Abstract
Bleomycin-induced lung injury triggers a profound and durable increase in tissue inhibitor of metalloproteinase (TIMP)-1 expression, suggesting a potential role for this antiproteinase in the regulation of lung inflammation and fibrosis. TIMP-1 protein induction is spatially restricted to areas of lung injury as determined by immunohistochemistry. Using TIMP-1 null mutation mice, we demonstrate that TIMP-1 deficiency amplifies acute lung injury as determined by exaggerated pulmonary neutrophilia, hemorrhage, and vascular permeability compared with wild-type littermates after bleomycin exposure. The augmented pulmonary neutrophilia observed in TIMP-1–deficient animals was not found in similarly treated TIMP-2–deficient mice. Using TIMP-1 bone marrow (BM) chimeric mice, we observed that the TIMP-1–deficient phenotype was abolished in wild-type recipients of TIMP-1–deficient BM but not in TIMP-1–deficient recipients of wild-type BM. Acute lung injury in TIMP-1–deficient mice was accompanied by exaggerated gelatinase-B activity in the alveolar compartment. TIMP-1 deficiency did not alter neutrophil chemotactic factor accumulation in the injured lung nor neutrophil migration in response to chemotactic stimuli in vivo or in vitro. Moreover, TIMP-1 deficiency did not modify collagen accumulation after bleomycin injury. Our results provide direct evidence that TIMP-1 contributes significantly to the regulation of acute lung injury, functioning to limit inflammation and lung permeability.
Keywords: inflammation, vascular permeability, hemorrhage
The response to acute lung injury is characterized by a series of events, including inflammation and extracellular matrix (ECM) revision, that can result in loss of the normal alveolar architecture. Throughout this process, ECM components are deposited, removed, or remodeled, enabling cell migration, neovascularization, and restructuring of the alveolar–capillary interface. The matrix metalloproteinase (MMP) gene family, a group of neutral proteinases collectively capable of degrading all components of the ECM, is thought to make important contributions to the inflammation response (1). Patients with acute respiratory distress syndrome (ARDS) or idiopathic pulmonary fibrosis (IPF) express increased levels of MMPs, suggesting their participation in the response to lung injury (2, 3). In experimental models of lung injury, MMPs contribute to leukocyte influx and altered vascular permeability at sites of inflammation (4–8).
The proteolytic activities of MMPs must be tightly controlled to restrict inflammation and ECM revision to areas of lung damage and to avoid destruction of healthy tissue. An important mechanism of MMP regulation is provided by the tissue inhibitors of metalloproteinases (TIMPs), which block MMP proteolytic activity. TIMPs are the major endogenous regulators of MMP activities in the tissue microenvironment. Four homologous TIMPs have been well characterized: TIMP-1,-2, -3, and -4 (reviewed by Gomez and coworkers [9]). Each inhibitor noncovalently binds to the MMP with 1:1 stoichiometry. Members of the TIMP family are distinguished by differences in their efficiency of MMP inhibition, their transcriptional regulation, and in their patterns of expression. In addition, TIMPs have been shown to possess biological functions that are independent of MMP-inhibitory activity, including stimulation of cell proliferation, induction or inhibition of apoptosis, and induction of MMP expression in vitro (10–13).
Indirect evidence suggests that members of the TIMP gene family may make important contributions to the response to lung injury. Levels of TIMP-1 are increased in the bronchoalveolar lavage (BAL) fluid recovered from patients with ARDS (2). Increased TIMP-1 immunoreactive protein levels have been identified in the lung biopsies of patients with ARDS or IPF (3, 14). Experimental models also suggest a potential role for TIMPs in the response to lung injury. We and others have found a significant and durable increase in TIMP-1 expression in bleomycin-induced lung injury (15, 16). However, direct evidence for the contribution of TIMP-1 in the response to acute lung injury has yet to be demonstrated.
To directly investigate the importance of TIMP-1 in acute lung injury, we have compared the inflammatory and fibrotic responses of TIMP-1 null mutation mice with those of wild-type littermates after bleomycin- or lipopolysaccharide-induced injury. The use of mice made deficient of TIMP-1 by targeted mutagenesis enables definitive evaluation of the role of TIMP-1 in the response to lung injury. We observed that TIMP-1 deficiency amplifies acute lung injury as determined by increased pulmonary neutrophilia, hemorrhage, and vascular permeability. TIMP-1 deficiency did not, however, alter the development of pulmonary fibrosis in the injured lung. Our results provide direct evidence that TIMP-1 contributes significantly to the regulation of acute lung injury, acting to limit inflammation and to preserve pulmonary vascular integrity.
MATERIALS AND METHODS
Mice
TIMP-1 null mutation (TIMP-1−/−) and wild-type (TIMP-1+/+) mice were bred from C57BL/6 mice heterozygous for a targeted disruption of exon 3 of the TIMP-1 gene (17). TIMP-2 null mutation (TIMP-2−/−) mice were bred from C57BL/6 mice heterozygous for a targeted disruption of exon 1 of the TIMP-2 gene (18). The genotypes of wild, TIMP-1−/− and TIMP-2−/− mice were confirmed by polymerase chain reaction (PCR) analysis performed on DNA prepared from the tails of 3-wk-old animals as previously described (17, 18). The null mutation phenotype was confirmed at the level of mRNA by quantitative real-time PCR using mouse TIMP-1– and TIMP-2–specific primer–probe sets and an ABI Prism 7000 Sequence Detection System following the manufacturer's protocols (Applied Biosystems Inc., Foster City, CA). All procedures involving the mice were approved by the Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee.
Bone Marrow Transplantation
To study in vivo effects of lung structural cell TIMP-1 deficiency or hematopoeitic cell TIMP-1 deficiency in bone marrow (BM) chimeric mice, TIMP-1−/− C57BL/6 (Ly5.2) mice were transplanted with TIMP-1+/+ C57BL/6 (Ly5.1) BM and TIMP-1+/+ C57BL/6 (Ly5.1) mice were transplanted with TIMP-1−/− C57BL/6 (Ly5.2) BM, respectively. The BM chimeras were prepared as previously described (19). In brief, recipient mice received 900 cGy of total body irradiation (TBI) in a single fraction from a linear accelerator at an exposure rate of 20 cGy/min on the day before transplantation. Donor marrow was obtained by femur flush. Chimerism was validated by FACS analysis of peripheral blood leukocytes using mAbs specific for Ly5.1 or Ly5.2 on Day 60 (Pharmingen, San Diego, CA). On Day 80 after transplantation, BM chimeric mice were treated with a single intratracheal injection of bleomycin (0.007 U/g body weight).
Bleomycin-Induced Lung Injury
Specific pathogen–free male 8- to 12-wk-old homozygous TIMP-1−/−, TIMP-2−/−, and wild-type mice received a single dose of 0.007 U/g body weight of bleomycin sulfate (Pharmacia, Inc., Kalamazoo, MI) in 50 μl of sterile saline via a tracheotomy under intraperitoneal avertin anesthesia (15). Control mice received saline alone. In experiments designed to assess survival to Day 30 or the development of lung fibrosis, a single intratracheal bleomycin dose of 0.0035 U/g body weight or saline was administered.
Lipopolysaccharide-Induced Lung Injury
TIMP-1−/− and wild-type mice received a single dose of 1 μg/g body weight of lipopolysaccharide (LPS) (Escherichia coli serotype 055:B5; Sigma Chemical Co., St. Louis, MO) in 50 μl of sterile saline via a tracheostomy under avertin anesthesia. Control mice received vehicle alone. The animals were killed at 4 h after instillation and the degree of lung inflammation was determined as described below.
BAL
The animals were killed by exsanguination under deep anesthesia at 0, 1, 2, 3, 5, and 7 d after instillation. After killing, the thorax was rapidly opened, the trachea cannulated with a 20-gauge catheter, and the lungs lavaged in situ with 1.0 ml sterile phosphate-buffered saline (PBS). The pulmonary circulation was perfused with 10 ml sterile PBS and the lungs were then either homogenized for cytokine or myeloperoxidase analysis, or inflation fixed in 4% paraformaldehyde for histologic examination.
An aliquot of the BAL fluid was immediately processed for total and differential cell count determination using a hemocytometer before and after hypotonic lysis of the red blood cells. The remainder of the BAL fluid was centrifuged at 500 × g to pellet cells, and the supernatants were stored in individual aliquots at −70°C. Leukocyte differential cell counts were performed on Wright-stained cytocentrifuge preparations (Leukostat; Fisher Scientific, Pittsburgh, PA), counting a total of 600 cells. BAL erythrocyte concentrations were calculated by subtracting the total leukocyte concentration from the total cell concentration for each specimen. BAL cell concentrations were expressed as cells per ml BAL fluid. IgM and albumin concentrations in the BAL fluid were quantified by ELISA (Bethyl Laboratories, Montgomery, TX).
Chemokine and Myeloperoxidase Measurement
The lungs were weighed and then homogenized in 1.0 ml of protease inhibitor cocktail (Roche Diagnostics Corp., Indianapolis, IN) using a hand-held homogenizer. The homogenate was divided into aliquots for cytokine or myeloperoxidase (MPO) measurements. For cytokine measurements, the aliquot was vigorously mixed with a buffer containing 0.5% Triton X-100, 150 mM NaCl, 15 mM Tris, 1 mM CaCl2, and 1 mM MgCl2 (pH 7.40), incubated for 30 min at 4°C, and then centrifuged at 10,000 × g for 20 min. The supernatants were stored at −70°C. Cytokine-induced neutrophll chemoattractant (KC), macrophage inflammatory protein (MIP)-2, tumor necrosis factor (TNF)-α, and LPS-induced CXC chemokine (LIX) concentrations in the BAL fluid and lung homogenates were quantified by ELISA (R&D Systems, Minneapolis, MN).
For MPO measurements, the homogenate aliquot was vigorously mixed with 50 mM potassium phosphate (pH 6.0), 5% hexadecyltrimethyl ammonium bromide (Sigma Chemical Co.), and 5 mM EDTA. The mixture was sonicated and spun at 12,0000 × g for 15 min at 25°C. The supernatants were stored at −70°C. The MPO activity of lung and skin was measured as previously described (20). Lung tissue was homogenized and sonicated in 2 ml of cold homogenization buffer (50 mM KH2PO4, 5 mM EDTA, 0.5% hexadecyl trimethylammonium bromide [HTAB; Sigma Chemical Co.]). After centrifugation (44,000 × g 15 min, 4°C) 200 μl supernatants were reacted with H2O2 (0.0005%) in the presence of 0-dianisidine dihydrochloride (0.167 mg/ml) for 15 min. The reaction was stopped by the addition of 50 μl of 10% sodium azide, and the optical density at 570 nm was measured. Protein concentrations of the tissue extracts were determined by the bicinchoninic acid assay (Pierce, Rockford, IL). The assays were performed in triplicate and MPO activity was expressed as the change in optical density/mg protein.
Morphometric Studies
For histologic analysis, the left lung was inflated with 4% neutral buffered paraformaldehyde instilled at 20 cm H2O pressure for 120 min. Lung sections from bleomycin-injured and control mice were stained with hematoxylin and eosin (H&E) or Masson trichrome stains. To confirm the identity of neutrophils, chloroacetate ester stain was performed on representative tissue sections. Total neutrophils, mononuclear inflammatory cells, and red blood cells within a 200 × 200 μm2 area of bronchovascular bundles were counted by light microscopic analysis of chloroacetate ester–stained sections using morphometric software (MetaMorph 4.6; Molecular Devices Corp., Sunnyvale, CA) to tabulate cells within 6,800 μm2 fields located around medium-sized airways (∼ 100 μm airway diameter). A total of three areas each measuring 40,000 μm2 were counted per specimen under ×1,000 magnification and the mean value calculated for each specimen. The morphometric studies were performed by an observer who was blinded to the experimental treatment groups.
Immunohistochemistry
Antigen retrieval was performed on 5-μm sections of mouse lung tissue using citrate buffer pH 6.0 at 85–90°C for 20 min. Tissue sections were blocked for endogenous avidin/biotin, endogenous peroxidase activity, and nonspecific protein (see online supplement). Sections were treated with affinity-purified goat polyclonal antibody to mouse recombinant TIMP-1 (1:40; R&D Systems) followed by biotinylated donkey anti-goat antibody (1:200; Jackson ImmunoResearch), which was visualized using an RTU Vectastain kit (Vector Laboratories, Burlingame, CA) and the Envision DAB kit (Dako Cytomation; Dako Corp., Carpinteria, CA). The sections were counterstained with hematoxylin. Digital photomicrographs were produced with a Nikon E600 photomicroscope and MetaMorph 4.6 software (Molecular Devices Corp.).
Gelatin Zymography
Gelatin zymography of BAL fluid (40 μl) was performed by electrophoresis on 10% SDS-polyacrylamide gel containing 1 mg/ml gelatin as previously described (21). The gel was washed in 2.5% Triton X-100 for 1 h and then incubated for 18 h in 50 mM Tris (pH 7.5), 200 mM NaCl, 5 mM CaCl2, and 0.02% Brij-35 at 37°C before staining with Coomasie blue. Digital images of the gels were captured (EagleEye System; Stratagene, La Jolla, CA) and the relative intensities of the lytic bands were determined using Image Quant software (Molecular Dynamics, Sunnyvale, CA).
PMN Emigration in the Skin
Intradermal injection of interleukin (IL)-8 induces the accumulation of neutrophils at the site of injection (22). A single intradermal injection of recombinant human IL-8 (50 ng in 50 μl saline; R&D Systems) was given to TIMP-1−/− and wild-type mice as previously described (22). In a separate site, each mouse received an injection of 50 μl of saline. After 4 h, the skin at each injection site was excised and either analyzed for MPO activity as a measure of neutrophil accumulation or fixed in 4% buffered paraformaldehyde for histologic assessment.
PMN Migration through Matrigel
The ability of murine BM-derived neutrophils to migrate in response to 5 nM recombinant KC through prototypic extracellular matrix secreted by the Engelbreth-Holm-Swarm tumor was evaluated using Biocoat Matrigel Cell Invasion Chambers (Becton Dickinson Labware, Bedford, MA) as previously described (23) (see online supplement). The number of cells that migrated to the underside of the filter in four random high-power fields (×400) was quantified for each of five filters for each genotype and condition. In addition, the total number of cells recovered from the lower compartment were counted on a hemocytometer in duplicate for each of five chambers for each genotype and condition.
Neutrophil Chemotaxis Assay
Neutrophil chemotaxis was measured with a fluorescent chemotaxis assay as previously described using calcein-labeled human neutrophils as the chemotactic targets (24) (see online supplement). All procedures using human neutrophils were approved by the Fred Hutchinson Cancer Research Center Human Subjects Committee.
Hydroxyproline Quantification
The mice were killed at Days 7, 28, 45, or 60 after instillation, and the degree of lung fibrosis was quantified by measurement of the lung hydroxyproline content of the right lung and by histologic analysis of the left lung. Total lung collagen content was measured by assaying lung hydroxyproline content after hydrolysis with 6 N HCl as previously described (25). Whole lung hydroxyproline values were measured in triplicate and expressed as μg/lung.
Blood Leukocyte Counts
Blood was collected by retro-orbital venous plexus sampling in silica-treated tubes containing sodium citrate. Complete blood counts were performed using a Celldyn 3500 automated cell counter (Abbott Labs, Santa Clara, CA). Leukocyte differentials were determined on Wright-stained blood smears.
Data Presentation and Statistical Analysis
All data are expressed as means ± SEM. Statistical differences between groups were determined using Student's t test assuming unequal variances. Differences were considered significant at the P < 0.05 level.
RESULTS
Neutrophil Inflammation Is Amplified in Bleomycin-Injured Lungs of TIMP-1–Deficient Mice
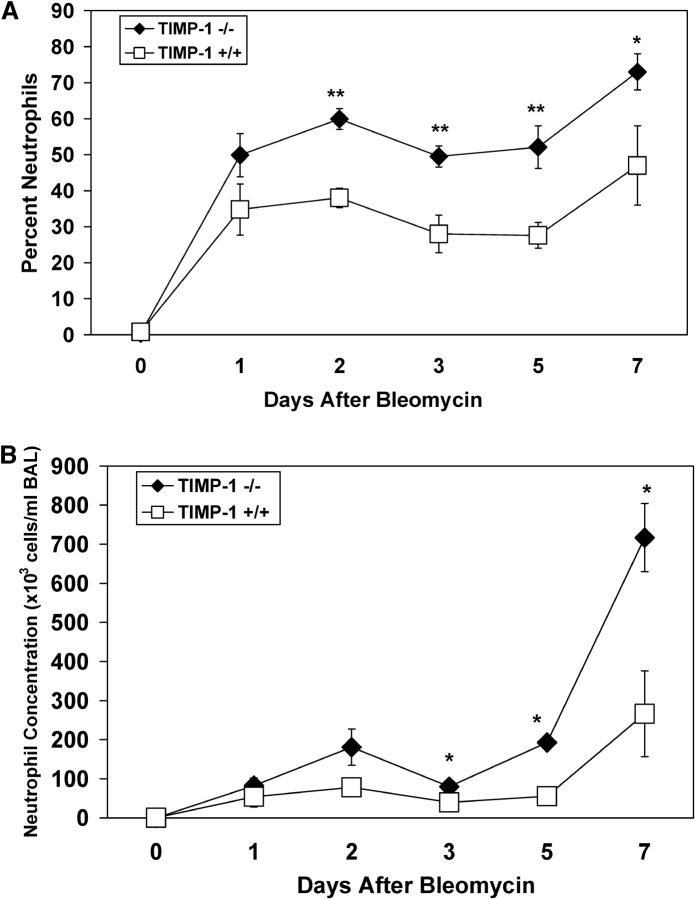
TIMP-1 mRNA and protein expression is selectively and markedly increased in bleomycin-induced lung injury (15). To determine whether the inflammatory response to lung injury was altered in the absence of TIMP-1, we measured the BAL leukocyte concentrations in TIMP-1–deficient and wild-type littermates after intratracheal bleomycin administration. Untreated mice of both genotypes had very low concentrations of neutrophils in the BAL (Figure 1). In the wild-type mice, there was a marked increase in the percentage and concentration of neutrophils in the BAL after bleomycin treatment. The neutrophil percentage and concentration in the BAL recovered from TIMP-1−/− mice were significantly higher than those of wild-type animals at comparable times after bleomycin instillation. By Day 3 after bleomycin treatment, the percentage and concentration of neutrophils in the BAL of TIMP-1−/− mice were ∼ 2.0-fold higher than those of similarly treated wild-type litter-mates. At Day 7 after bleomycin administration, the percentage and concentration of neutrophils in the BAL recovered from TIMP-1−/− mice were 1.5-fold and over 3.5-fold higher than that of the wild-type mice. We measured MPO concentrations in whole lung to quantify neutrophil accumulation in the lung parenchyma itself. At Day 7 after bleomycin exposure, the mean MPO activity in the lung homogenates of TIMP-1−/− mice was 1.9 ± 0.46 compared with 0.6 ± 0.13 OD/mg protein of wild-type animals (P = 0.03).
Figure 1.
Temporal changes in neutrophil percent and concentration in the BAL recovered from wild-type and TIMP-1−/− mice after bleomycin administration. Mean values (± SE) for neutrophil percent (A) and neutrophil concentration (B) in the BAL of TIMP-1+/+ (open squares) and TIMP-1−/− (filled diamonds) mice before or after a single intratracheal dose of bleomycin. Six TIMP-1+/+ and six TIMP-1−/− mice were analyzed at each time point. *P < 0.05, **P < 0.01. Saline-instilled mice had < 6,000 PMN/ml recovered in the BAL at Day 7 for each genotype.
In contrast, we found no significant difference in the concentrations of alveolar macrophages or lymphocytes recovered in the BAL from injured TIMP-1−/− versus wild-type mice. Furthermore, there was no difference in the total circulating leukocyte counts and the percentages of neutrophils, lymphocytes, monocytes, and eosinophils in the peripheral blood between the two genotypes at baseline or Day 7 after bleomycin administration (Table 1).
TABLE 1.
Circulating leukocytes in TIMP-1–deficient and wild-type mice (mean ± sem).
| Genotype | Condition | Total Number of Leukocytes (× 10−3/μl) |
Neutrophils (%) | Lymphocytes (%) | Monocytes (%) | Eosinophils (%) |
|---|---|---|---|---|---|---|
| TIMP-1 WT (n = 6) | Control | 7.6 ± 0.47 | 16.0 ± 3.4 | 78.0 ± 2.7 | 0 | 5.0 ± 1.1 |
| TIMP-1 KO (n = 8) | Control | 6.8 ± 0.36 | 16.0 ± 1.5 | 81.0 ± 1.6 | 0 | 3.0 ± 0.7 |
| P value | 0.2 | 0.83 | 0.38 | NS | 0.10 | |
| TIMP-1 WT (n = 5) | Day 7 Bleomycin | 5.4 ± 0.76 | 50.0 ± 8.5 | 47.6 ± 8.5 | 1 ± 0.8 | 0.8 ± 0.5 |
| TIMP-1 KO (n = 6) | Day 7 Bleomycin | 5.2 ± 0.78 | 52.3 ± 7.9 | 44.7 ± 6.8 | 1.8 ± 0.9 | 1.2 ± 0.7 |
| P value | 0.82 | 0.87 | 0.79 | 0.50 | 0.66 |
Definition of abbreviations: KO, knockout; TIMP-1, tissue inhibitor of metalloproteinase-1; WT, wild type.
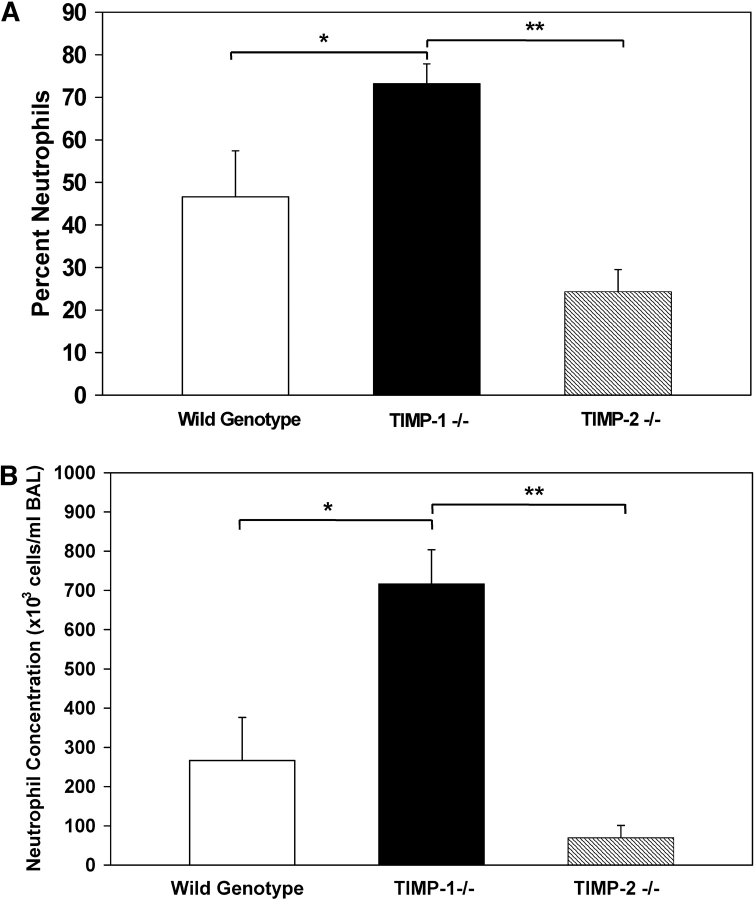
The exaggerated pulmonary neutrophilia observed in the injured TIMP-1−/−animals was not found in TIMP-2−/− mice. At Day 7 after bleomycin administration, the BAL neutrophil percentage and concentration of TIMP-1−/− mice were 3-fold and 10-fold higher, respectively, than those of similarly treated TIMP-2−/− animals (Figure 2). By comparison, the concentration and percentage of neutrophils recovered in the BAL of TIMP-2−/− mice were not statistically different from those of wild-type mice at Day 7. Our results suggest that the exaggerated pulmonary neutrophilia is specific for TIMP-1 deficiency.
Figure 2.
Neutrophil percent and concentration in the BAL of wild-type, TIMP-1−/−, and TIMP-2−/− mice at Day 7 after bleomycin administration. Mean values (± SE) for neutrophil percent (A) and neutrophil concentration (B) in the BAL of wild-type (white column), TIMP-1−/− (black column), and TIMP-2−/− (striped column) mice at Day 7 after a single intratracheal dose of bleomycin. Six mice were analyzed for each genotype. *P < 0.05, comparison of TIMP-1+/+ with TIMP-1−/−; **P < 0.001 comparison of TIMP-1−/− with TIMP-2−/−.
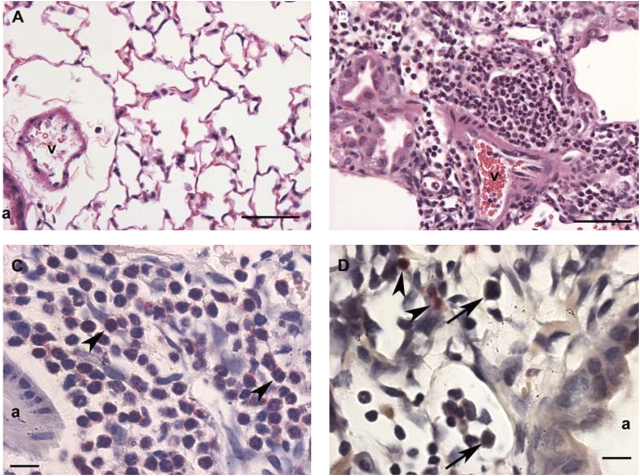
Lung histology performed on tissue sections recovered at Day 7 after bleomycin treatment showed a striking increase in neutrophil accumulation in TIMP-1−/− mice compared with wild-type animals (Figure 3). As anticipated, TIMP-1−/− mouse lungs had little evidence of inflammation after saline instillation (Figure 3A). In contrast, extensive inflammatory cell accumulation was observed in the peri-airway regions of TIMP-1−/− lungs after bleomycin treatment (Figure 3B). Neutrophil-specific granule staining with chloroacetate ester of bleomycin injured TIMP-1−/− mouse lungs showed abundant neutrophil accumulation in the areas surrounding bronchovascular bundles and in the alveolar compartment (Figure 3C). By comparison, wild-type mouse lungs at Day 7 after bleomycin administration showed predominantly a mononuclear cell infiltration in the same regions (Figure 3D).
Figure 3.
Lung histology for TIMP-1−/− and wild-type mice at Day 7 after saline or bleomycin administration. (A) TIMP-1−/− mouse lung at Day 7 after saline instillation shows little evidence of inflammation. a, airway; v, blood vessel (H&E, magnification: ×400; bar = 50 μm). (B) TIMP-1−/− mouse lung at Day 7 after bleomycin instillation shows extensive inflammatory cell accumulation in the peri-airway regions (H&E, magnification: ×400; bar = 50 μm). (C) Higher magnification of image in B stained with chloroacetate ester shows extensive neutrophil accumulation (arrowheads) in the alveolar compartment at Day 7 after bleomycin administration to TIMP-1−/− mice. (Leder stain, magnification: ×1,000; bar = 10 μm). (D) Wild-type mouse lung at Day 7 after bleomycin instillation shows predominantly mononuclear cell infiltration (arrows) rather than neutrophil accumulation (arrowheads) in the alveolar compartment. (Leder stain, magnification: ×1,000; bar = 10 μm).
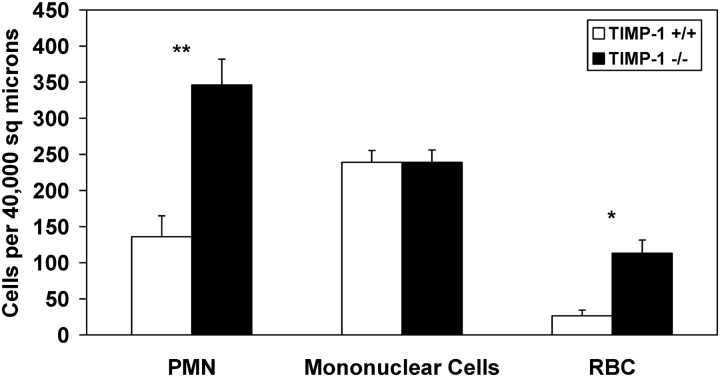
Morphometric studies were performed to quantify the differences in neutrophil accumulation found in the lung tissue of TIMP-1−/− compared with wild-type mice after bleomycin injury. We counted the total number of inflammatory cells and erythrocytes that were present in 40,000 μm2 areas centered around medium-sized bronchovascular bundles (airway diameters ∼ 100 μm). We found a 2.5-fold increase in the number of neutrophils per unit area of lung tissue in the bronchovascular regions of injured TIMP-1−/− mice compared with similarly treated wild-type animals (Figure 4). In contrast, we found no significant difference in the number of mononuclear inflammatory cells per unit area in the injured lungs of TIMP-1−/− compared with wild-type mice. These results were consistent with the exaggerated pulmonary neutrophilia found in the BAL and lung homogenate specimens of injured TIMP-1−/− mice.
Figure 4.
Morphometric analysis of leukocyte and erythrocyte concentrations in the lungs of TIMP-1−/− and wild-type mice at Day 7 after bleomycin instillation. Mean values (± SE) for neutrophils, mononuclear inflammatory cells, and erythocytes within 40,000 μm2 areas centered on medium-sized bronchovascular bundles (airway diameter ∼ 100 μm) for TIMP-1+/+ (white columns) and TIMP-1−/− (black columns) at Day 7 after bleomycin instillation. Three 40,000 μm2 areas were analyzed for each of five TIMP-1+/+ and five TIMP-1−/− mice. *P < 0.001, **P < 0.01.
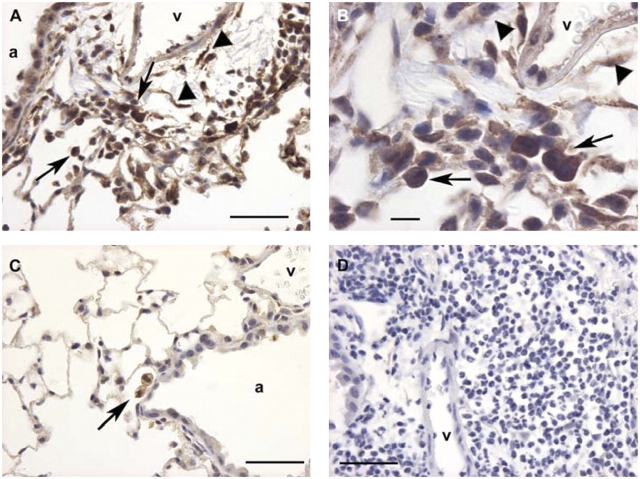
Our previous in situ hybridization studies demonstrated that TIMP-1 transcripts localized to inflammatory leukocytes as well as structural cells of the lung after bleomycin injury (15). To examine the location of TIMP-1 protein in the injured lung, we performed immunohistochemistry on lung tissue recovered from saline- or bleomycin-treated wild-type mice. At 5 d after saline instillation we found infrequent, faint TIMP-1 staining of alveolar macrophages (data not shown). In contrast, we observed strong TIMP-1 protein staining of alveolar macrophages, airway epithelial cells, and lung interstitial cells in areas of injury at 5 d after bleomycin treatment (Figures 5A and 5B). In areas remote from bleomycin damage, faint TIMP-1 staining was detected infrequently in alveolar macrophages (Figure 5C). Specificity of the TIMP-1 antibody was confirmed using an irrelevant isotype antibody (Figure 5D).
Figure 5.
Localization of TIMP-1 protein by immunohistochemistry in bleomycin-injured and control mouse lung. (A) Wild-type mouse lung at Day 5 after bleomycin instillation incubated with antibody to murine TIMP-1 demonstrates strong TIMP-1 immunostaining of airway epithelial cells, alveolar macrophages (arrows), and lung interstitial cells (arrowheads) within a focus of injury in the peri-airway region. a, airway; v, blood vessel (magnification: ×400; bar = 50 μm). (B) Higher magnification of A shows that TIMP-1 protein localizes to alveolar macrophages (arrows) and lung interstitial cells (arrowheads) within the focus of lung injury (magnification: ×1,000; bar = 10 μm). (C) Wild-type mouse lung at Day 5 after bleomycin instillation incubated with murine TIMP-1 antibody demonstrates only occasional immunostaining of macrophages (arrow) in an area of lung remote from damage (magnification: ×400; bar = 50 μm). (D) Wild-type mouse lung at Day 5 after bleomycin administration incubated with isotype control antibody shows no background staining within a focus of injury (magnification: ×400; bar = 50 μm).
TIMP-1 Expression by Parenchymal Cells Is Crucial in Regulating Pulmonary Neutrophilia
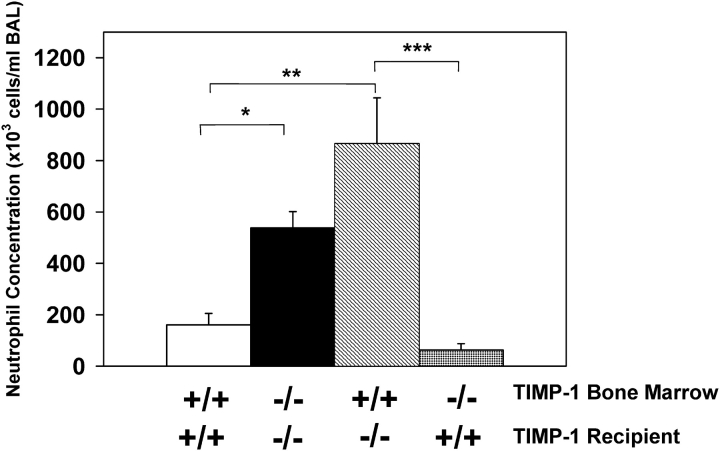
To determine whether TIMP-1 deficiency in either inflammatory cells or in lung parenchymal cells was sufficient to produce exaggerated pulmonary neutrophilia, bleomycin lung injury was performed in BM chimeric mice that lacked TIMP-1 either in the hematopoeitic cell compartment or in the lung structural cells. At Day 7 after bleomycin instillation, TIMP-1−/− recipient mice of TIMP-1−/− BM had a > 3-fold increase in BAL neutrophil concentration compared with wild-type recipient mice of wild-type BM, consistent with our previous observations (Figure 6). We found a > 5-fold increase in BAL neutrophil concentration at Day 7 after bleomycin administration to TIMP-1−/− recipient mice of wild-type BM compared with similarly exposed wild-type recipient mice of wild-type BM. Moreover, there was a 1.6-fold increase in BAL neutrophil concentration of TIMP-1−/− recipient mice of wild-type BM compared with TIMP-1−/− recipient mice of TIMP-1−/− BM, although this difference was not statistically significant. In contrast, the BAL neutrophil concentration found in wild-type recipients of TIMP-1−/− BM was no different than that of wild-type recipient mice of wild-type BM after bleomycin treatment. From these data, we conclude that TIMP-1 production by lung parenchymal cells at sites of bleomycin injury is crucial in regulating the inflammatory response characteristic of the TIMP-1–deficient phenotype. Furthermore, TIMP-1 expression by inflammatory cells may facilitate neutrophil egress into the alveolar compartment when parenchymal cells are deficient of TIMP-1.
Figure 6.
Neutrophil concentration in the BAL of TIMP-1 BM chimeric mice at Day 7 after bleomycin administration. Mean values (± SE) for neutrophil concentration in the BAL of TIMP-1+/+ recipients of TIMP-1+/+ BM (white column), TIMP-1−/− recipients of TIMP-1−/− BM (black column), TIMP-1−/− recipients of TIMP-1 +/+ BM (striped column), and TIMP-1+/+ recipients of TIMP-1−/− BM (checked column) at Day 7 after a single intratracheal dose of bleomycin. At least seven mice were analyzed per group. *P < 0.05, **P < 0.01, ***P < 0.005.
Pulmonary Neutrophilia Is Exaggerated in LPS-Injured Lungs of TIMP-1–Deficient Mice
An exaggerated pulmonary neutrophilia was also observed in TIMP-1–deficient mice after the intratracheal administration of LPS. At 4 h after a single intratracheal dose of LPS, the concentrations of total leukocytes and neutrophils in the BAL recovered from TIMP-1−/− mice were > 3-fold higher than those of similarly treated wild-type animals (Figure E1 in the online supplement). We found no significant difference in the concentration of BAL macrophages or lymphocytes between the two genotypes after LPS exposure. These results indicate that the amplified pulmonary neutrophilia observed in TIMP-1–deficient lungs occurs in response to different inflammatory stimuli.
Lung Hemorrhage Is Increased in the Injured Lungs of TIMP-1–Deficient Mice
To determine whether TIMP-1 deficiency increases the intensity of the lung injury, we measured erythrocyte concentrations in the BAL recovered from control and bleomycin-injured TIMP-1−/− and wild-type mice. As expected, animals that received saline alone had very low concentrations of erythrocytes recovered in the BAL (data not shown). In the injured wild-type mice we observed a marked increase in the BAL erythrocyte concentration at Day 7 after bleomycin instillation (Figure E2). At the same time point after injury, the erythrocyte concentration in BAL recovered from TIMP-1−/− mice was > 3-fold higher than that of the wild-type animals. Likewise, the number of erythrocytes within alveoli per unit area of lung was > 4-fold higher in bleomycin injured lungs of TIMP-1−/− versus wild-type mice at Day 7 (Figure 4).
Pulmonary Vascular Permeability Is Increased in the Injured Lungs of TIMP-1–Deficient Mice
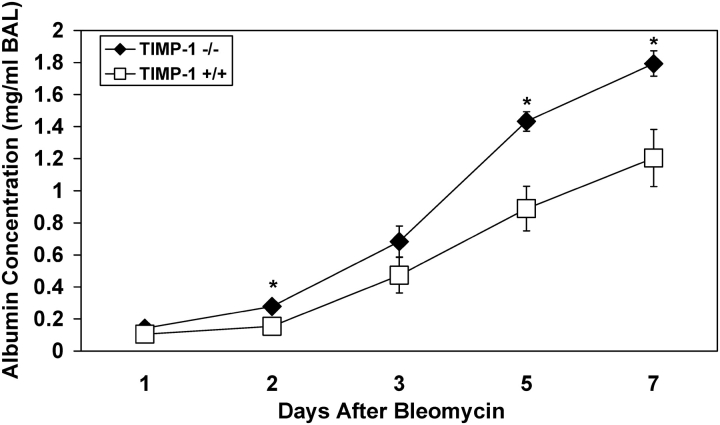
Changes in pulmonary vascular permeability were evaluated by quantifying the concentrations of the serum macromolecules albumin and IgM in the BAL after bleomycin exposure of TIMP-1−/− and wild-type mice. As expected, there was a large increase in BAL albumin and IgM in the injured lungs of wild-type mice as compared with saline control animals, reflecting increased vascular permeability. BAL fluid from TIMP-1−/− mice had a significantly higher concentration of serum macromolecules compared with wild-type animals after bleomycin instillation. Albumin concentrations in the BAL fluid of TIMP-1−/− mice were increased > 1.5-fold at Days 2, 5, and 7 after bleomycin instillation compared with similarly treated wild-type mice (Figure 7). Likewise, there was an increase in IgM concentrations in the BAL of TIMP-1−/− mice during the first week after bleomycin treatment compared with wild-type mice; however, this difference did not reach statistical significance (Figure E3). The observed differences in BAL albumin and IgM concentrations between TIMP-1−/− and wild-type mice are consistent with increased vascular permeability for serum macromolecules in the injured lung when TIMP-1 is absent.
Figure 7.
Temporal changes in albumin concentration in the BAL recovered from wild-type and TIMP-1−/− mice after bleomycin administration. Mean values (± SE) for albumin concentration in the BAL of TIMP-1+/+ (open squares) and TIMP-1−/− (solid diamonds) mice before or after a single intratracheal dose of bleomycin. Six TIMP-1+/+ and six TIMP-1−/− mice were analyzed at each time point. *P < 0.03.
Weight Loss and Mortality Are Increased in TIMP-1–Deficient Mice after Lung Injury
To determine the biological significance of TIMP-1 deficiency after acute lung injury, we tracked weight changes and survival following bleomycin exposure. TIMP-1−/− mice developed a 33.3 ± 0.8% reduction in body weight compared with a 26.6 ± 3.0% decrease for wild-type animals at Day 7 after bleomycin administration (P < 0.05) (Figure E4A). Likewise, TIMP-1−/− mice had a survival of 76% compared with 100% for wild-type littermates by Day 30 after bleomycin treatment (P = 0.056) (Figure E4B). The differences in weight loss and survival observed between TIMP-1−/− and wild-type mice were consistent with the exaggerated lung injury of the TIMP-1−/− animals.
Gelatinase B Activity Is Amplified in the Alveolar Compartment of Injured TIMP-1–Deficient Mice
To assess the presence of gelatinase A and B in the alveolar compartment of bleomycin-injured TIMP-1–deficient and wild-type mice, we analyzed BAL fluid by substrate gel electrophoresis using gelatin. At Day 3 after bleomycin instillation, low levels of the pro- and mature isoforms of gelatinase B (MMP-9) were identified in the BAL fluid recovered from both genotype mice (Figure E5). At this time point no gelatinase A (MMP-2) was detectable in the samples. By Day 7 after bleomycin exposure, gelatinase B levels in the BAL fluid recovered from TIMP-1 −/− mice were more than twice that recovered from comparably treated wild-type littermates. Likewise, we observed 3-fold higher levels of gelatinase B proenzyme in the BAL specimens collected from injured TIMP-1−/− mice compared with wild-type animals; however, this difference did not achieve statistical significance (P = 0.07). Gelatinolytic activity was inhibited by development of the zymogram in the presence of 10 mM 1,10 phenathroline, confirming the presence of metalloproteinase activity in the BAL fluids (data not shown). In contrast to gelatinase B, BAL fluid from wild-type mice contained 1.3-fold higher (P < 0.03) levels of gelatinase A compared with TIMP-1−/− mice at Day 7 after bleomycin administration. Thus, acute lung injury in TIMP-1–deficient mice was accompanied by exaggerated gelatinase B but diminished gelatinase A activity in the alveolar compartment relative to similarly treated wild-type littermates.
TIMP-1 Deficiency Does Not Affect Neutrophil Chemotactic Activity
To determine if the exaggerated neutrophil accumulation observed in the absence of TIMP-1 was due to differences in neutrophil chemotactic activity, we quantified KC, MIP-2, TNF-α, and LIX concentrations in the BAL fluid and lung homogenates of bleomycin-injured wild-type and TIMP-1−/− mice at Days 1, 2, 3, 5, and 7 (Figure E6). The concentrations of KC and MIP-2 in the BAL and lung homogenates increased significantly at Day 1 after bleomycin administration, but were similar for the wild-type and TIMP-1−/− mice. The concentration of TNF-α in the BAL increased significantly at Day 2 after bleomycin treatment; however, the increase was comparable for wild-type and TIMP-1−/− animals. Likewise, the concentrations of TNF-α in lung homogenates were similar for wild-type and TIMP-1−/− mice after bleomycin exposure. The concentrations of LIX in the BAL and lung homogenates also were comparable between the two genotypes at baseline and after bleomycin injury. These results were consistent with our observation that the in vitro neutrophil chemotactic activity present in the BAL fluid at Days 3 and 7 after bleomycin instillation was similar for TIMP-1−/− and wild-type mice (data not shown).
A second possible explanation for the exaggerated pulmonary neutrophilia found in the injured lungs of TIMP-1–deficient mice might be due to differences between the two genotypes with respect to chemotactic responses of the neutrophils. To address this possibility, we measured neutrophil recruitment into the skin after intradermal injection of recombinant human IL-8, a well-established neutrophil chemoattractant. Neutrophil recruitment at 4 h in response to IL-8 was predominantly extravascular, so measurement of whole skin MPO activity was considered indicative of neutrophil emigration. Skin MPO activity increased more than 5-fold by IL-8 injection and was comparable for both wild-type and TIMP-1−/− mice (data not shown). These results suggest that the in vivo chemotactic response of neutrophils in uninjured tissue is unaffected by TIMP-1 deficiency. Similarly, the in vitro migration of neutrophils through matrigel membrane in response to the chemoattractant KC was no different for TIMP-1–deficient compared with wild-type neutrophils (data not shown).
Pulmonary Fibrosis Is Unaltered in the Absence of TIMP-1
To determine if the fibrotic response to lung injury was altered in the absence of TIMP-1, we measured lung hydroxyproline content of the injured lungs at Days 7, 28, 45, and 60 after bleomycin administration. The lung hydroxyproline content of bleomycin-injured lungs was significantly higher than that of saline-treated controls at Days 28, 45, and 60 for both genotypes. However, there was no significant difference in lung hydroxyproline content for TIMP-1−/− mice compared with similarly treated wild-type mice at any time point (Figure E7). These results suggest that TIMP-1 deficiency does not significantly alter collagen accumulation after bleomycin-induced lung injury.
DISCUSSION
The major goal of this study was to investigate the role of TIMP-1 in the response to acute lung injury. Our strategy was to determine whether inflammatory cell and serum macromolecule accumulation in the acutely injured lung were amplified in mice genetically engineered to lack TIMP-1. We found that pulmonary neutrophilia, hemorrhage, and vascular permeability were significantly higher after bleomycin injury in TIMP-1–deficient mice than in wild-type littermates. We demonstrated that TIMP-1 expression by lung parenchymal cells, but not inflammatory cells, was crucial in limiting the response to acute injury. Furthermore, the TIMP-1–deficient phenotype in response to acute lung injury was not found in TIMP-2–deficient mice. We observed that the level of gelatinase B activity in the alveolar lining fluid of injured lungs was significantly greater in the absence of TIMP-1. In contrast, there was no significant difference in collagen accumulation and lung fibrosis between TIMP-1–deficient and wild-type mice after bleomycin treatment. Finally, we found that the absence of TIMP-1 did not alter neutrophil chemotactic factor accumulation in the alveolar lining fluid of the injured lung nor neutrophil migration in response to chemotactic stimuli in vitro or in vivo.
Our study provides direct evidence that TIMP-1 contributes significantly to the regulation of the response to acute lung injury. The increase in neutrophil, erythrocyte, and serum macromolecule accumulation in injured TIMP-1−/− lungs supports the concept that TIMP-1 functions to limit the injury response. Our finding that TIMP-1 deficiency results in increased lung permeability and hemorrhage after injury is novel. These results are consistent with our previous findings that TIMP-1 expression was induced in bleomycin-exposed mouse lungs and localized to areas of acute injury (15).
Our finding of amplified pulmonary neutrophilia after bleomycin injury in TIMP-1−/− mice is consistent with the observation that TIMP-1 deficiency augments the corneal response to infection. Kernacki and coworkers reported increased corneal opacification and neutrophil accumulation in response to Pseudomonas aeruginosa infection in mice treated systemically with anti–TIMP-1 neutralizing antibody (26). Osiewicz and colleagues observed that TIMP-1−/− mice were hyper-resistant to corneal infection with P. aeruginosa and that the increased resistance to infection was neutrophil and complement dependent (27). Similar to our study, these authors found that TIMP-2−/− mice did not display the hyper-resistant phenotype. However, in contrast to our findings, Osiewicz reported that the hyper-resistance to corneal infection was suppressed by TIMP-1 expression by either hematopoeitic cells or ocular parenchymal cells. In that model, the hyper-resistant phenotype was dependent on the presence of gelatinase B, stromelysin-1, and matrilysin (28).
The enhanced response to lung injury in the absence of TIMP-1 was the opposite of the effect observed in animals with targeted disruption of matrix metalloproteinase genes. Bleomycin lung injury in matrilysin (MMP-7)-deficient mice significantly reduced neutrophil recruitment to the alveolar compartment by blocking the release of neutrophil chemotactic factor KC from the epithelial cell surface (4). Mice deficient in stromelysin-1 (MMP-3) or gelatinase B (MMP-9) developed less severe lung injury, as determined by pulmonary hemorrhage and changes in vascular permeability, compared with wild-type animals after immune complex instillation (5). The reduction in lung injury observed in stromelysin-deficient mice was associated with reduced neutrophil accumulation in the injured lungs. Similarly, gelatinase A (MMP-2)–deficient mice developed less inflammatory cell accumulation and lower concentrations of the chemokine CCL11 in the alveolar compartment compared with wild-type animals after allergen challenge (6). In contrast to animals deficient in matrilysin or gelatinase A, TIMP-1−/− mice did not show a difference in BAL chemotactic activity for neutrophils nor chemotactic factor concentrations compared with wild-type littermates after lung injury.
The increased levels of gelatinase B in the BAL fluid of injured TIMP-1−/− mice compared with wild-type littermates suggest the presence of increased proteinase activity in the alveolar compartment. The TIMP-1–deficient phenotype observed following lung injury may be due, in part, to increased gelatinase B activity, because this MMP has been shown to contribute to pulmonary hemorrhage and edema in the injured lung (5). However, the exaggerated neutrophilia found in injured TIMP-1−/− lungs cannot be attributed to increased gelatinase B activity, because neutrophil influx is independent of this proteinase (5, 29). The amplified neutrophil accumulation is more likely the result of increased proteolytic activity of other MMPs in the absence of TIMP-1.
The amplified pulmonary neutrophilia, hemorrhage, and vascular permeability observed in injured TIMP-1−/− lungs suggests that TIMP-1 functions to preserve the alveolar–capillary barrier, possibly by inhibiting proteolytic degradation of intercellular junctions or the alveolar basement membrane. MMP inhibitors are thought to enhance cell adhesion by preventing cadherin ectodomain cleavage, thus stabilizing cadherin-mediated cell–cell contact. In support of this possible mechanism, MMP inhibitors have been shown to reduce VE cadherin shedding by cultured endothelial cells (30) and to induce cadherin expression and cell–cell contact by fibroblasts in vitro (31). In response to bleomycin injury, matrilysin has been shown to mediate E cadherin shedding from airway and alveolar epithelia (32). MMP inhibitors have also been reported to stabilize focal adhesion contacts and promote p125FAK phosphorylation, suggesting that TIMP-1 may also function to preserve integrin–extracellular matrix adhesion and focal contact assembly (31).
TIMP-1 deficiency did not alter lung collagen accumulation or pulmonary fibrosis after bleomycin injury. This finding was not unanticipated because other investigators have reported that the lack of TIMP-1 did not alter renal interstitial fibrosis in murine models (33). We have previously shown that bleomycin injury induces the durable expression of both TIMP-1 and TIMP-2 in the alveolar and interstitial compartments (15). The production of TIMP-2 by TIMP-1−/− mice may serve a compensatory function in regulating collagenase activity in bleomycin-injured TIMP-1–deficient lungs.
The TIMP-1–deficient phenotype we observed after bleomycin lung injury differed from that reported by Osiewicz and coworkers (27) after pseudomonal corneal infection with respect to suppression of the phenotype only by TIMP-1 expression by lung parenchymal cells. This distinction suggests that the injury response differs with respect to the organ involved and the cellular sources of TIMP-1. We infer from our results that parenchymal cell TIMP-1 plays a crucial role in regulating the response to lung injury. Our previously published work using in situ hybridization indicated that lung fibroblasts were a prominent source of TIMP-1 expression in areas of bleomycin-injured lung (15). In addition, TIMP-1 is expressed by fibroblasts in the lung biopsies of patients with ARDS (3). Taken together, these findings suggest that fibroblast-derived TIMP-1 is important in the regulation of the response to acute lung injury.
Our results also suggest that TIMP-1 expression by inflammatory cells may facilitate neutrophil egress into an area of injury when parenchymal cells are deficient of TIMP-1. In support of this possibility, Anthwal and colleagues have shown that TIMP-1 is inducibly expressed on the neutrophil surface and is required for MMP-9 binding to the neutrophil cell surface (34). Their results indicate that membrane-bound TIMP-1 functions to anchor MMP-9 to the leukocyte surface, thereby enhancing MMP-9 activity in the pericellular environment of the neutrophil. In our chimeric mice that expressed inflammatory cell–derived TIMP-1 in the absence of parenchymal cell–derived TIMP-1, neutrophil extravasation may have been amplified by enhanced MMP-9 activity in the pericellular environment that was unopposed in the absence of lung structural cell TIMP-1 secretion.
Our finding that TIMP-2−/− mice displayed the same level of pulmonary neutrophil accumulation as wild-type mice after bleomycin exposure stands in contrast to the observation of Gipson and coworkers (35). In their study, antibody-induced neutralization of endogenous TIMP-2 resulted in intensified lung damage as measured by lung albumin and neutrophil accumulation after immune complex instillation in rats. The apparent discrepancy between these studies may be due to differences in the species and injury models used.
In conclusion, our studies demonstrate that TIMP-1 deficiency amplifies acute lung injury as manifested by increased pulmonary neutrophilia, hemorrhage, and serum macromolecule extravasation. The amplification of acute lung injury is specific to TIMP-1 deficiency and suggests that TIMP-1 functions to preserve the alveolar–capillary barrier. TIMP-1–deficient parenchymal cells appear to be primarily responsible for this amplified response to acute injury, indicating that TIMP-1 expression by lung structural cells makes a crucial contribution to limiting acute injury of the lung.
Supplementary Material
Acknowledgments
None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
The authors thank Dr. Joan G. Clark, Dr. William C. Parks, and Dr. John M. Harlan for their helpful discussions. They also thank Lisa McLemore and Venus Wong for excellent technical assistance.
This study was supported by an American Heart Association Research grant 0151159Z (D.K.M.), an American Lung Association of Washington Research Grant (D.K.M.), and grant HL63994 to two authors (D.K.M. and R.C.H.) from the National Institutes of Health.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
References
- 1.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617–629. [DOI] [PubMed] [Google Scholar]
- 2.Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, Dayer J-M. Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med 1996;154:346–352. [DOI] [PubMed] [Google Scholar]
- 3.Hayashi T, Stetler-Stevenson WG, Fleming MV, Fishback N, Koss MN, Liotta LA, Ferrans VJ, Travis WD. Immunohistochemical study of metalloproteinases and their tissue inhibitors in the lungs of patients with diffuse alveolar damage and idiopathic pulmonary fibrosis. Am J Pathol 1996;149:1241–1256. [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell 2002;111:635–646. [DOI] [PubMed] [Google Scholar]
- 5.Warner RL, Beltran L, Younkin EM, Lewis CS, Weiss SJ, Varani J, Johnson KJ. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am J Respir Cell Mol Biol 2001;24:537–544. [DOI] [PubMed] [Google Scholar]
- 6.Corry DB, Rishi K, Kanellis J, Kiss A, Song LZ, Xu J, Feng L, Werb Z, Kheradmand F. Decreased allergic lung inflammatory cell egression and increased susceptibility to asphyxiation in MMP2-deficiency. Nat Immunol 2002;3:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mulligan MS, Desrochers PE, Chinnaiyan AM, Gibbs DF, Varani J, Johnson KJ, Weiss SJ. In vivo suppression of immune complex-induced alveolitis by secretory leukoproteinase inhibitor and tissue inhibitor of metalloproteinases 2. Proc Natl Acad Sci USA 1993;90:11523–11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbs DF, Warner RL, Weiss SJ, Johnson KJ, Varani J. Characterization of matrix metalloproteinases produced by rat alvelolar macrophages. Am J Respir Cell Mol Biol 1999;20:1136–1144. [DOI] [PubMed] [Google Scholar]
- 9.Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol 1997;74:111–122. [PubMed] [Google Scholar]
- 10.Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. FEBS Lett 1992;298:29–32. [DOI] [PubMed] [Google Scholar]
- 11.Hayakawa T, Yamashita K, Ohuchi E, Shinagawa A. Cell growth-promoting activity of tissue inhibitor of metalloproteinases-2 (TIMP-2). J Cell Sci 1994;107:2373–2379. [DOI] [PubMed] [Google Scholar]
- 12.Guedez L, Stetler-Stevenson WG, Wolff L, Wang J, Fukushima P, Monsoor A. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J Clin Invest 1998;102:2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clark IM, Powell LK, Cawston TE. Tissue inhibitor of metalloproteinases (TIMP-1) stimulates the secretion of collagenase from human skin fibroblasts. Biochem Biophys Res Commun 1994;203:874–880. [DOI] [PubMed] [Google Scholar]
- 14.Selman M, Ruiz V, Cabrera S, Segura L, Ramírez R, Barrios R, Pardo A. TIMP-1, -2, -3, and -4 in idiopathic pulmonary fibrosis: a prevailing nondegradative lung microenvironment? Am J Physiol Lung Cell Mol Physiol 2000;279:L562–L574. [DOI] [PubMed] [Google Scholar]
- 15.Madtes DK, Elston AL, Kaback LA, Clark JG. Selective induction of tissue inhibitor of metalloproteinase-1 in bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2001;24:599–607. [DOI] [PubMed] [Google Scholar]
- 16.Swiderski RE, Dencoff JE, Floerchinger CS, Shapiro SD, Hunninghake GW. Differential expression of extracellular matrix remodeling genes in a murine model of bleomycin-induced pulmonary fibrosis. Am J Pathol 1998;152:821–828. [PMC free article] [PubMed] [Google Scholar]
- 17.Soloway PD, Alexander CM, Werb Z, Jaenisch R. Targeted mutagenesis of TIMP-1 reveals that lung tumor invasion is influenced by TIMP-1 genotype of the tumor but not by that of the host. Oncogene 1996;13:2307–2314. [PubMed] [Google Scholar]
- 18.Wang Z, Juttermann R, Soloway PD. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem 2000;275:26411–26415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen W, Chatta GS, Rubin WD, Clark JG, Hackman RC, Madtes DK, Liggitt DH, Kusunoki Y, Martin PJ, Cheever MA. T cells specific for a polymorphic segment of CD45 induce graft-versus-host disease with predominant pulmonary vasculitis. J Immunol 1998;161:909–918. [PubMed] [Google Scholar]
- 20.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982;78:206–209. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Stetler-Stevenson WG. Quantitative zymography: detection of picogram quantities of gelatinases. Anal Biochem 1994;218:325–329. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, Till GO, Diaz LA. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest 1997;100:1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delclaux C, Delacourt C, D'Ortho MP, Boyer V, Lafuma C, Harf A. Role of Gelatinase B and Elastase in human polymorphonuclear neutrophil migration across basement membrane. Am J Respir Cell Mol Biol 1996;14:288–295. [DOI] [PubMed] [Google Scholar]
- 24.Frevert CW, Wong VA, Goodman RB, Goodwin R, Martin TR. Rapid fluorescence-based measurement of neutrophil migration in vitro. J Immunol Methods 1998;213:41–52. [DOI] [PubMed] [Google Scholar]
- 25.Madtes DK, Elston AL, Hackman RC, Dunn AR, Clark JG. Transforming growth factor-α deficiency reduces pulmonary fibrosis in transgenic mice. Am J Respir Cell Mol Biol 1999;20:924–934. [DOI] [PubMed] [Google Scholar]
- 26.Kernacki KA, Barrett R, Hazlett LD. Evidence for TIMP-1 protection against P. aeruginosa-induced corneal ulceration and perforation. Invest Ophthalmol Vis Sci 1999;40:3168–3176. [PubMed] [Google Scholar]
- 27.Osiewicz K, McGarry M, Soloway PD. Hyper-resistance to infection in TIMP-1-deficient mice is neutrophil dependent but not immune cell autonomous. Ann NY Acad Sci 1999;878:494–496. [DOI] [PubMed] [Google Scholar]
- 28.Lee MM, Yoon BJ, Osiewicz K, Preston M, Bundy B, van Heeckeren AM, Werb Z, Soloway PD. Tissue inhibitor of metalloproteinase-1 regulates resistance to infection. Infect Immun 2005;73:661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Betsuyaku T, Shipley JM, Liu Z, Senior RM. Neutrophil emigration in the lungs, peritoneum, and skin does not require gelatinase B. Am J Respir Cell Mol Biol 1999;20:1303–1309. [DOI] [PubMed] [Google Scholar]
- 30.Herren B, Levkau B, Raines EW, Ross R. Cleavage of β-catenin and plakoglobin and shedding of VD-cadherin during endothelial apoptosis: evidence for a role for caspases and metalloproteinases. Mol Biol Cell 1998;9:1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ho AT, Voura EB, Soloway PD, Watson KLM, Khokha R. MMP inhibitors augment fibroblast adhesion through stabilization of focal adhesion contacts and up-regulation of cadherin function. J Biol Chem 2001;276:40215–40224. [DOI] [PubMed] [Google Scholar]
- 32.McGuire JK, Li Q, Parks WC. Matrilysin (Matrix Metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol 2003;162:1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim H, Oda T, López-Guisa J, Wing D, Edwards DR, Soloway PD, Eddy AA. TIMP-1 deficiency does not attenuate interstitial fibrosis in obstructive nephropathy. J Am Soc Nephrol 2001;12:736–748. [DOI] [PubMed] [Google Scholar]
- 34.Anthwal S, Xu J, Opdenakker G, Soloway PD, Shapiro SD, Owen CA. Membrane-bound TIMP-1 on PMN has a counter intuitive role in anchoring MMP-9 to the cell surface and promoting cell associated MMP activity. Am J Respir Crit Care Med 2004;169:A867. (Abstr.) [Google Scholar]
- 35.Gipson TS, Bless NM, Shanley TP, Crouch LD, Bleavins MR, Younkin EM, Sarma V, Gibbs DF, Tefera W, McConnell PC, et al. Regulatory effects of endogenous protease inhibitors in acute lung inflammatory injury. J Immunol 1999;162:3653–3662. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.