Abstract
Activated T cells have been implicated in chronic rhinosinusitis (CRS) and asthma and physically interact with epithelial cells in the airways. We now report that human airway epithelial cells display significant constitutive cell-surface expression of costimulatory ligands, B7-H1, B7-H2, B7-H3, and B7-DC. Expression of B7-H1 and B7-DC was selectively induced by stimulation of either BEAS2B or primary nasal epithelial cells (PNEC) with interferon (IFN)-γ (100 ng/ml). The combination of IFN-γ and tumor necrosis factor-α (100 ng/ml) selectively induced expression better than IFN-γ alone. Fluticasone treatment (10−7 M) reduced the baseline expression and inhibited the induction of B7-H1 and B7-DC in BEAS2B cells. In vitro exposure of PNEC to IFN-γ also resulted in selective induction of B7-H1 and B7-DC. Monoclonal antibody blockade of B7-H1 or B7-DC enhanced IFN-γ expression by purified T cells in co-culture experiments, suggesting that these two B7 homologs inhibit T cell responses at the mucosal surface. Immunohistochemical staining of human sinonasal surgical tissue confirmed the presence of B7-H1, B7-H2, and B7-H3 in the epithelial cell layer, especially in samples from patients diagnosed with Samter's Triad, a severe form of CRS. Real-time PCR analysis of sinonasal tissue revealed elevated levels of B7-H1 and B7-DC in CRS compared with controls. These results demonstrate that epithelial cells express functional B7 costimulatory molecules and that expression of selected B7 family members is inducible in vitro and in vivo. Epithelial B7 homologs could play a role in regulation of lymphocytic activity at mucosal surfaces.
Keywords: cell-surface molecules, costimulation, human airway epithelial cells, inflammation
Epithelial cells line the mucosal surface of airways, forming a mechanical barrier that is important in repulsion and removal of particulates and microorganisms by means of the mucociliary escalator. Although it has been clear for some time that airway epithelium plays a role in airway inflammation by virtue of the production of cytokines and chemokines (1–4), recent studies indicate that epithelial cells express Toll-like receptors and can be directly activated to release these mediators by pathogen-associated molecular patterns, suggesting that they may also participate in innate immunity (5, 6). Whether epithelial cells play a role in adaptive immunity is uncertain. Epithelial cells have many of the cell surface molecules associated with antigen presentation, including major histocompatibility complex (MHC) class I and class II molecules (7–10) and CD40 (11–16). The adhesion molecule ICAM-1 is expressed on epithelial cells, and could serve as a ligand for leukocyte function–associated molecule-1 (LFA-1) expressed by T cells (17). Unlike peripheral blood T cells, nasal polyp T cells and long-lived intraepithelial T cells express αEβ7 integrin (CD103), a potential ligand for epithelial E-cadherin, suggesting that this molecule may help retain T cells in the mucosa (18).
It is also notable that substantial numbers of T cells are found in association with epithelium in the respiratory and gastrointestinal systems (19). Several studies have shown that epithelial cells have the capacity to present antigens to T lymphocytes and to stimulate in vitro (8, 20, 21). The mechanism of this effect has been unclear because epithelial cells lack expression of the important costimulators B7.1 or B7.2, which play critical roles in the priming of T cell responses (22, 23).
It is now accepted that optimal activation of T cells requires both costimulation and TCR engagement (24–28). Antigen presentation in the absence of costimulation may lead to T cell anergy. Costimulatory interactions between the B7 family ligands expressed on antigen-presenting cells (APC) and their receptors on T cells play critical roles in the growth, differentiation, and death of T cells (24–28). Engagement of the T cell costimulatory receptor CD28 by its ligands B7-1 (CD80) and B7-2 (CD86) augments activation of T cells and promotes T cell survival. In contrast, binding of B7-1 or B7-2 with CTLA-4, a homolog of CD28, may inhibit T cell responses by delivering a putative negative signal (27, 29–32). Over the last few years, work by many different investigators has identified the existence of numerous homologs of the B7 costimulatory molecules, and it is now clear that the individual members of this family have specialized roles in antigen presentation and T cell stimualtion.
Two of these homologs, B7-H1 (33) and B7-DC (34) (also referred to as PDL-1 and PDL-2), are ligands for PD-1, an inhibitory receptor on T cells (35, 36). B7-H2 is detected on B cells and macrophages, and is a ligand for the inducible co-stimulator (ICOS) expressed on antigen-primed T cells. Costimulatory signals via ICOS activate memory T cells with some preference for Th2 responses (37–42). B7-H3, which is not usually detectable in normal tissues, can be induced on dendritic cells and monocytes by inflammatory cytokines and costimulates proliferation of both CD4+ and CD8+ T cells, enhances the induction of cytotoxic T cells, and selectively stimulates the production of a key Th1-type cytokine, interferon (IFN)-γ (43).
In a recent report, we demonstrated by flow cytometry that one of the recently recognized B7 homologs, B7-H2, was constitutively and strongly expressed on both the immortalized bronchial epithelial cell line BEAS-2B and primary human bronchial epithelial cells (PBEC), whereas B7-1 and B7-2 were undetectable on either epithelial cell type (23). Expression of B7-H2 was confirmed in vitro by Western blot in cultured epithelial cells and was confirmed in vivo by immunohistochemical analysis of airway tissue derived from autopsies. These findings support the hypothesis that airway epithelial cells may play a role in some instances as APC during the development of airway inflammatory and immune responses.
The purpose of the present study was to further characterize the phenotype of airway epithelial cells with respect to expression of B7 homologs both in vitro and in human subjects. We now report that human airway epithelial cells express B7-H1, B7-H2, B7-H3, and B7-DC in vitro and in vivo. Two of these B7 homologs, B7-H1 and B7-DC, were found to be inducible by stimulation with cytokines, especially the combination of IFN-γ and tumor necrosis factor (TNF)-α. Flow cytometric analysis of airway epithelial cells collected by brushing of the upper airways of human subjects revealed expression of B7-H1, B7-H2, B7-H3, and B7-DC. Immunohistochemical analysis of tissue specimens from patients undergoing nasal surgery confirmed expression of these B7 homologs in vivo and real-time PCR analysis demonstrated elevated levels of B7-H1 and B7-DC in tissue from patients with chronic rhinosinusitis. These results suggest that airway epithelial cells express a variety of costimulatory molecules in the B7 family and provide a potential mechanism by which epithelial cells may have a role in regulating mucosal responses of T cells.
MATERIALS AND METHODS
Collection of Nasal Scrapings and Surgical Samples
Nasal epithelial cells were collected from the inferior nasal turbinate by curettage with a Rhinoprobe (Arlington Scientific, Inc., Springville, UT) or cytology brush (Wampole, Harrisburg, PA) under a Johns Hopkins Medicine Institutional Review Board (JHMIRB)-approved human subjects research protocol. Care was taken to take 10 uniform passes of mucosal scrapings. A portion of the specimen was placed immediately in RNAlater (Ambion, Austin, TX) for subsequent isolation of mRNA, and the remainder was placed in Ca2+ and Mg2+–free Ham's F12/Dulbecco's modified Eagle's medium (DMEM) containing penicillin (100 U/ml), streptomycin (100 U/ml), fungizone (1 μg/ml), and L-glutamine (GIBCO-BRL, Gaithersburg, MD) for immediate flow cytometric analysis or for cell culture. Each nasal scraping specimen yielded ∼ 1–2 × 106 cells, of which > 90% were confirmed to be epithelial cells by Wright's stain and cytokeratin immunofluorescence, as described previously (23). All surgical sinus tissue from CRS and normal control subjects were obtained through a JHMIRB-approved human subjects research protocol.
Culture of BEAS-2B Cells and Primary Nasal Epithelial Cells
The BEAS-2B cell line, derived from human bronchial epithelium transformed by an adenovirus 12-SV40 virus, was kindly supplied by Dr. Curtis Harris of the NIH (44). BEAS-2B cells were cultured in Ham's F12/DMEM. Primary nasal epithelial cells (PNEC) were cultured in bronchial epithelial basal medium (Biosource, Camarillo, TX) containing bovine pituitary extract, hydrocortisone, human recombinant epidermal growth factor, epinephrine, transferrin, insulin, retinoic acid, triiodothyronine, bovine serum albumin–fatty acid free, gentamicin, and amphotericin-B. BEAS-2B cells and PNEC were cultured in 6-well culture plates either uncoated (BEAS-2B) or coated with collagen (PNEC; Vitrogen, Collagen Biomaterials, Palo Alto, CA). BEAS-2B cells were used from passages 38–43. PNEC were used only at their first passage. Cells were cultured at 37°C with 5% CO2 in humidified air.
When cells reached 80% confluence, they were stimulated with 100 ng/ml of human recombinant TNF-α (R&D Systems, Minneapolis, MN), 100 ng/ml of IFN-γ (Genzyme, Cambridge, MA), or 50 ng/ml of interleukin (IL)-4 (R&D Systems), in the presence or absence of the glucocorticoid fluticasone (10−7 M). The cells were incubated for 24 h, and then washed three times with 0.05% trypsin/0.53 mM EDTA in HBSS (BEAS-2B) or with 0.02% EDTA in HBSS (PNEC), and then removed for analysis of mRNA expression or for flow cytometry. Generally, the cell recovery was ∼ 5–8 million cells/6-well plate. The viability of both BEAS-2B cells and primary nasal epithelial cells at the time of cell harvest was assessed by erythrosin B staining or propidium iodide exclusion and was consistently > 95% of the cells harvested.
Isolation of mRNA
Fresh epithelial cells from nasal scrapings were placed directly into 500 μl of RNAlater (Ambion) for isolation of total RNA using RNeasy kits (Qiagen, Valencia, CA). Contaminating genomic DNA was removed using RNase-Free DNase Set Protocol (Qiagen). Integrity of isolated RNA was evaluated using 1% agarose gels and levels of input RNA were calculated based upon absorbance at 260 nm. RNA was stored at −80°C until use. BEAS-2B cells and PNEC were washed with ice-cold HBSS and total cellular RNA was extracted using the TRIzol reagent (GIBCO BRL) according to the manufacturer's instructions. Briefly, cells were lysed in the presence of TRIzol and chloroform. The RNA was precipitated at −20°C in isopropanol overnight. The RNA pellet was recovered by centrifugation at 4°C, washed once in 70% ethanol, dried, and suspended in DEPC-treated water. The resulting RNA was quantitated using spectrophotometry. The RNA was stored at −80°C until use. Total mRNA from sinus tissue was isolated using the TRIzol (0.8 ml) reagent (Invitrogen, Carlsbad, CA) as previously described (45). DNA was removed using DNAfree (Ambion) per the manufacturer's protocol. Isolated mRNA was reverse transcribed using a poly(dT)15 primer (Roche, Indianapolis, IN) and Moloney murine leukemia virus reverse transcriptase (Applied Biosystems, Foster City, CA) using conditions provided by the manufacturer. Integrity of samples was confirmed using agarose gel electrophoresis.
Flow Cytometry
The monoclonal antibodies used for flow cytometry are listed in Table 1. A fluorescein isothiocyanate (FITC)-conjugated goat F (ab′)2 anti-mouse IgG (Pharmingen, Palo Alto, CA) or FITC-conjugated goat F (ab′)2 anti-hamster IgG (Biosource, Camarillo, CA) was used as the secondary antibody. Typically, 5–10 × 104 cells were incubated in 60 μl of PBS/0.2% BSA/0.1% NaN3 containing saturating concentrations of each monoclonal antibody and 4 mg/ml of human IgG (to reduce nonspecific binding; Sigma Chemicals, St. Louis, MO) on ice for 30 min. The cells were washed, resuspended in saturating amounts of secondary antibody for another 30 min, and then washed again, resuspended in the buffer, and immediately analyzed with a FACS Calibur flow cytometer (Becton Dickinson, Mountainview, CA) using CellQuest software. Fluorescence was determined on all cells for each sample after debris, dead cells, and aggregates were excluded by forward angle and side scatter gating after propidium iodide staining. Mean fluorescence intensity (MFI) was compared with control staining using an irrelevant isotype-matched mouse monoclonal antibody. For each sample, at least 10,000 events were collected, and histograms were generated. Data were expressed as mean ± SEM.
TABLE 1.
Primary and secondary antibodies used in flow cytometry
| Target | Type | Species | Isotype | Source |
|---|---|---|---|---|
| Human B7-H1 | Monoclonal | Mouse | IgG1 | Ebioscience (San Diego) |
| Human B7-H2 | Monoclonal | Mouse | IgG2B Clone 2D3 | L.C. (41) |
| Human B7-H3 | Monoclonal | Hamster | IgG Clone 9C11 | L.C. (43) |
| Human B7-H4 | Monoclonal | Mouse | IgG Clone H4.1 | L.C. (58) |
| Human B7-DC | Monoclonal | Mouse | IgG | D.P. (55) |
| Secondary mouse | Monoclonal | Goat | IgG | Biosource (Camarillo) |
| Secondary hamster | Monoclonal | Mouse | IgG | Pharmingen (Palo Alto) |
Quantitative Taqman Real-Time PCR
Messenger RNA was reverse transcribed to cDNA using standard protocols (23). PCR amplification in the presence of specific primers and a fluorescently labeled probe selected using Primer Express software was monitored by real-time analysis using an Applied Biosystems Model 7700 Sequence Detector. Table 2 lists the genes that were analyzed and the probes and primers used. The probes used were labeled with FAM (reporter dye) on the 5′ end and with TAMRA (quencher dye) on the 3′ end. A probe and PCR primers were designed for human GAPDH and 18 s RNA (Applied Biosystems), incorporating JOE as the reporter dye. Using the Sequence Detector software, the reporter dye emission was compared with the quenching dye emission generating a peak normalized reporter fluorescence value generated by each cycle of PCR (46). The peak normalized reporter fluorescence values (ΔRn) were plotted versus the cycle number. The computer algorithm was then used to calculate the threshold cycle (CT), that is, the point at which the amplification plot crossed a defined fluorescence threshold, set by the algorithm at 10 times the SD of the baseline value obtained during the first 10–15 cycles. Data are expressed as fold change in CT after normalization to GAPDH with each unit change of CT value corresponding to a doubling of the level of target mRNA.
TABLE 2.
Primer and probe sequences for real-time pcr
| Target/Accession No. | Primer/Probe Sequence | ||
|---|---|---|---|
| B7-H1 | NM014143 | Forward | 5′-ACTGGCATTTGCTGAACGCA-3′ |
| Reverse | 3′ TACACCCCTGCATCCTGCAAT-5′ | ||
| Probe | 5′-CTGTCACGGTTCCCA-3′ | ||
| B7-H2 | AF289028 | Forward | 5′-GCAAACCAGTGAGTCGAAAACC-3′ |
| Reverse | 3′-GGTGACATCAGGGCTCGGT | ||
| Probe | 5′-CTACCACATCCCACAGAACAGCTCCTTGG-3′ | ||
| B7-H3 | NM025240 | Forward | 5′ AGCACTGTGGTTCTGCCTCACA |
| Reverse | 3′ CACCAGCTGTTTGGTATCTGTCAG | ||
| Probe | 5′-CCGATGCCACCCTGT-3′ | ||
| B7-DC | AF344424 | Forward | 5′ GCCTCGTTCCACATACCTCAAG |
| Reverse | 3′ GTGGCTTTAGGCGCAGAACACT | ||
| Probe | 5′-TACCAATGCATAATCATCTAT-3′ | ||
| B7-H4 | AY280972 | Forward | 5′-TTAGGCTTGGTCCATGAGTTCA-3′ |
| Reverse | 3′-TCTGTGAGTTGCACGTTTTTCAG-5′ | ||
| Probe | 5′-AAGTGATAGTTGGCAATGC-3′ | ||
T cell Isolation
Buffy coat fractions were obtained from leukopheresed subjects belonging to the NIH (Bethesda, MD) normal donor pool. PBMC were isolated from buffy coat fractions by density gradient centrifugation using lymphocyte separation media as previously described (47). T lymphocytes were fractionated by negative magnetic immunoselection using T cell enrichment cocktail (Stem Cell Technologies, Vancouver, BC, Canada) containing anti-CD14, -CD16, -CD19, -CD56, and –glycophorin A–linked magnetic cell sorting beads and MACS Separation Column (Miltenyi Biotec, Auburn, CA) as described in the manufacturer's instructions.
T Cell Activation in Co-Culture with BEAS2B Cells
BEAS2B cells suspended in Ham's F12/DMEM media were placed in 96-well plates at 37°C for 3 h with or without blocking antibody to B7-H1 (1 μg/ml; 48), B7-H2 (1 μg/ml; eBioscience, San Diego, CA), B7-H4 (1 μg/ml; 49), B7-DC (1 μg/ml; eBioscience) or IgG1 kappa isotype control (1 μg/ml; eBioscience). Each antibody was titrated to optimal binding concentration for BEAS2B cells using FACS analysis. Then T cells (105) suspended in RPMI were added to each well and incubated with BEAS2B cells at 37°C for 48 h at a final volume of 200 μl. After 48 h, cell supernatants were assayed for IFN-γ levels by commercially available ELISA (eBioscience).
Standard Cytochemistry and Immunohistochemistry
Surgical sinonasal tissue was immediately fixed in 4% formaldehyde in PBS (4°C, 4 h) and then rinsed with PBS. Tissues were cryoprotected overnight in 18% sucrose in PBS, covered with mounting medium, and frozen in liquid nitrogen. Sections (10 μm) were cut on a cryostat, collected on lysine-coated slides and air-dried. Sections were incubated with blocking solution containing 1% BSA, 10% normal goat serum, and 0.1% Tween 20 in PBS for 1 h at room temperature, and then incubated (24 h, 4°C) with 1 μg/ml of monoclonal antibody to B7 homologs or other markers listed in Table 1. Slides were washed three times in 0.3% Triton-X100/1% BSA in PBS and labeled for 10 min with biotinylated secondary antibody, washed again, and developed with ABC–peroxidase complex (Vector Laboratories, Burlingame, CA) and Vector diaminobenzidine substrate. The slides were then counterstained in Mayer's hematoxylin for 30 s, dehydrated, and coverslipped. We used a four-level grading system: 0 (stain absent), 1 (weak staining), 2 (moderate), or 3 (strong), and the location was noted. The degree of staining was determined by a blinded observer.
Statistical Analysis
All data are expressed as mean ± SEM unless otherwise indicated. The statistical significance of the effect of IFN-γ, TNF-α, IL-4, and fluticasone propionate on RNA expression and cell surface expression of B7 homologs was determined using Student's t test for paired samples. Differences were considered significant for values of P < 0.05.
RESULTS
Flow Cytometric Analysis of B7 Homologs on BEAS2B Cells
Previous studies from this laboratory had demonstrated expression of B7-H2 mRNA and protein in BEAS2B and primary epithelial cells (23). The classical costimulatory molecules B7-1 and B7-2 were not significantly detected in either BEAS2B cells or in primary bronchial epithelial cells by PCR or flow cytometric analysis (23). In the present studies, we set out to extend these results to determine whether epithelial cells express additional related costimulators B7-H1, B7-H3, and B7-DC. Data in Figure 1 show that BEAS2B cells displayed significant surface expression of B7-H1, B7-H2, B7-H3, and B7-DC. The pattern of expression was bimodal for B7-H3 with apparent low and high expressing populations, similar to that shown previously for B7-H2 (23). Although such comparisons are qualitative, the relative rank order of cell surface expression was B7-H1 ∼ B7-H3 > > B7-H2 ∼ B7-DC.
Figure 1.
Flow cytometric analysis of cell surface expression of B7 homologs in BEAS2B airway epithelial cells. Cells were cultured and stained with specific antibodies to indicated B7 homologs or an isotype-matched control mAb (IgG Control) as described in MATERIALS AND METHODS. The histograms shown are from a representative example and values in parentheses represent mean ± SEM % positive staining cells from the indicated number of experiments: B7-H1 (n = 12), B7-H2 (n = 4), B7-H3 (n = 6), and B7-DC (n = 7). *P < 0.05, **P < 0.01, and +P < 0.2 as compared with IgG Control.
Effects of TNF-α, IFN-γ, and IL-4 on B7 Homolog Expression
To test the hypothesis that mucosal inflammation may regulate B7 homolog expression, we examined the influence of various proinflammatory cytokines and the anti-inflammatory glucocorticoid fluticasone on B7 homolog expression in BEAS2B cells. We had previously demonstrated that the cytokines TNF-α, IFN-γ, and IL-4 had no effect or slightly decreased B7-H2 expression (23). We examined the effect of these same cytokines on epithelial cell expression of B7-H1, B7-H3, and B7-DC. Data in the upper left-hand panel of Figure 2 show that cell surface expression of B7-H1 was induced in response to 24 h stimulation with IFN-γ (100 ng/ml). Although TNF-α (100 ng/ml) alone did not induce B7-H1, the combination of TNF-α and IFN-γ induced B7-H1 better than IFN-γ alone. IL-4 had no effect on expression of B7-H1. Exposure to the potent glucocorticoid fluticasone (10−7 M) resulted in greater than 50% inhibition of the induction of B7-H1 expression by TNF-α and IFN-γ. Similar results were also obtained from analysis of B7-H1 mRNA expression (Figure 2, upper right-hand panel). These data were generated by Taqman real-time PCR and are expressed compared with levels in unstimulated cells. Though not visible in the figure, it is interesting to note that fluticasone reduced baseline expression of B7-H1 mRNA by 70% (P < 0.05).
Figure 2.
Effect of 24 h exposure of BEAS2B cells to TNF-α (100 ng/ml), IFN-γ (100 ng/ml), IL-4 (10 ng/ml), and control medium on cell surface expression of B7-H1 (upper left panel) and mRNA levels of B7-H1 (upper right panel, n = 5–9), B7-DC (lower left panel, n = 5), and B7-H3 (lower right panel, n = 3). Cells were stimulated as described in MATERIALS AND METHODS in the presence or absence of fluticasone (10−7 M), and then processed for flow cytometry or analysis by real-time PCR. Values shown are mean ± SEM fluorescence intensity or mean ± SEM fold change in mRNA from medium control. *P < 0.05 compared with control condition. +P < 0.05 compared with no steroid. **P < 0.01 compared with control condition. ++P < 0.01 compared with no steroid.
The effect of cytokines and fluticasone on expression of mRNA for B7-DC and B7-H3 were also examined. Both TNF-α and IFN-γ significantly induced the expression of B7-DC mRNA (Figure 2, lower left-hand panel). As was observed with B7-H1, the combination of TNF-α and IFN-γ induced B7-DC to a greater extent than either cytokine alone. In addition, fluticasone inhibited the appearance of cell surface B7-DC induced by TNF-α and IFN-γ by > 50%. As was observed with B7-H1, fluticasone also reduced baseline expression of B7-DC by ∼ 80% (P < 0.05). A modest induction of B7-DC was observed with IL-4 stimulation. In contrast, the cytokines tested and fluticasone had little or no effect on levels of mRNA for B7-H3, as shown in Figure 2 in the lower right-hand panel.
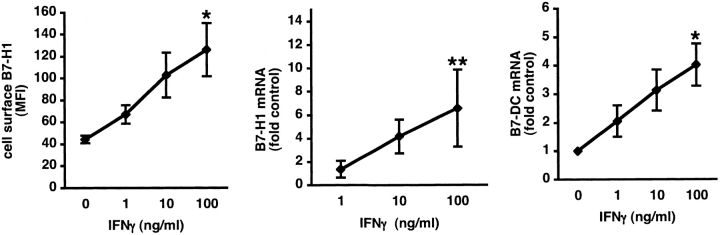
Flow cytometry and real-time PCR were next used to determine the concentration-dependence of induction of B7-H1 and B7-DC expression by IFN-γ. The data in Figure 3 show that IFN-γ exposure resulted in a concentration-dependent increase in B7-H1 cell-surface protein and mRNA and in B7-DC mRNA. Thus, epithelial cells were found to express all four B7 homologs tested, and expression of B7-H1 and B7-DC was regulated by inflammatory cytokines and anti-inflammatory glucocorticoids, whereas the expression of B7-H3 and B7-H2 was not influenced by these modulators.
Figure 3.
Concentration-dependent induction of B7-H1 and B7-DC by IFNγ in BEAS2B cells. Cells were cultured in the presence of control medium or the indicated concentrations of IFN-γ for 24 h. Expression of cell surface B7-H1 was analyzed by flow cytometry (left, n = 4) and expression of mRNA for B7-H1 (middle, n = 9), and B7-DC (right, n = 4) was analyzed by real-time PCR. *P < 0.05 compared with control condition without IFN-γ. **P < 0.01 compared with control condition without IFN-γ.
Comparison of B7 Homolog mRNA Expression from Three Different Cell Preparations
The BEAS2B cell line is a transformed cell line and may have a different phenotype than normal cells. Previous studies had shown that B7-H2 is constitutively expressed in bronchial epithelial cells as detected by immunohistochemistry of samples taken from autopsy lungs (23). To determine whether normal primary cells express B7 homologs, we set out to assess expression in sinonasal epithelial cells. Nasal epithelial cells were collected by scraping or brushing of the nasal mucosa of human subjects. We compared the mRNA levels of B7-H1, B7-H2, B7-H3, and B7-DC in BEAS2B and nasal epithelial cells by Taqman real-time PCR analysis. Messenger RNA was extracted from the BEAS2B cell line, fresh nasal epithelial cell scrapings taken directly from control subjects (nasal scrapings), and primary human nasal epithelial cells harvested from surgical tissue and expanded in cell culture as described in Materials and Methods (PNEC). Data in Figure 4 demonstrate that mRNA for all four B7 homologs was detected in BEAS2B cells. The data shown in Figure 4 are expressed as CT, which is the number of PCR cycles required to generate a threshold signal in real-time PCR. This is a direct reflection of the amount of input target mRNA, and a change of CT value of 1 unit is equal to a doubling, or halving, of the level of target mRNA. The higher the CT value, the lower the level of target mRNA. Also shown are the levels of GAPDH, an internal control for the amount of input mRNA in each cell type. The results of this analysis were highly reproducible, as shown by the small standard error bars. These data suggest that the phenotype of cultured nasal epithelial cells is similar to that of freshly isolated cells with respect to B7 homologs. Although the BEAS2B cells show a generally similar pattern, there are noticeable differences, particularly with respect to B7-DC expression. In general, BEAS2B cells expressed higher levels of B7 homologs compared with fresh or cultured primary epithelial cells. While fresh nasal epithelial cells taken directly from nasal scrapings and cultured primary cells displayed minimal to undetectable levels of B7-DC mRNA, BEAS2B cells constitutively expressed B7-DC. The patterns of expression of mRNA of other B7 homologs were quite similar for all cell sources examined.
Figure 4.
Real-time PCR analysis of expression of mRNA for B7-H1, B7-H2, B7-H3, B7-DC, and GAPDH in BEAS2B cells (left, n = 6), epithelial cells from nasal scrapings (center, n = 8), and cultured primary nasal epithelial cells (right, n = 3). Values shown are mean ± SEM CT from Taqman analysis and reflect target mRNA. The Y scale is inverted so that higher bars represent higher levels of mRNA. Probes, primers and assays used are described in MATERIALS AND METHODS.
Flow Cytometric Analysis of B7 Homologs on Cultured Primary Nasal Epithelial Cells
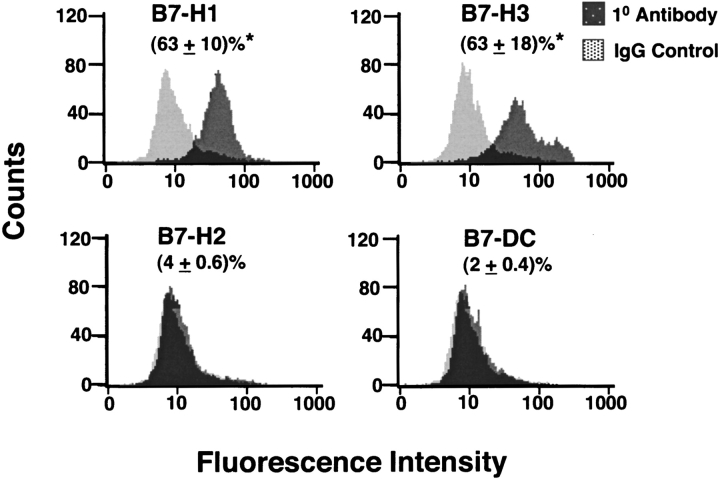
We next performed flow cytometric analysis of cultured primary nasal epithelial cells from freshly derived nasal scrapings from four normal control subjects. The results in Figure 5 demonstrate that at least two of the B7 homologs were detectable by flow cytometry. Expression of B7H1 and B7-H3 was readily detectable, whereas expression of B7-H2 and B7-DC was barely detected. As was observed at the level of mRNA, the levels of cell surface expression of all four B7 homologs were somewhat lower than those seen in the BEAS2B cell line. The relative rank order of cell surface expression was B7-H3 ≃ B7-H1 ≫B7-H2 ≃ B7-DC. Both the BEAS2B cell line and PNEC expressed high constitutive levels of B7-H1 and B7-H3 and significantly lower levels B7-H2 and B7-DC on the cell surface.
Figure 5.
Flow cytometric analysis of the expression of B7-H1, B7-H2, B7-H3, and B7-DC on cultured primary nasal epithelial cells collected by nasal scrapings. Cells were cultured and stained with specific antibodies to B7 homologs or an isotype-matched control mAb (Control IgG) as described in MATERIALS AND METHODS. Histograms shown are a representative example and values shown in parentheses are mean ± SEM % positive staining cells from four separate experiments. *P < 0.05 as compared with IgG Control.
We next examined the in vitro effect of IFN-γ and fluticasone on cultured primary epithelial cells grown from nasal scrapings of normal control human subjects. Only first passage cells were used. As shown in Figure 6, IFN-γ exposure of PNEC for 24 h significantly induced B7-H1 and B7-DC cell surface expression, but had no effect on B7-H2 and B7-H3, similar to results seen in BEAS2B cells. Interestingly, fluticasone treatment of PNEC only slightly reduced the induction of B7-H1 and B7-DC by IFN-γ.
Figure 6.
Effect of IFN-γ and fluticasone on cell surface expression of B7 homologs in cultured PNECs (n = 4). Cells were stimulated with 100 ng/ml of IFN-γ in the presence or absence of fluticasone propionate (FP, 10−7 M) for 24 h. The cells were then processed for flow cytometry as described in MATERIALS AND METHODS. The data are expressed as mean ± SEM fluorescence intensity (MFI). *P < 0.05 compared with control condition.
In Vitro Activation of T Cells by B7 Homologs on BEAS2B Cells
To explore the functional significance of the presence of B7 homologs on the surface of BEAS2B cells, we examined whether human airway epithelial cells could directly activate T cells in vitro. To this end, BEAS2B cells were co-cultured with primary human T cells isolated from donors using a negative selection affinity column. After purification, the T cell population was routinely found to be 95% pure by flow cytometric confirmation of CD3+ labeling. Because recent work has shown that BEAS2B cells lack the capacity to express mRNA for IFN-γ (50), IFN-γ production was used as a measure of T cell activation in our co-culture cell system. As shown in Figure 7, left panel, exposure of a constant number of purified human T cells to varied numbers of BEAS2B cells resulted in significant release of T cell–derived IFN-γ. Neither BEAS2B cells alone nor T cells alone in culture produced significant levels of IFN-γ. Optimal IFN-γ production occurred when cells were co-cultured using a 1:1 ratio of BEAS2B to purified primary human T cells. To test the role of B7 homologs on BEAS2B cells in this effect, the ability of specific blocking antibodies against B7-H1, B7-H2, B7-H4, and B7-DC to affect epithelial cell stimulation of IFN-γ production from T cells was examined. As shown in Figure 7, right panel, addition of B7-H1 or B7-DC blocking antibodies to BEAS2B cells and T cells in co-culture resulted in significant further induction of BEAS2B cell–dependent IFN-γ production from T cells (31% or 67% increase, respectively). In contrast, blocking antibody to B7-H2, B7-H4, or IgG isotype control had no effect. The combination of anti–B7-H1 and anti–B7-DC antibodies together did not increase IFN-γ release from T cells greater than either antibody alone. These results suggest that the activation of T cells is regulated, at least in part, by the presence of cell-surface co-stimulatory molecules on BEAS2B cells.
Figure 7.
In vitro activation of primary human T cells by BEAS2B cells; role of B7 homologs. Left panel: Induction of T cell–derived IFN-γ by BEAS2B cells in co-culture. Primary human T cells (105) were isolated from different donors and were co-cultured with increasing seeding concentrations of BEAS2B cells for 48 h as described in MATERIALS AND METHODS (n = 5). Values shown are the mean ± SEM of IFN-γ (pg/ml). *P < 0.05 as compared with BEAS2B cells alone. Right panel: Effect of blocking antibodies to B7 homologs on BEAS2B cell activation of T cell IFN-γ production. BEAS2B cells (105) were exposed to saturating amounts (1 μg/ml) of blocking antibody to B7-H1, B7-H2, B7-H4, B7-DC, or IgG control and co-cultured with 105 primary human T cells (n = 4) for 48 h as described in MATERIALS AND METHODS. Data are expressed as mean ± SEM of % of maximal IFN-γ produced in the absence of blocking antibody. *P < 0.05 and **P < 0.005 as compared with IgG control.
Analysis of B7 Homolog Expression in Human Sinonasal Tissue
To explore the presence of B7 homologs in sinonasal tissue further, we analyzed B7 homolog mRNA levels in sinonasal surgical samples from control subjects and subjects with CRS with nasal polyps. In both cases, tissue was derived from the ethmoid mucosa. Data in Figure 8 show that levels of 18 s RNA did not differ in these two groups. Levels of mRNA for B7-H1 and B7-DC were significantly elevated in tissue from patients with CRS (∼ 3–4 Ct values, which corresponds to 8- to 16- fold). Levels of other B7 homologs did not differ in the two groups.
Figure 8.
Real-time PCR analysis of expression of mRNA for B7-H1, B7-H2, B7-H3, B7-H4, B7-DC, and 18 sRNA in human sinonasal tissue from control subjects (n = 7) and subjects with CRS (CRS, n = 5). Values shown are mean ± SEM CT from Taqman analysis and reflect target mRNA. The Y scale is inverted so that higher bars represent higher levels of mRNA. Probes, primers, and assays used are described in MATERIALS AND METHODS. *P < 0.05 as compared with control subjects.
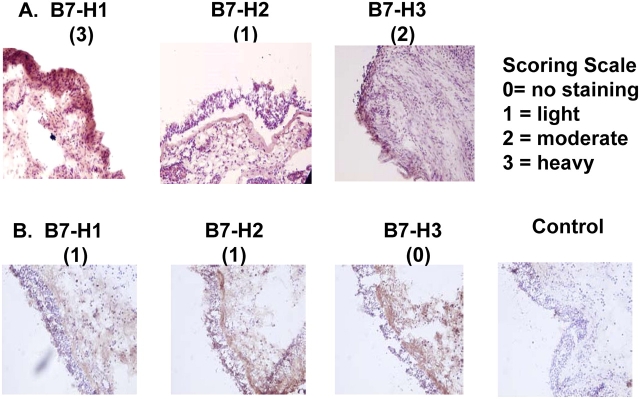
The data obtained by mRNA and flow cytometric analysis are novel and strongly suggest that nasal epithelial cells express B7 homologs. To confirm the presence of B7 homologs on epithelium in vivo, immunohistochemical staining was performed on nasal surgical samples using monoclonal antibodies against B7-H1, B7-H2, and B7-H3. In these studies, sinonasal tissue from patients with Samter's Triad or chronic rhinosinusitis (CRS) were tested and the staining intensity of epithelial cells evaluated using the following semiquantitative scale: 0 = no staining, 1 = light staining, 2 = staining positive in most epithelial cells, and 3 = heavy staining. Data in Figure 9 show that all three B7 homologs, B7-H1, B7-H2, and B7-H3, were detected in a subset of samples from human subjects with sinonasal disease. The intensity of staining varied among subjects. Samples depicted in Panel A were from a patient with Samter's Triad and displayed intense staining for B7-H1, light staining for B7-H2, and moderate staining for B7-H3. Samples from a patient with CRS alone are depicted in Panel B and displayed noticeable but light staining for B7-H1 and B7-H2, and no detectable staining for B7-H3.
Figure 9.
In vivo expression of B7 homologs by epithelial cells in sinonasal tissue. Surgical sinonasal tissue from two subjects (A and B) was immediately placed in 4% paraformaldehyde and processed for immunohistochemical staining of B7-H1, B7-H2, and B7-H3 as described in MATERIALS AND METHODS. Staining intensity was determined according to the indicated scoring scale.
Table 3 summarizes the pattern of immunostaining in the individual subjects tested to date and according to patient group. Although these are preliminary results, the heaviest staining for B7 homologs was generally observed in sinus tissue specimens from patients with Samter's Triad as compared with patients with CRS alone or the single control subject. In addition, B7 homolog staining was observed primarily, but not exclusively in the epithelial cell layer (e.g., see Figure 9A, B7-H3).
TABLE 3.
Immunohistochemical analysis of B7 homolog expression in human sinonasal tissue
| Subject | B7 Homolog | Patient Group | ||
|---|---|---|---|---|
| H1 | H2 | H3 | ||
| 1 | 2 | 2 | 2 | Samter's Triad* |
| 2 | 3 | 1 | 2 | Samter's Triad |
| 3 | 1 | 2 | 1 | Samter's Triad |
| 4 | 0 | 2 | 1 | CRS alone |
| 5 | 1 | 1 | 0 | CRS alone |
| 6 | 0 | 0 | 1 | CRS alone |
| 7 | 1 | 0 | 1 | CRS alone |
| 8 | 0 | 0 | 1 | CRS alone |
| 9 | ND | ND | 1 | Control |
Definition of abbreviations: CRS, chronic rhinosinusitis; ND, not done.
CRS plus asthma plus aspirin hypersensitivity.
DISCUSSION
Recent evidence suggests that airway epithelial cells might act as immune effector cells in response to endogenous or exogenous stimuli (1–5, 52). As the primary surface that comes into contact with antigenic materials, epithelial cells may play a key role in the interaction between the lung and external environment in immune and inflammatory responses. Several studies have shown that airway epithelial cells express and secrete various molecules involved in inflammation and immunity, including MHC class I (6, 32), CD40 (37), inflammatory lipids (45), oxygen radicals (46), adhesion molecules (47, 50, 51), and a wide variety of cytokines and chemokines (1–5, 52).
In previous studies we demonstrated expression of B7-H2 mRNA and protein in BEAS2B and primary epithelial cells (23). The classical costimulatory molecules B7-1 and B7-2 were not significantly detected in either BEAS2B cells nor in primary bronchial epithelial cells by real-time PCR or flow cytometric analysis (23). In this study, we found that both BEAS2B and primary cells display relatively higher cell-surface and mRNA expression of B7-H1 and B7-H3 than B7-H2 and B7-DC. These studies thus demonstrate that human airway epithelial cells constitutively express all four B7 homologs.
We examined the effect of TNF-α, IFN-γ, and IL-4 on epithelial cell expression of mRNA and protein for B7-H1, B7-H3, and B7-DC and found that both B7-H1 and B7-DC mRNA and protein were induced in response to 24 h stimulation with TNF-α (100 ng/ml) and IFN-γ (100 ng/ml). The combination of TNF-α and IFN-γ induced B7-H1 and B7-DC better than either cytokine alone, and the response to IFN-γ was similar in magnitude between the BEAS2B and primary cells used. Cytokines tested had little or no effect on levels of B7-H2 or B7-H3 mRNA or protein. We also found that the potent glucocorticoid fluticasone decreased baseline expression and inhibited the induction of B7-H1. Although fluticasone exposure had a tendency to diminish the IFN-γ induction of B7-DC, the inhibition of induction by fluticasone did not reach statistical significance. Finally, IL-4 had little or no effect on expression of any of the B7 homologs. Among the stimuli we tested, IFN-γ was most effective and resulted in a concentration-dependent increase in B7-H1 mRNA and cell-surface expression as assessed by flow cytometry. Thus, epithelial cells appear to express all four B7 homologs and expression of at least two B7 homologs are regulated by inflammatory cytokines (B7-H1 and B7-DC) and anti-inflammatory steroids (B7-H1).
Attempts to validate expression of B7 homologs by nasal epithelial cells in vitro using flow cytometry and mRNA analysis of freshly cultured human nasal epithelial cells revealed a noticeable difference between fresh cultured cells and BEAS2B cells with respect to B7-DC mRNA expression. Both fresh nasal epithelial cells taken directly from nasal brushings and cultured primary nasal epithelial cells displayed minimal to undetectable levels of mRNA for B7-DC, as compared with the BEAS2B immortalized cell line, which constitutively expressed B7-DC. Because the primary nasal epithelial cells studied from fresh nasal scrapings were found to express similar levels of B7-homologs to those seen in cultured PNEC, this suggests that B7 homologs may have been induced as a result of the process by which the BEAS2B cells were immortalized. These studies emphasize the need to reproduce in vitro studies on BEAS2B cells with studies on cultured primary nasal epithelial cells. Aside from B7-DC, the pattern of expression of mRNA for B7 homologs was similar among the various epithelial cell sources. The relative rank order of cell surface expression for both BEAS2B and cultured primary nasal epithelial cells was B7-H1 ≃ B7-H3 ≫ B7-H2 ≃ B7-DC (Figures 1 and 5), and the similar pattern of inducibility of B7-H1 and B7-DC by IFN-γ and TNF-α (Figures 2 and 6) indicate that findings in BEAS2B cells are of value in predicting behavior of primary cells.
Induction of the expression of B7-H1 and B7-DC in BEAS2B cells was inhibited by the potent glucocorticoid fluticasone. Interestingly, fluticasone failed to inhibit the induction of B7-H1 and B7-DC in PNEC. Regulation of expression of costimulatory molecules is one mechanism by which topical steroids could influence the function of T cells in the airways. The explanation for why PNEC are less steroid responsive with respect to B7 homolog expression than the immortalized BEAS2B cell line has not yet been determined, but could reflect differences in expression of glucocorticoid receptor, glucocorticoid receptor function, or previous exposure to glucocorticoids in vivo.
To address the functional significance of the presence of B7 homologs on airway epithelial cells, we used an in vitro co-culture system to study allogenic stimulation of T cells by BEAS2B cells. BEAS2B cells were found to induce substantial IFN-γ production by T cells. Interestingly, addition of blocking antibodies to two putative inhibitory costimulatory ligands, B7-H1 and B7-DC, resulted in enhanced IFN-γ production. Thus, in our co-culture system, B7-H1 and B7-DC appear to have inhibitory effects on T cell activation and inhibition of these ligands by functionally blocking antibodies resulted in greater IFN-γ production from T cells. It is unclear why exposure to the combination of both antibodies to B7-H1 and B7-DC did not result in additive enhancement of IFN-γ release.
We further assessed expression of B7 homologs in CRS using sinonasal surgical samples. We found that tissue from patients with CRS expressed significantly higher levels of mRNA for B7-H1 and B7-DC than tissues from control subjects. Because these tissues contain many cell types, it is not possible to attribute this response to epithelial cells. It is notable, however, that these are the only two B7 homologs that we have found to be inducible on epithelial cells in vitro. To further assess the presence of B7 homologs on epithelium in vivo, immunohistochemical staining was performed on nasal surgical samples using monoclonal antibodies against B7-H1 and B7-H2, and a polyclonal antibody against B7-H3. Although the intensity of staining varied among subjects, all three B7 homologs, B7-H1, B7-H2, and B7-H3, were detected in several human sinonasal tissue samples. Interestingly, the intensity of staining appeared to vary among different groups of patients according to disease; all three patients with Samter's had increased expression compared with the five subjects with CRS alone and the single control sample. Although these studies are preliminary and are not conclusive with respect to the influence of disease activity on B7 homolog expression, these findings confirm expression of B7 homologs by epithelial cells in vivo in human subjects. We note that minimal or no expression of B7 homologs was observed in nasal samples from several subjects with CRS and from a normal subject (Table 3). It is possible that expression of some B7 homologs in vivo may be low or absent and costimulators are only induced during immune and inflammatory responses in the airways. Preliminary results in a rhinovirus challenge model support this contention (51).
Mucosal inflammation is a hallmark of several diseases of the airways, including asthma and CRS. A growing literature suggests that both of these diseases are characterized by activation of T cells and epithelial cells (52, 53). Activated T cells are widely believed to be central orchestrating cells, and interactions between airway epithelial cells and T cells are likely to be important in the pathogenesis of asthma and CRS. For example, IL-4 and IL-13, products of Th2 cells, have been shown to trigger STAT6-dependent inflammatory processes in airway epithelium, including production of eotaxin and other CC chemokines. These responses are associated with alterations of epithelial morphology, including desquamation of superficial columnar cells, goblet cell hyperplasia, squamous metaplasia, basement membrane thickening and mucosal edema. Whether epithelial cells reciprocate to alter T cell responses is less clear. In the present report, we demonstrate that epithelial cells express significant constitutive levels of several newly recognized members of the B7 costimulatory molecule family, including B7-H1, B7-H2, B7-H3, and B7-DC, suggesting that epithelial cells express costimulatory molecules necessary to regulate T cells. Both B7-H2 and B7-H3 are thought to be positive costimulatory ligands in that engagement results in activation of T cell function. Engagement of B7-H2 results in activation of T-helper memory cells with a bias toward Th2 cytokine production, such as IL-4 and IL-13 (43). B7-H3 is expressed in nonlymphoid tissue, and its expression can be induced on lymphoid cells by inflammatory cytokines. Of the costimulatory molecules examined in our studies, B7-H3 was the most abundant costimulatory molecule detected by flow cytometry on both BEAS2B and cultured primary nasal epithelial cells. Engagement B7-H3 ligands results in proliferation of CD4+ and CD8+ T cells, a bias toward Th1 cytokine production, and primary cytotoxic T cell activation. Thus the presence of B7 homologs on epithelial cells may play a role in driving expression of the Th1 and Th2 cytokines observed in asthma and CRS. B7-H1 (PD-L1) and B7-DC (PD-L2) have been identified in both lymphoid and several nonlymphoid tissues, as well as in several tumor cell lines, and are putative inhibitory co-stimulatory ligands (36). Interaction of these ligands with the counter-receptor PD-1 can result in inhibition of T and B cell responses (35, 54). Among the costimulatory molecules studied, B7-DC displayed the lowest level of cell surface expression in unstimulated BEAS2B and cultured PNEC. However, both of these putative inhibitory costimulatory ligands (B7-H1 and B7-DC) were found to be sensitive to induction by IFN-γ. More recent findings suggest that there may exist several mechanisms of PD-1–mediated immune regulation. For example, Yamazaki and coworkers recently reported finding PD-1 on activated B cells and B7-H1 on activated T cells (55). This suggested that the engagement of PD-1 on B cells by B7-H1 on T cells may constitute a novel mechanism of T cell–mediated B cell suppression. These costimulators may exert activating influences as well as inhibitory effects. For example, B7-DC engagement on dendritic cells was found to costimulate T cell proliferation more efficiently than B7-1 and induce secretion of IFN-γ, but not IL-4 or IL-10, from isolated naïve T cells, suggesting that signaling through PD-1 by B7-H1 and B7-DC may sometimes be stimulatory (55, 56). In monocytes that are treated with IFN-γ, B7-H1 expression was found to precede B7-DC expression (35, 57). Thus the presence of B7-H1 and B7-DC on airway epithelial cells may provide either positive or negative feedback signals to T cells in the airways. Although our functional studies with co-cultured epithelial cells and T cells suggest that B7-H1 and B7-DC exert inhibitory effects, due to the nature of the in vitro system, it is not possible to definitively conclude whether these homologs are activating or inhibitory in vivo. In the context of CRS, we found increased expression of these same inhibitory co-stimulatory molecules in CRS sinus tissue. Our preliminary immunohistochemical studies also show that staining intensity increases with severity of disease. Taken together, these data suggest that CRS may represent a state in which inhibitory co-stimulatory molecules, B7-H1 and B7-DC, are induced as a response to inflammation within the mucosa.
Because T cell infiltration of the epithelium and submucosa are striking features of both asthma and CRS (18), and considering the breadth of evidence for direct physical interactions between T cells and epithelial cells in the airways, further characterization of the phenotype of epithelial cells would be of value. In light of the data presented herein, we anticipate that T lymphocytes, which express ICOS, PD-1, and other costimulatory receptors, may encounter and interact with B7 homologs expressed on airway epithelial cells, resulting in regulation of the function or survival of T cells in the airways in the presence of antigen exposure. Identification of B7 homolog–expressing resident cells within the airway tract other than alveolar macrophages and dendritic cells expands the possible mechanisms that underlie T cell activation in airway inflammation.
Acknowledgments
The authors thank Ms. Bonnie Hebden for her secretarial assistance in the preparation of this manuscript and Mr. Jim Plitt and Mrs. Carol Bickel for their excellent technical assistance.
This work was supported by National Institutes of Health grants (AI57400, M01-RR-02719, AI37168, AI50530, and HL68546) and FAMRI.
Conflict of Interest Statement: J.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.C.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; L.C. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; D.P. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; Q.-A.T.-T. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; A.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; J.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; L.F. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript; R.P.S. has received the following consultancy fees: from GlaxoSmithKline, $2,500 in 09/2002 and $4,000 in 06/2003; from Aventis Pharmaceuticals, $3,000 in 03/2002 and $4,000 in 02/2003; from AstraZeneca, $3,000 in 2004; and from Altana, $3,000 in 2005. He also received a sponsored grant of $60,000 from GlaxoSmithKline in 12/2002, and $48,000 in 06/2003.
References
- 1.Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy 1999;29:90–95. [DOI] [PubMed] [Google Scholar]
- 2.Schwiebert LM, Stellato C, Schleimer RP. The epithelium as a target of glucocorticoid action in the treatment of asthma. Am J Respir Crit Care Med 1996;154:S16–S19. [DOI] [PubMed] [Google Scholar]
- 3.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP III, Toews GB, Westwick J, Strieter RM. Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest 1990;86:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stellato C, Beck LA, Gorgone GA, Proud D, Schall TJ, Ono SJ, Lichtenstein LM, Schleimer RP. Expression of the chemokine RANTES by a human bronchial epithelial cell line: modulation by cytokines and glucocorticoids. J Immunol 1995;155:410–418. [PubMed] [Google Scholar]
- 5.Diamond G, Legarda D, Ryan LK. The innate immune response of the respiratory epithelium. Immunol Rev 2000;173:27–38. [DOI] [PubMed] [Google Scholar]
- 6.Sha Q, Plitt J, Heller N, Beck LA, Imani F, Schleimer RP. Airway epithelial cell activation and toll-like receptors (TLR). J Allergy Clin Immunol 2003;111:S212. [Google Scholar]
- 7.Glanville AR, Tazelaar HD, Theodore J, Imoto E, Rouse RV, Baldwin JC, Robin ED. The distribution of MHC class I and II antigens on bronchial epithelium. Am Rev Respir Dis 1989;139:330–334. [DOI] [PubMed] [Google Scholar]
- 8.Kalb TH, Chuang MT, Marom Z, Mayer L. Evidence for accessory cell function by class II MHC antigen-expressing airway epithelial cells. Am J Respir Cell Mol Biol 1991;4:320–329. [DOI] [PubMed] [Google Scholar]
- 9.Mezzetti M, Soloperto M, Fasoli A, Mattoli S. Human bronchial epithelial cells modulate CD3 and mitogen-induced DNA synthesis in T cells but function poorly as antigen-presenting cells compared to pulmonary macrophages. J Allergy Clin Immunol 1991;87:930–938. [DOI] [PubMed] [Google Scholar]
- 10.Papi A, Stanciu LA, Papadopoulos NG, Teran LM, Holgate ST, Johnston SL. Rhinovirus infection induces major histocompatibility complex class I and costimulatory molecule upregulation on respiratory epithelial cells. J Infect Dis 2000;181:1780–1784. [DOI] [PubMed] [Google Scholar]
- 11.Atsuta J, Sterbinsky SA, Plitt J, Schwiebert LM, Bochner BS, Schleimer RP. Phenotyping and cytokine regulation of the BEAS-2B human bronchial epithelial cell: demonstration of inducible expression of the adhesion molecules VCAM-1 and ICAM-1. Am J Respir Cell Mol Biol 1997;17:571–582. [DOI] [PubMed] [Google Scholar]
- 12.Companjen AR, van der Wel LI, Boon L, Prens EP, Laman JD. CD40 ligation-induced cytokine production in human skin explants is partly mediated via IL-1. Int Immunol 2002;14:669–676. [DOI] [PubMed] [Google Scholar]
- 13.Denfeld RW, Hollenbaugh D, Fehrenbach A, Weiss JM, von Leoprechting A, Mai B, Voith U, Schopf E, Aruffo A, Simon JC. CD40 is functionally expressed on human keratinocytes. Eur J Immunol 1996;26:2329–2334. [DOI] [PubMed] [Google Scholar]
- 14.Propst SM, Estell K, Schwiebert LM. CD40-mediated activation of NF-kappa B in airway epithelial cells. J Biol Chem 2002;277:37054–37063. [DOI] [PubMed] [Google Scholar]
- 15.Propst SM, Denson R, Rothstein E, Estell K, Schwiebert LM. Proinflammatory and Th2-derived cytokines modulate CD40-mediated expression of inflammatory mediators in airway epithelia: implications for the role of epithelial CD40 in airway inflammation. J Immunol 2000;165:2214–2221. [DOI] [PubMed] [Google Scholar]
- 16.van Kooten C, Banchereau J. CD40–CD40 ligand. J Leukoc Biol 2000;67:2–17. [DOI] [PubMed] [Google Scholar]
- 17.Atsuta J, Plitt J, Bochner BS, Schleimer RP. Inhibition of VCAM-1 expression in human bronchial epithelial cells by glucocorticoids. Am J Respir Cell Mol Biol 1999;20:643–650. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Segura A, Brieva JA, Rodriguez C. T lymphocytes that infiltrate nasal polyps have a specialized phenotype and produce a mixed TH1/TH2 pattern of cytokines. J Allergy Clin Immunol 1998;102:953–960. [DOI] [PubMed] [Google Scholar]
- 19.Jahnsen FL, Farstad IN, Aanesen JP, Brandtzaeg P. Phenotypic distribution of T cells in human nasal mucosa differs from that in the gut. Am J Respir Cell Mol Biol 1998;18:392–401. [DOI] [PubMed] [Google Scholar]
- 20.Kalb TH, Yio XY, Mayer L. Human airway epithelial cells stimulate T-lymphocyte lck and fyn tyrosine kinase. Am J Respir Cell Mol Biol 1997;17:561–570. [DOI] [PubMed] [Google Scholar]
- 21.Salik E, Tyorkin M, Mohan S, George I, Becker K, Oei E, Kalb T, Sperber K. Antigen trafficking and accessory cell function in respiratory epithelial cells. Am J Respir Cell Mol Biol 1999;21:365–379. [DOI] [PubMed] [Google Scholar]
- 22.Cunningham AC, Zhang JG, Moy JV, Ali S, Kirby JA. A comparison of the antigen-presenting capabilities of class II MHC-expressing human lung epithelial and endothelial cells. Immunology 1997;91:458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurosawa S, Myers AC, Chen L, Wang S, Ni J, Plitt JR, Heller NM, Bochner BS, Schleimer RP. Expression of the costimulatory molecule B7–H2 (inducible costimulator ligand) by human airway epithelial cells. Am J Respir Cell Mol Biol 2003;28:563–573. [DOI] [PubMed] [Google Scholar]
- 24.Fraser JD, Irving BA, Crabtree GR, Weiss A. Regulation of interleukin-2 gene enhancer activity by the T cell accessory molecule CD28. Science 1991;251:313–316. [DOI] [PubMed] [Google Scholar]
- 25.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 1992;356:607–609. [DOI] [PubMed] [Google Scholar]
- 26.June CH, Ledbetter JA, Gillespie MM, Lindsten T, Thompson CB. T-cell proliferation involving the CD28 pathway is associated with cyclosporine-resistant interleukin 2 gene expression. Mol Cell Biol 1987;7:4472–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers CA, Allison JP. Co-stimulation in T cell responses. Curr Opin Immunol 1997;9:396–404. [DOI] [PubMed] [Google Scholar]
- 28.Gause WC, Mitro V, Via C, Linsley P, Urban JF Jr, Greenwald RJ. Do effector and memory T helper cells also need B7 ligand costimulatory signals? J Immunol 1997;159:1055–1058. [PubMed] [Google Scholar]
- 29.Bugeon L, Dallman MJ. Costimulation of T cells. Am J Respir Crit Care Med 2000;162:S164–S168. [DOI] [PubMed] [Google Scholar]
- 30.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med 1996;183:2541–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994;1:405–413. [DOI] [PubMed] [Google Scholar]
- 33.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 1999;5:1365–1369. [DOI] [PubMed] [Google Scholar]
- 34.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J. Exp Med 2001;193:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med 2000;192:1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol 2001;2:261–268. [DOI] [PubMed] [Google Scholar]
- 37.Coyle AJ, Lehar S, Lloyd C, Tian J, Delaney T, Manning S, Nguyen T, Burwell T, Schneider H, Gonzalo JA, et al. The CD28-related molecule ICOS is required for effective T cell-dependent immune responses. Immunity 2000;13:95–105. [DOI] [PubMed] [Google Scholar]
- 38.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol 2000;165:5035–5040. [DOI] [PubMed] [Google Scholar]
- 39.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature 2001;409:102–105. [DOI] [PubMed] [Google Scholar]
- 40.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 1999;397:263–266. [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Zhu G, Chapoval AI, Dong H, Tamada K, Ni J, Chen L. Costimulation of T cells by B7–H2, a B7-like molecule that binds ICOS. Blood 2000;96:2808–2813. [PubMed] [Google Scholar]
- 42.Yoshinaga SK, Zhang M, Pistillo J, Horan T, Khare SD, Miner K, Sonnenberg M, Boone T, Brankow D, Dai T, et al. Characterization of a new human B7-related protein: B7RP-1 is the ligand to the co-stimulatory protein ICOS. Int Immunol 2000;12:1439–1447. [DOI] [PubMed] [Google Scholar]
- 43.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, et al. B7–H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol 2001;2:269–274. [DOI] [PubMed] [Google Scholar]
- 44.Reddel RR, Ke Y, Gerwin BI, McMenamin MG, Lechner JF, Su RT, Brash DE, Park JB, Rhim JS, Harris CC. Transformation of human bronchial epithelial cells by infection with SV40 or adenovirus-12 SV40 hybrid virus, or transfection via strontium phosphate coprecipitation with a plasmid containing SV40 early region genes. Cancer Res 1988;48:1904–1909. [PubMed] [Google Scholar]
- 45.Vandermeer J, Sha Q, Lane AP, Schleimer RP. Innate immunity of the sinonasal cavity: expression of messenger RNA for complement cascade components and toll-like receptors. Arch Otolaryngol Head Neck Surg 2004;130:1374–1380. [DOI] [PubMed] [Google Scholar]
- 46.Gibson UE, Heid CA, Williams PM. A novel method for real time quantitative RT-PCR. Genome Res 1996;6:995–1001. [DOI] [PubMed] [Google Scholar]
- 47.McDyer JF, Li Z, John S, Yu X, Wu CY, Ragheb JA. IL-2 receptor blockade inhibits late, but not early, IFN-gamma and CD40 ligand expression in human T cells: disruption of both IL-12-dependent and -independent pathways of IFN-gamma production. J Immunol 2002;169:2736–2746. [DOI] [PubMed] [Google Scholar]
- 48.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002;8:793–800. [DOI] [PubMed] [Google Scholar]
- 49.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity 2004;20:327–336. [DOI] [PubMed] [Google Scholar]
- 50.Spurrell JCL, Wiehler S, Zaheer RS, Sanders SP, Proud D. Human airway epithelial cells produce IP-10 (CXCL10) in vitro and in vivo upon rhinovirus infection. Am J Physiol Lung Cell Mol Physiol 2005;289:L85–L95. [DOI] [PubMed] [Google Scholar]
- 51.Kim J, Plitt J, Myers A, Schleimer RP. Expression of B7 homolog costimulatory molecules in airway epithelial cells. Denver: American Association of Immunology; 2003.
- 52.Berger G, Kattan A, Bernheim J, Ophir D, Finkelstein Y. Acute sinusitis: a histopathological and immunohistochemical study. Laryngoscope 2000;110:2089–2094. [DOI] [PubMed] [Google Scholar]
- 53.Grevers G, Klemens A, Menauer F, Sturm C. Involvement of inferior turbinate mucosa in chronic sinusitis–localization of T-cell subset. Allergy 2000;55:1155–1162. [DOI] [PubMed] [Google Scholar]
- 54.Nishimura H, Honjo T. PD-1: an inhibitory immunoreceptor involved in peripheral tolerance. Trends Immunol 2001;22:265–268. [DOI] [PubMed] [Google Scholar]
- 55.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol 2002;169:5538–5545. [DOI] [PubMed] [Google Scholar]
- 56.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, Shalabi A, Shin T, Pardoll DM, Tsuchiya H. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med 2001;193:839–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aicher A, Hayden-Ledbetter M, Brady WA, Pezzutto A, Richter G, Magaletti D, Buckwalter S, Ledbetter JA, Clark EA. Characterization of human inducible costimulator ligand expression and function. J Immunol 2000;164:4689–4696. [DOI] [PubMed] [Google Scholar]
- 58.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, Zhu Y, Flies DB, Tamada K, Chen L. Genomic organization and expression analysis of b7-h4, an immune inhibitory molecule of the b7 family. J Immunol 2003;171:4650–4654. [DOI] [PubMed] [Google Scholar]