within recruited eosinophils (Figure 3A). Moreover, allergen-induced eosinophil influx and lipid body formation within recruited eosinophils were dramatically inhibited by the pretreatments with neutralizing antibodies (Figure 3B), indicating that endogenous eotaxin and RANTES, acting via CCR3, elicited in vivo lipid body formation within infiltrating eosinophils.
Figure 3.
Role of CCR3-activating chemokines RANTES and eotaxin on allergen-induced lipid body formation and eosinophil influx. In A, naive mice received intrathoracic injection of RANTES (30 pmol/cavity) or eotaxin (30 pmol/cavity). Analyses of pleural leukocytes were performed 6 h after chemokine administration. In B, treatments were administered intraperitoneally 30 min before allergic challenge. Analyses were performed 24 h after allergic intrathoracic challenge. Results were expressed as the means ± SEM from at least five animals. *P ⩽ 0.05 compared with control group. +P ⩽ 0.05 compared with allergen-challenged mice.
Chemotaxis Does Not Interfere with Eosinophil Lipid Body Formation
To investigate the role of migratory processes in lipid body formation observed within recruited eosinophils during an allergic inflammatory reaction, we compared the magnitude of in vitro formation of new lipid bodies within migrating versus nonmigrating murine bone marrow eosinophils induced directly by eotaxin or PAF. As shown in Figure 4A, both eotaxin and PAF were able to induce eosinophil chemotaxis. Interestingly, the ability of either eotaxin or PAF to induce lipid body formation was not modified by such locomotory activity induced in parallel by each stimuli (Figure 4B).
Figure 4.

Eotaxin- or PAF-induced in vitro chemotaxis of murine eosinophils does not interfere with concurrent induction of lipid body formation. In A, bone marrow eosinophils from naive mice which migrated toward eotaxin (10 ng/ml) or PAF (10 μM) during 4 h were recovered from bottom chambers of the Transwell system and counted. In B, the lipid body analysis of bone marrow migrated eosinophils (cells recovered from bottom chambers after crossing the Transwell; hatched bars) versus non-migrated eosinophils (cells incubated with the stimuli in the bottom chambers; open bars) are shown. Results were expressed as the means ± SEM from three pools of bone marrow granulocytes. Each pool is prepared from two femurs' bone marrows of at least three animals. *P ⩽ 0.05 compared with appropriated nonstimulated condition.
In Vivo Elicited Lipid Bodies Control LTC4 Metabolism in Recruited Eosinophils
Eosinophils are capable of elaborating the cysLT LTC4 (7, 8). Within eosinophils, the enzymatic machinery of LTC4 synthesis can be found at two distinct compartments, the perinuclear membrane or lipid bodies, depending on the stimulatory condition (24). Thus, we first evaluated whether a key LT-forming enzyme was localized at eosinophil lipid bodies after in vivo allergic stimulation. Employing conditions of leukocyte fixation and permeabilization that prevent dissolution of lipid bodies, the intracellular compartmentalization of 5-LO was analyzed by immunofluorescence. Eosinophils stained with rabbit polyclonal antibody to 5-LO (Figure 5) showed punctate cytoplasmic localizations, proximate to but separate from the nucleus, and fully consistent in size, form, number, and distribution with lipid bodies. We have confirmed that 5-LO was, indeed, specifically localized within cytoplasmic lipid bodies of infiltrating leukocytes by performing a double-labeling with the anti–5-LO and an antibody against a protein marker of these organelles named ADRP (not shown). Of note, ADRP is a structural protein that surrounds lipid bodies of different cell types (25), including leukocytes (C. M. Maya-Monteiro and coworkers, unpublished observations). 5-LO–labeled lipid bodies were found predominantly in infiltrating eosinophils, although some mononuclear cells also showed specific staining for this enzyme. There was no immunoreactivity when control nonimmune rabbit serum was used as control for 5-LO antibody (Figure 5). Of note, just a few lipid bodies distributed within eosinophil cytoplasm were 5-LO–positive, indicating that only part of the newly formed lipid bodies are able to produce LTs.
Figure 5.
5-LO immunolocalization within cytoplasmic lipid bodies of infiltrating eosinophils. Phase contrast (A and C) and fluorescent (B and D) images of identical fields depict pleural leukocytes recovered from sensitized mice 24 h after allergic stimulation. Pleural leukocytes were stained with rabbit anti–5-LO (B) or nonimmune rabbit (D) serums followed by a Cy3-labeled donkey anti-rabbit IgG as described in MATERIALS AND METHODS. EOS, eosinophil; Mφ, macrophage; arrow, lipid body.
Inasmuch as our data show that lipid bodies of allergen-recruited eosinophils are sites of intracellular localization of the LT-forming enzyme 5-LO, we analyzed whether allergen-elicited lipid bodies within infiltrating eosinophils are also discrete and specific sites of LTC4 synthesis. As previously described (15), to evaluate intracellular production of LTC4, pleural leukocytes were fixed with carbodiimide and immunostained with a rabbit anti-LTC4 antibody plus a Cy2-labeled anti-rabbit IgG. As shown in Figures 6A and 6D, eosinophils infiltrating pleural space after allergic challenge yielded focal immunofluorescent staining for LTC4 in virtually all eosinophils. Again, the lipid body localization of newly formed LTC4 within infiltrating leukocytes was ascertained by the co-localization with ADRP (Figures 6B and 6E). The specificity of LTC4 immunostaining within cytoplasmic lipid bodies was validated, because: (1) leukocytes from sensitized nonchallenged mice exhibited no immunofluorescent staining for LTC4 (Figure 6N), showing neither background staining nor detection of preformed LTC4, as expected in the absence of cell stimulation; (2) the pretreatment with zileuton, which blocks 5-LO, completely abolished immunofluorescent staining for LTC4 within infiltrating eosinophils (Figure 6G); (3) mononuclear cells, also found in the pleural space after allergic challenge in sensitized mice, did not show any staining for intracellular LTC4 (Figure 6D, arrowhead); and (4) infiltrating neutrophils, found in the pleural space of LPS-stimulated mice, did not show any staining for intracellular LTC4 (not shown). Therefore, eosinophil lipid bodies represent the major compartment responsible for LTC4 production in a murine model of allergic inflammation. But it is noteworthy that similar to the extent of 5-LO immunolocalization, only a fraction of eosinophil lipid bodies were LTC4-positive, indicating that not every cytoplasmic lipid body functions as a LTC4 site of synthesis.
Figure 6.
Lipid bodies are intracellular sites of LTC4 synthesis within infiltrating eosinophils. Anti-LTC4 (left), anti-ADRP (middle), and merged (right) immunofluorescent microscopic images of identical fields of pleural leukocytes recovered from sensitized mice 24 h after saline (N, O, P) or allergic stimulation (A–M). A–C show a representative infiltrating eosinophil with LTC4–immunoreactive lipid bodies. Cells shown on images from G and H were obtained from zileuton-treated animals according to the protocol described in MATERIALS AND METHODS. In J, a nonimmune rabbit serum replaced anti-LTC4 antibody. Arrowheads, macrophage; arrows, eosinophil.
DISCUSSION
Increased marrow production of eosinophils and the subsequent circulating and tissue eosinophilia are hallmark events of allergic inflammatory disorders. Moreover, recruited eosinophils found at inflammatory sites show an activated pattern, featuring for instance an increased capacity to release lipid mediators. Expression upregulation of LTC4-forming enzymes within eosinophils, including 5-LO, FLAP, and LTC4 synthase, may be involved in the increased LT synthesis observed in individuals with asthma and in murine models of asthma (7, 26, 27). Also relevant to this specific ability is another important characteristic of activated eosinophils—the presence of increased numbers of enlarged lipid bodies within the cytoplasm. These cytoplasmic organelles appear to have significant function in a variety of intracellular metabolic and signaling processes (28–32), including eicosanoid metabolism by leukocytes (16). The genesis of new lipid bodies is a rapid and highly regulated event by specific signaling pathways that depends on proper stimuli and the cell type. Specifically concerning human eosinophils, in vitro studies revealed that lipid bodies are specialized intracellular domains for LTC4 synthesis (15). Although this function did not have any support from in vivo studies, it seemed reasonable to speculate that the eosinophils attracted to inflammatory sites of an allergic reaction would contain an increased number of lipid bodies, which would correspond to the compartment of LTC4 production within eosinophils in vivo. In this study, we demonstrated that allergic stimulation induced formation of new lipid bodies within recruited eosinophils, via a mechanism dependent on CCR3 activation by endogenously produced eotaxin and RANTES. Further, the present findings are the first to show that in vivo newly formed lipid bodies function as the intracellular loci of 5-LO–driven LTC4 synthesis within eosinophils attracted to the site of allergic reaction in vivo.
High numbers of cytoplasmic lipid bodies have been noted within cells engaged in inflammatory disorders. For instance, increased lipid body numbers were found within leukocytes from inflammatory arthritis (33, 34), bronchoalveolar lavage of patients with acute respiratory distress syndrome, and from blood of patients with sepsis (35, 36). Even though eosinophils from patients with asthma have not yet been analyzed, circulating eosinophils from patients with hypereosinophilic syndrome or Crohn's disease show several lipid bodies within their cytoplasm (17, 18, 37). In addition, it was also observed that new lipid bodies are formed in vivo within leukocytes during the development of experimental inflammatory responses, such as oxidized low-density lipoprotein (ox-LDL)– (38, 39) and LPS-induced pleurisies (36). Similarly, our experimental models of murine allergic reaction yielded formation of new lipid bodies within the eosinophils recruited to the focus of inflammatory reaction of sensitized mice. Of note, the sensitization processes per se (allergic pleurisy model) did not modify the cytoplasmic lipid body numbers of those bone marrow eosinophils that may reach the allergic pleural inflammatory site after challenge. Yet, the bone marrow eosinophils of allergen-challenged sensitized animals displayed a discrete but significant increase in lipid body numbers (∼ 7 lipid bodies per cell), indicating that mediators (likely cytokines, such as IL-5) produced locally at bone marrow may regulate the genesis of new lipid bodies through a process that is not dependent on cell migration. Nevertheless, because the number of lipid bodies found within infiltrating pleural eosinophils was much higher (∼ 25 per cell) than the marrow ones, it seems that the migration process and/or local activation of recruited eosinophils control the biogenesis of new lipid bodies in vivo during allergic reaction. Further studying these potential mechanisms involved in in vivo induction of lipid body formation, we verified that the chemotactic process—an important component of allergic eosinophil accumulation—did not modulate lipid body formation induced by key eosinophil chemoatractants (eotaxin and PAF) in an in vitro chemotaxis assay. However, the contribution of other events involved in a more complex in vivo migration process (e.g., priming at blood compartment, adhesion, and/or transmigration) still needs to be investigated.
The mechanism of in vivo formation of lipid bodies within recruited eosinophils was also investigated. From in vitro studies we have learned that several specific agonists can directly trigger signaling events that rapidly stimulate the formation of new lipid bodies within human eosinophils. For instance, PAF, acting via its G protein–lined receptor, induces lipid body formation via a downstream signaling involving, sequentially, 5-LO activation to generate 5-HETE that per se activates its G protein–linked receptor and protein kinase C activation (40). Alternatively, chemokines acting via CCR3 receptors, including RANTES, eotaxin, eotaxin-2, and eotaxin-3, can initiate intracellular signaling that also culminate in de novo formation of lipid bodies (15, 41). CCR3 signals through phosphoinositide 3-kinase (PI3K) and the ERK1/2 and p38 MAP kinases (15). Here, by using neutralizing antibodies against eotaxin, RANTES, and CCR3, we demonstrated that besides controlling in vivo eosinophil trafficking to the site of allergic reaction, eotaxin/CCR3 systems largely regulate in vivo lipid body formation within recruited eosinophils triggered by intrapleural allergic challenge in sensitized mice. Of note, a treatment with an anti-eotaxin antibody also blocked lipid body formation within BAL eosinophils in the asthma model (not shown). Likely, other eosinophil receptor systems (e.g., PAF and IL-5 receptors) may contribute to allergic in vivo formation of lipid bodies, inasmuch as anti-CCR3 did not completely inhibit lipid body formation. Indeed, specific PAF receptor antagonists were also able to partially inhibit lipid body formation within recruited eosinophils triggered by allergic challenge in sensitized mice (data not shown). Still, direct and selective activation of CCR3 appears to be sufficient to elicit the lipid body formation within eosinophils in vivo, because administration of eotaxin or RANTES also promoted lipid body formation in vivo within recruited eosinophils.
Little is known about the composition and functions of lipid bodies in vivo. In leukocytes and other cells, lipid bodies are surrounded by a monolayer of phospholipids, are typically rich in triacylglycerides, and contain arachidonyl-phospholipids (42, 43); however, lipid bodies are not formed exclusively by lipids. Recent studies focusing on the protein content of these organelles have revealed how broad lipid body functions can be. Lipid bodies are formed by the structural PAT proteins (perilipin, ADRP, and TIP) (25); and by compartmentalizing fatty acid metabolic enzymes, proteins of Rab family, small GTPases (30–32), and kinases (28, 29), lipid bodies may control lipid metabolism, membrane trafficking, and intracellular signaling. By analyzing specifically lipid bodies of human eosinophils, we have learned that these lipid-rich organelles have inflammatory functions because they contain: (1) enzymes needed for eicosanoid synthesis, such as cPLA2, 5-LO, 15-LO, COX, and LTC4 synthase (13, 18, 28); (2) cytokines/chemokines, such as RANTES, TNF-α, and IL-16 (37, 44); and (3) structural proteins with circumferential immunolocalization, such as ADRP, which can be used as a lipid body marker (C. M. Maya-Monteiro and colleagues, unpublished data). In vivo experimentally induced lipid bodies, in both ox-LDL– and LPS-induced models of inflammation, have 5-LO and COX-2 enzymes within recruited leukocytes (36, 38). Similarly, eosinophils attracted to the site of allergic reaction showed 5-LO immunoreactivity within newly formed lipid bodies. The recurring localization of eicosanoid-forming enzymes within lipid bodies of different leukocytes associated with different types of inflammatory responses, observed during the last decade, have suggested that these organelles have roles on the generation of these inflammatory lipid mediators. Indeed, increases in lipid body numbers correlated with increased release of eicosanoids by leukocytes when activated with submaximal concentration of calcium ionophore (13, 15, 40). Conversely, agents that inhibit lipid body formation in vitro inhibited the priming response for enhanced eicosanoid release from leukocytes (13, 40).
It is noteworthy that specific immunolocalization of the integral membrane eicosanoid-forming enzymes FLAP, 5-LO, and LTC4 synthase at the nuclear envelope of activated leukocytes have identified the perinuclear membrane as an alternative and/or complementary intracellular site of eicosanoid biosynthesis (14, 45). Interestingly, based on studies of enucleated eosinophil cytoplasts, it seemed that perinuclear membranes were not essential for LTC4 synthesis by human eosinophils (13). To definitively characterize the intracellular compartment of eicosanoid synthesis within leukocytes, a methodology was developed to directly immunolocalize the newly formed eicosanoid at its site of synthesis (15). Using such direct approach to specifically immunolocalize LTC4 within human eosinophils, it was demonstrated that the intracellular domain synthesizing LTC4 varies according with the different stimulus used. For instance, in vitro eotaxin- or RANTES-stimulated human eosinophils not only formed new lipid bodies, but also synthesized LTC4 at these lipid bodies. Distinctly, eosinophils stimulated with calcium ionophore (15) or by cross-linking cell surface orphan receptors (e.g., CD9 or leukocyte immunoglobulin-like receptor 7) (46) synthesized LTC4 at the perinuclear membrane. Our current study is the first to demonstrate the actual intracellular site of LTC4 synthesis during the development of an allergic inflammatory response. Infiltrating eosinophils of allergen-challenged sensitized mice showed immunolocalization of newly formed LTC4 at cytoplasmic punctate structures compatible in number, size, localization, and form with lipid bodies. Indeed, we confirmed the identity of LTC4 immunoreactive structures by immunostaining them with a specific marker of lipid bodies, ADRP. Of note, the green fluorescent punctate immunoreactivity found within infiltrating eosinophils was confirmed as a 5-LO metabolite and therefore LTC4, as 5-LO inhibitor zileuton impaired its detection within eosinophils, which preferentially produce LTC4. No perinuclear immunostaining for LTC4 was found within infiltrating eosinophils, suggesting that under a CCR3-driven response, like in vitro stimulation with eotaxin or RANTES (15) or in vivo allergic inflammatory reaction, synthesis of LTC4 takes place at lipid bodies. Lately, it has become clear that the specific localization of eicosanoid synthesis at distinct intracellular compartments is crucial in governing subsequent eicosanoid functions. For instance, LTC4 produced in vitro within lipid bodies of eosinophils activated via CCR3 have intracrine functions regulating differential release of IL-4 by eosinophils (47). Therefore, in vivo synthesis of lipid body-derived LTC4 after allergic stimulation, besides acting as paracrine mediators of allergic inflammatory processes, may also have intracrine functions that need to be identified and may represent a new target for alternative anti-allergic therapies.
Altogether our results demonstrated that allergic challenge elicits formation of new cytoplasmic lipid bodies within recruited eosinophils—a CCR3-regulated phenomenon that can be easily used as a marker of cell activation. Moreover, our findings indicate that lipid bodies formed in vivo within infiltrating eosinophils in response to allergic challenge are sites of 5-LO–driven LTC4 synthesis.
This work was supported by Fogarty International Center-NIH (TW05890) and the NIH grant AI22571; Howard Hughes Medical Institute (to P.T.B.), PRONEX-MCT, Conselho Nacional de Pesquisa (CNPq, Brazil), PROFIX (to C.B.M.), and Fundação de Amparo à Pesquisa do Rio de Janeiro (FAPERJ, Brazil).
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wardlaw AJ. Molecular basis for selective eosinophil trafficking in asthma: a multistep paradigm. J Allergy Clin Immunol 1999;104:917–926. [DOI] [PubMed] [Google Scholar]
- 2.Weller PF. Human eosinophils. J Allergy Clin Immunol 1997;100:283–287. [DOI] [PubMed] [Google Scholar]
- 3.Costa JJ, Weller PF, Galli SJ. The cells of the allergic response: mast cells, basophils, and eosinophils. JAMA 1997;278:1815–1822. [PubMed] [Google Scholar]
- 4.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 5.Lee JJ, Dimina D, Macias MP, Ochkur SI, McGarry MP, O'Neill KR, Protheroe C, Pero R, Nguyen T, Cormier SA, et al. Defining a link with asthma in mice congenitally deficient in eosinophils. Science 2004;305:1773–1776. [DOI] [PubMed] [Google Scholar]
- 6.Hodges MK, Weller PF, Gerard NP, Ackerman SJ, Drazen JM. Heterogeneity of leukotriene C4 production by eosinophils from asthmatic and from normal subjects. Am Rev Respir Dis 1988;138:799–804. [DOI] [PubMed] [Google Scholar]
- 7.Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, Lam BK, Penrose JF, Austen FK, Holgate ST, et al. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest 1998;101:834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weller PF, Lee CW, Foster DW, Corey EJ, Austen KF, Lewis RA. Generation and metabolism of 5-lipoxygenase pathway leukotrienes by human eosinophils: predominant production of leukotriene C4. Proc Natl Acad Sci USA 1983;80:7626–7630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med 1990;323:645–655. [DOI] [PubMed] [Google Scholar]
- 10.Laitinen LA, Laitinen A, Haahtela T, Vilkka V, Spur BW, Lee TH. Leukotriene E4 and granulocytic infiltration into asthmatic airways. Lancet 1993;341:989–990. [DOI] [PubMed] [Google Scholar]
- 11.Lam BK, Xu K, Atkins MB, Austen KF. Leukotriene C4 uses a probenecid-sensitive export carrier that does not recognize leukotriene B4. Proc Natl Acad Sci USA 1992;89:11598–11602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol 2004;173:1503–1510. [DOI] [PubMed] [Google Scholar]
- 13.Bozza PT, Yu W, Penrose JF, Morgan ES, Dvorak AM, Weller PF. Eosinophil lipid bodies: specific, inducible intracellular sites for enhanced eicosanoid formation. J Exp Med 1997;186:909–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brock TG, Anderson JA, Fries FP, Peters-Golden M, Sporn PH. Decreased leukotriene C4 synthesis accompanies adherence-dependent nuclear import of 5-lipoxygenase in human blood eosinophils. J Immunol 1999;162:1669–1676. [PubMed] [Google Scholar]
- 15.Bandeira-Melo C, Phoofolo M, Weller PF. Extranuclear lipid bodies, elicited by CCR3-mediated signaling pathways, are the sites of chemokine-enhanced leukotriene C4 production in eosinophils and basophils. J Biol Chem 2001;276:22779–22787. [DOI] [PubMed] [Google Scholar]
- 16.Bozza PT, Bandeira-Melo C. Mechanisms of leukocyte lipid body formation and function in inflammation. Mem Inst Oswaldo Cruz 2005;100:113–120. [DOI] [PubMed] [Google Scholar]
- 17.Solley GO, Maldonado JE, Gleich GJ, Giuliani ER, Hoagland HC, Pierre RV, Brown AL Jr. Endomyocardiopathy with eosinophilia. Mayo Clin Proc 1976;51:697–708. [PubMed] [Google Scholar]
- 18.Bozza PT, Yu W, Cassara J, Weller PF. Pathways for eosinophil lipid body induction: differing signal transduction in cells from normal and hypereosinophilic subjects. J Leukoc Biol 1998;64:563–569. [DOI] [PubMed] [Google Scholar]
- 19.Bartemes KR, McKinney S, Gleich GJ, Kita H. Endogenous platelet-activating factor is critically involved in effector functions of eosinophils stimulated with IL-5 or IgG. J Immunol 1999;162:2982–2989. [PubMed] [Google Scholar]
- 20.Lloyd CM, Gonzalo JA, Nguyen T, Delaney T, Tian J, Oettgen H, Coyle AJ, Gutierrez-Ramos JC. Resolution of bronchial hyperresponsiveness and pulmonary inflammation is associated with IL-3 and tissue leukocyte apoptosis. J Immunol 2001;166:2033–2040. [DOI] [PubMed] [Google Scholar]
- 21.Penido C, Castro-Faria-Neto HC, Vieira-de-Abreu A, Figueiredo RT, Pelled A, Martins MA, Jose PJ, Williams TJ, Bozza PT. LPS induces eosinophil migration via CCR3 signaling through a mechanism independent of RANTES and Eotaxin. Am J Respir Cell Mol Biol 2001;25:707–716. [DOI] [PubMed] [Google Scholar]
- 22.Rothenberg ME. Eotaxin: an essential mediator of eosinophil trafficking into mucosal tissues. Am J Respir Cell Mol Biol 1999;21:291–295. [DOI] [PubMed] [Google Scholar]
- 23.Pease JE, Williams TJ. Eotaxin and asthma. Curr Opin Pharmacol 2001;1:248–253. [DOI] [PubMed] [Google Scholar]
- 24.Bandeira-Melo C, Weller PF. Eosinophils and cysteinyl leukotrienes. Prostaglandins Leukot Essent Fatty Acids 2003;69:135–143. [DOI] [PubMed] [Google Scholar]
- 25.Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res 1998;294:309–321. [DOI] [PubMed] [Google Scholar]
- 26.Chu SJ, Tang LO, Watney E, Chi EY, Henderson WR Jr. In situ amplification of 5-lipoxygenase and 5-lipoxygenase-activating protein in allergic airway inflammation and inhibition by leukotriene blockade. J Immunol 2000;165:4640–4648. [DOI] [PubMed] [Google Scholar]
- 27.Koshino T, Takano S, Houjo T, Sano Y, Kudo K, Kihara H, Kitani S, Takaishi T, Hirai K, Ito K, et al. Expression of 5-lipoxygenase and 5-lipoxygenase-activating protein mRNAs in the peripheral blood leukocytes of asthmatics. Biochem Biophys Res Commun 1998;247:510–513. [DOI] [PubMed] [Google Scholar]
- 28.Yu W, Bozza PT, Tzizik DM, Gray JP, Cassara J, Dvorak AM, Weller PF. Co-compartmentalization of MAP kinases and cytosolic phospholipase A2 at cytoplasmic arachidonate-rich lipid bodies. Am J Pathol 1998;152:759–769. [PMC free article] [PubMed] [Google Scholar]
- 29.Yu W, Cassara J, Weller PF. Phosphatidylinositide 3-kinase localizes to cytoplasmic lipid bodies in human polymorphonuclear leukocytes and other myeloid-derived cells. Blood 2000;95:1078–1085. [PubMed] [Google Scholar]
- 30.Fujimoto Y, Itabe H, Sakai J, Makita M, Noda J, Mori M, Higashi Y, Kojima S, Takano T. Identification of major proteins in the lipid droplet-enriched fraction isolated from the human hepatocyte cell line HuH7. Biochim Biophys Acta 2004;1644:47–59. [DOI] [PubMed] [Google Scholar]
- 31.Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem 2004;279:3787–3792. [DOI] [PubMed] [Google Scholar]
- 32.Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically-stimulated 3T3–L1 adipocytes. J Biol Chem 2004;274:46835–46842. [DOI] [PubMed] [Google Scholar]
- 33.Weinstein J. Synovial fluid leukocytosis associated with intracellular lipid inclusions. Arch Intern Med 1980;140:560–561. [PubMed] [Google Scholar]
- 34.Bozza PT, Payne JL, Morham SG, Langenbach R, Smithies O, Weller PF. Leukocyte lipid body formation and eicosanoid generation: cyclooxygenase-independent inhibition by aspirin. Proc Natl Acad Sci USA 1996;93:11091–11096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Triggiani M, Oriente A, Seeds MC, Bass DA, Marone G, Chilton FH. Migration of human inflammatory cells into the lung results in the remodeling of arachidonic acid into a triglyceride pool. J Exp Med 1995;182:1181–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacheco P, Bozza FA, Gomes RN, Bozza M, Weller PF, Castro-Faria-Neto HC, Bozza PT. Lipopolysaccharide-induced leukocyte lipid body formation in vivo: innate immunity elicited intracellular Loci involved in eicosanoid metabolism. J Immunol 2002;169:6498–6506. [DOI] [PubMed] [Google Scholar]
- 37.Beil WJ, Weller PF, Peppercorn MA, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of subcellular sites of TNF-alpha in colonic Crohn's disease. J Leukoc Biol 1995;58:284–298. [DOI] [PubMed] [Google Scholar]
- 38.de Assis EF, Silva AR, Caiado LF, Marathe GK, Zimmerman GA, Prescott SM, McIntyre TM, Bozza PT, de Castro-Faria-Neto HC. Synergism between platelet-activating factor-like phospholipids and peroxisome proliferator-activated receptor gamma agonists generated during low density lipoprotein oxidation that induces lipid body formation in leukocytes. J Immunol 2003;171:2090–2098. [DOI] [PubMed] [Google Scholar]
- 39.Silva AR, de Assis EF, Caiado LF, Marathe GK, Bozza MT, McIntyre TM, Zimmerman GA, Prescott SM, Bozza PT, Castro-Faria-Neto HC. Monocyte chemoattractant protein-1 and 5-lipoxygenase products recruit leukocytes in response to platelet-activating factor-like lipids in oxidized low-density lipoprotein. J Immunol 2002;168:4112–4120. [DOI] [PubMed] [Google Scholar]
- 40.Bozza PT, Payne JL, Goulet JL, Weller PF. Mechanisms of platelet-activating factor-induced lipid body formation: requisite roles for 5-lipoxygenase and de novo protein synthesis in the compartmentalization of neutrophil lipids. J Exp Med 1996;183:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandeira-Melo C, Herbst A, Weller PF. Eotaxins: contributing to the diversity of eosinophil recruitment and activation. Am J Respir Cell Mol Biol 2001;24:653–657. [DOI] [PubMed] [Google Scholar]
- 42.Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique fatty acid composition. J Biol Chem 2002;277:44507–44512. [DOI] [PubMed] [Google Scholar]
- 43.Weller PF, Dvorak AM. Arachidonic acid incorporation by cytoplasmic lipid bodies of human eosinophils. Blood 1985;65:1269–1274. [PubMed] [Google Scholar]
- 44.Lim KG, Wan HC, Bozza PT, Resnick MB, Wong DT, Cruikshank WW, Kornfeld H, Center DM, Weller PF. Human eosinophils elaborate the lymphocyte chemoattractants. IL-16 (lymphocyte chemoattractant factor) and RANTES. J Immunol 1996;156:2566–2570. [PubMed] [Google Scholar]
- 45.Woods JW, Evans JF, Ethier D, Scott S, Vickers PJ, Hearn L, Heibein JA, Charleson S, Singer II. 5-lipoxygenase and 5-lipoxygenase-activating protein are localized in the nuclear envelope of activated human leukocytes. J Exp Med 1993;178:1935–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tedla N, Bandeira-Melo C, Tassinari P, Sloane DE, Samplaski M, Cosman D, Borges L, Weller PF, Arm JP. Activation of human eosinophils through leukocyte immunoglobulin-like receptor 7. Proc Natl Acad Sci USA 2003;100:1174–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bandeira-Melo C, Woods LJ, Phoofolo M, Weller PF. Intracrine cysteinyl leukotriene receptor-mediated signaling of eosinophil vesicular transport-mediated interleukin-4 secretion. J Exp Med 2002;196:841–850. [DOI] [PMC free article] [PubMed] [Google Scholar]