The Emperor asked, “When a child in arms has a ‘wind within’ and fever, when its breathing is troubled and it wheezes while resting its shoulders, what is then the condition of the pulse?”
Ch'i Po answered: “When there is troubled breathing and wheezing while resting the shoulders, the pulse is large and full. When it is slow it means life; when it is rapid it means death…. Those who do not rest and whose breathing is noisy have disorders in the region of the Yang Ming (the ‘sunlight’).” (1)
—Huang Ti (2698–2598 bce)
The Chief commented, “Treat the inflammation.”
—Anonymous (2005 ce)
The clinical description of asthma dates back to ancient times and, over this long period, multiple paradigms for asthma pathogenesis have been proposed and revised. In honor of the ATS centennial anniversary, we were pleased to provide a historical overview of asthma pathogenesis and to highlight the major advances in the last 100 years. Whenever possible, we will also provide a broader historical context, suggesting that at times, what appears new may instead be borrowed anew. The review is organized chronologically with seemingly distinct phases of research activities, but new asthma paradigms often emerged from previous models and usually shared significant temporal overlap. In addition, many laboratories often contributed in parallel to develop new paradigms. The pseudopodial approach to science was often invoked, whereby one lab reached out to find something new and related labs quickly followed in the same direction. We also acknowledge that assigning paradigms to individuals is difficult because of temporal and spatial overlap among researchers and limitations related to literature availability, translational bias, publication bias, publication lag, and historical interpretation. Nonetheless, we submit that a useful trail of discovery can be found in the story of asthma and related airway diseases.
BRONCHOCONSTRICTOR PARADIGM
The key observations leading to the development of the bronchoconstrictor paradigm for asthma appear to be derived from personal experiences with asthma as well as detailed clinical observations. A liberal interpretation of the medical literature identifies the first clinical description of a subject with presumed asthma in a Chinese textbook of internal medicine from around 2600 bce (1–3). The word asthma derived from the Greek word meaning “to exhale with open mouth, to pant” and appeared in the English language, as we know it today, around 1600 (4, 5). Up until the mid-1600s, asthma was a nonspecific descriptor of any clinical condition associated with dyspnea. In 1662, the Belgian physician Jean van Helmont, who suffered from asthma, provided a detailed account of the asthma phenotype and offered one of the first pathophysiologic mechanisms of asthma: “the lungs are contracted or drawn together” (3, 5). Asthma was clearly distinguished from other respiratory disorders in 1698 by the English physician Sir John Floyer. Dr. Floyer, who developed asthma following a respiratory infection, provided detailed accounts of asthma signs and symptoms, treatment, prevention, and prognosis. He also described a hereditary component of asthma, and numerous exacerbating factors such as air pollution, infection, cold air, exercise, sleep, psychological stress, and tobacco smoke, and astutely observed the benefits of clean air and environmental change.
By the early 1900s, the physiologic process of bronchial narrowing from constriction of airway smooth muscle had been experimentally documented, and Brodie and Dixon proposed that this process caused asthma (6). In support of this possibility, individuals with asthma were later documented to show an exaggerated bronchoconstrictor response to a variety of agents (7). Indeed, airway hyperreactivity is now a critical component in the consensus definition of asthma and remains a major focus of asthma research. Because this airway hyperreactivity could not consistently be associated with inherent differences in airway smooth muscle behavior between subjects with asthma and normal subjects, it was proposed that “extra-muscular” events caused the development of airway hyperreactivity in asthma and other chronic inflammatory airway diseases (8). Indeed, the development of airway hyperreactivity in other conditions, such as acute and chronic bronchitis, has led to proposals of a shared pathogenic mechanism between asthma and other airway inflammatory diseases and conditions (9–11).
Some recent studies have identified differences in airway smooth muscle cells in animal models of asthma and human subjects with asthma. For example, subjects with asthma have increased levels of smooth muscle myosin light chain kinase (a member of the smooth muscle cell contractile apparatus) and decreased levels of CCAAT/enhancer binding protein a (a transcription factor implicated in the control of smooth muscle cell proliferation) (12, 13). These new findings suggest that inherent differences in the smooth muscle of individuals with asthma may also contribute to increased degrees of bronchoconstriction in asthma, but as developed below, the major focus of further asthma research was aimed at other factors that might influence the function of airway smooth muscle as well as other cell types that control airway behavior.
NERVOUS SYSTEM PARADIGM
The search for factors that control airway caliber began at least by the late 1600s. At that time, a new model was put forth that extended the bronchoconstrictor paradigm; an abnormal nervous system was postulated to cause bronchial constriction and mucosal edema. In 1684, Thomas Willis proposed that asthma was “stirr'd up by the default partly of the Lungs ill-fram'd and partly by default of the Nerves and nervous Fibers appertaining to the breathing parts.” (14). Although originally described in the seventeenth century, this neurogenic hypothesis did not gain much popularity until the descriptions by the English physician Henry Hyde Salter. Dr. Salter suffered from asthma after acquiring whooping cough in his infancy, and in 1868 he proposed that a “perverted nervous action” was the underlying pathophysiologic mechanism that caused the airways to constrict. He also noted a strong association between asthma and hay fever and the importance of inhaled environmental irritants in precipitating paroxysms of asthma (15, 16). This model for asthma pathogenesis remained popular throughout the first part of the twentieth century and continues in some aspects to the present day (17, 18).
Over the past century, work in the field of respiratory neurobiology has determined that the lungs are innervated by the sympathetic, parasympathetic, and nonadrenergic noncholinergic (NANC) nervous systems. In general, the bronchial tone of the airway is determined, at least in part, by a balance of bronchodilating and bronchoconstricting influences of these three systems. Although the sympathetic nervous system has little, if any, direct innervation of the airways, the bronchial smooth muscle cells contain β2-adrenergic receptors that are activated by circulating catacholamines to produce smooth muscle relaxation and bronchodilatation. By contrast, the parasympathetic nervous system directly innervates the airways and activation of this system (at least in some species) results in bronchoconstriction and increased mucous secretion. With these opposing physiologic responses in mind, models that incorporate either a relative deficiency of β-adrenergic bronchodilator action or an overactive parasympathetic nervous system were both proposed as the cause of the abnormal bronchoconstriction in asthma (19, 20).
Interest in the neurogenic paradigm continues in large part from the relatively recent discovery for the influence of the NANC nervous system on airway behavior. The NANC afferent nerves are nociceptive sensory nerves that respond to noxious stimulants and upon activation release a series of excitatory neuropeptides (tachykinins, calcitonin gene-related peptide, and gastrin-releasing peptide). These excitatory neuropeptides are capable of causing bronchoconstriction, vasodilatation, and mucus secretion (21, 22). In addition, NANC efferent nerves can release inhibitory neuropeptides (vasoactive intestinal peptide, peptide with histidine at the N-terminal and methionine at the C-terminal and nitric oxide) that can result in bronchial and vascular dilation. The primary role of the NANC nervous system in asthma pathogenesis still needs to be defined, but models have been proposed that attribute asthma pathogenesis to an imbalance of excitatory and inhibitory NANC neuropeptides (23, 24). This topic also remains of interest as a cause of cough, both in the context of treatment with ACE inhibitors and during airway inflammation.
ALLERGIC PARADIGM
The allergic paradigm for asthma was likely first proposed because of clinical and histopathologic similarities between asthma and allergic conditions. In particular, there is clear overlap in the clinical manifestations of asthma with those of allergic rhinitis (hay fever) and anaphylaxis. In addition, the high incidence of allergen skin-test reactivity and tissue eosinophilia in both asthma and allergic diseases further supports the concept that asthma itself is an allergic disease. Even today, asthma and allergy remain closely linked, and this association has often placed asthma and its pathogenesis within the spectrum of research on allergy.
The proposal that asthma should be considered an allergic disease took scientific shape in the early twentieth century. In 1906, experimental exposure to purified allergens was found to reproduce the symptoms of hay fever and so established this condition as an allergic hypersensitivity to foreign antigen (25, 26). By extrapolation, allergy was also considered to drive asthma pathogenesis, because exposure to inhaled allergen was associated with asthma exacerbations. Although the initial experiments that reproduced the anaphylactic response were performed in 1839, the word anaphylaxis was not formally introduced until 1902, when it was used to describe the response to antigen challenge in a previously sensitized animal (27–29). Shortly thereafter, Samuel Meltzer reported that sensitization and subsequent challenge of a guinea pig produced a lethal anaphylaxis model. In this model, the pathologic findings of constricted and edematous airways were strikingly similar to asthma, thus leading to the conclusion that asthma was an anaphylactic phenomenon (30, 31).
These experimental observations were quickly translated into studies of human subjects. Skin-test reactivity to specific antigens could be associated with allergen-induced exacerbations of asthma (25), and eosinophil recruitment, a hallmark of an allergic response, was identified in the sputum, blood, and airways of subjects with asthma (18, 32, 33). Allergen skin-testing not only aided the physician in diagnosing an allergic diathesis but also enabled the use of environmental avoidance and allergen desensitization to treat allergic disease (33–35). At this time, the allergic paradigm had developed to the point where it was felt that asthma attacks occurred in sensitized subjects but that some other factor was also needed to precipitate an asthma attack.
Analysis of the precipitating factors for asthma soon indicated that some subjects with asthma exhibited an allergic diathesis, whereas other subjects did not. From a case series of 648 individuals with asthma, subjects were broadly categorized as having either extrinsic or intrinsic asthma depending on the origin of the precipitating factor (36). Extrinsic asthma occurred in hypersensitive subjects, and exacerbations were induced by exposure to inhaled allergens that originated from outside the body. This condition was also associated with hay fever, skin-test reactivity, sputum eosinophils, a genetic predisposition, and sensitivity to inhaled animal dander, pollen, or vegetable dust. Alternatively, intrinsic asthma was precipitated by something inside the body, rather than an extrinsic allergen, and was associated with respiratory infection (bacterial or viral), a reflex from upper airway irritation, or neurosis such as anxiety (36). This clinical classification scheme suggested the possibility that asthma is a heterogeneous disease, and indeed, the relative contribution of allergic and nonallergic mechanisms to asthma pathogenesis remains an active area of research.
A seminal event in the definition of allergy came with the discovery and characterization of immunoglobulin (Ig) E. Based on what we might now call passive transfer experiments, IgE was originally described as a humoral factor (designated “reagin”) that could transfer specific allergen sensitivity to a nonallergic individual (37). The protein was finally purified in 1966 and later was found to be a product of B cells. Functional studies demonstrated that IgE was capable of stimulating mast cells and basophils to release mediators that could produce some of the pathophysiologic features of asthma. Findings for IgE-dependent release of histamine, leukotrienes, prostacyclins, and cytokines from mast cells and basophils provided a critical step toward establishing the role of cellular mediators in asthma pathogenesis (38).
MEDIATOR PARADIGM
The mediator paradigm for asthma derived from the proposal that specific cellular mediators are the critical agents that produce the downstream cellular processes responsible for the asthma phenotype. Rapid advances in protein and lipid biochemistry allowed for the purification, synthesis, and detection of these biologically active agents as well as the development of specific inhibitors. The ability to experimentally manipulate these mediators during in vitro and in vivo experiments provided a powerful new approach to define the cellular mechanisms that resulted in bronchoconstriction, vasodilatation, increased capillary permeability, and enhanced mucous secretion. The search for asthma mediators as well as specific and therapeutic inhibitors of mediator action continues in earnest today.
Perhaps the earliest proposal for a mediator of asthma was histamine. As noted above, histamine was implicated in asthma pathogenesis because increased histamine levels were present in animal models of allergic anaphylaxis (27, 39), blood cells from sensitized subjects released histamine in response to allergen (40), and the manifestations of allergic asthma could be imitated by administration of histamine (41). However, it became evident that histamine could not account for all of the manifestations of allergy. Additional effects were attributed to a slow-reacting substance of anaphylaxis (SRS-A) that could cause the pathophysiologic abnormalities of anaphylaxis and, like histamine, could also cause bronchoconstriction, increased capillary permeability, increased mucous secretion, and enhanced eosinophil recruitment. Pioneering studies by Charles Parker, Robert Orange, and others eventually gave way to lipid biochemistry by Matts Hamberg, Bengt Samuelsson, and others that led to the discovery of a family of arachidonic acid products designated as leukotrienes (42). The development and application of high-performance liquid chromatography and gas chromatography-mass spectrometry allowed for the structural elucidation of cysteinyl leukotrienes and their identity as the SRS-A (43, 44).
Additional research has subsequently identified series upon series of lipid, protein, peptide, nucleotide, and nucleic acid mediators implicated in asthma pathogenesis. These discoveries often led to the development of molecular cascades that attempted to define the time course of mediator generation, release, and action (45). Collectively, the framework of the mediator paradigm led to the identification of specific targets involved in asthma pathogenesis. Importantly, this paradigm shifted the research focus of asthma pathogenesis from pathophysiologic processes (such as bronchoconstriction, mucous hypersecretion, and edema) to the biochemical signals that caused these abnormalities. The next paradigms for asthma pathogenesis tried to integrate these cell and molecular events with the growing fields of immunology, microbiology, and genetics.
INFLAMMATORY PARADIGM
Perhaps the most widely accepted paradigm for asthma pathogenesis is one based on airway inflammation. This paradigm rests on the proposal that excessive and possibly inappropriate airway inflammation is the major pathophysiologic process that drives the asthma phenotype. As developed below, this concept was formalized in animal models of asthma, but before and certainly after this experimental work, several lines of evidence from human subjects also supported the role for airway inflammation in asthma pathogenesis. Thus, tissue from autopsy cases and later through fiberoptic bronchoscopy invariably demonstrated airway mucosal inflammation in subjects with asthma. In addition, the extent of airway inflammation often correlated with exacerbations of asthma and disease severity. Moreover, anti-inflammatory treatment with glucocorticoids often decreased airway inflammation and improved manifestations of asthma, while discontinuation of anti-inflammatory treatment often led to increased mucosal inflammation and worsening of asthma traits.
We typically assign the origins of the inflammatory paradigm of asthma to the early 1980s, and indeed this was the time when the model was formally developed. One of the first studies that quantified the degree of experimental airway inflammation also suggested that nonallergic inflammation directed by airway epithelial cells may be responsible for airway hyperreactivity (46). While these observations and others served to focus modern asthma research on inflammation in first broad and then narrow strokes, previous work as early as 1868 had also incorporated vascular and inflammatory abnormalities as an inciting process in asthma pathogenesis. At that time, Dr. Salter wrote that “…the inflammation or congestion of the mucous surface appears to be the stimulus that, through the nerves of air-tubes, excites the muscular wall to contract” (15, 16). The presence of airway inflammation was also described in detail in a large autopsy series of subjects that died from status asthmaticus in 1922. This series described the clinical presentation and classic histopathologic features of asthma that include: hypertrophy of the bronchial glands and muscles, thickening of the basement membrane, airway epithelial cell injury, mucous cell metaplasia, and leukocyte accumulation in the subepithelial and epithelial layers of the airway. These inflammatory cells included eosinophils as well as neutrophils, small mononuclear round cells (likely lymphocytes), and large mononuclear pigmented cells (likely macrophages). In this series, not all subjects were found to have eosinophilic infiltration of the airways, suggesting to the authors that there may also be nonallergic subtypes of asthma (32).
The development of the inflammation paradigm was dependent on characterizing the immune cell infiltrate into airway tissue and so was significantly advanced by the use of fiberoptic bronchoscopy. This approach provided a relatively noninvasive tool that enabled researchers to sample and analyze lung tissue and bronchoalveolar lavage fluid from living subjects with asthma. In addition, the use of bronchoscopy in research allowed for comparison of airway samples from individuals with different disease severity (mild to severe), disease activity (stable versus flare), treatment modalities (on versus off glucocorticoid treatment), and experimental interventions (pre- versus post-allergen challenge). In general, these studies confirmed previous autopsy findings by demonstrating the presence of mucosal inflammation in subjects with asthma (47, 48). In addition to the presence of mucosal inflammation, in some studies the extent of inflammation correlated with disease severity (49) and the inflammatory cell accumulation was increased during spontaneous and experimentally induced flares (50–52).
Of particular interest to the issue of airway inflammation was (and still is) better definition and understanding of the mechanisms responsible for the beneficial actions of glucocorticoids in asthma. Indeed, early studies of glucocorticoid effect on airway tissue helped to develop the concept that inflammation was an important cause of the asthma phenotype. For example, experimental exposure of sensitized individuals with asthma to inhaled allergens can produce a delayed phase of airflow limitation, referred to as the late asthmatic response (53). Bronchoscopic studies indicated that this late response is also associated with the accumulation of inflammatory cells and bronchospastic mediators, and that glucocorticoids can blunt both the accumulation of inflammatory cells and airflow limitation associated with the late response (54–56). Furthermore, glucocorticoid administration decreases the inflammatory cell accumulation in the airway in naturally occurring asthma, and this decrease in inflammatory cell accumulation correlates with improved airway hyperreactivity and symptoms (57). Conversely, withdrawal of glucocorticoids can result in increased mucosal inflammation and worsening of airway hyperreactivity and asthma symptoms (58–61). These relationships between anti-inflammatory treatment, extent of inflammation, and disease symptoms provided additional proof-of-concept to support the role of airway inflammation as a major factor in asthma pathogenesis.
TH2-STYLE INFLAMMATORY PARADIGM
Coincident with mucosal inflammation being identified as a key component of asthma pathogenesis, it also became evident that there were distinct patterns of airway inflammation. Based on the behavior of the adaptive immune response in mice, these inflammatory patterns were categorized as T helper type 1 (Th1) or type 2 (Th2), and were distinguished from each other by development of distinct subsets of CD4+ T cells (62). This phase of asthma research (which is still developing) is highly influenced by immunology and immunogenetics developed in the murine system. In that system, the Th2 inflammatory pattern is driven by interleukin (IL)-4 and is characterized by CD4+ T cell secretion of IL-4, IL-5, and IL-13, plasma cell production of IgE, and eosinophil infiltration of airway tissue. This type of response is often typical of parasitic infection. By contrast, the Th1 inflammatory pattern is driven by IL-12 and is characterized by CD4+ T cell secretion of interferon (IFN)-γ. This type of response is typical of infections with intracellular pathogens (especially viruses). Under some circumstances, the two types of T cell responses display reciprocal inhibition such that IL-4 inhibits the development of Th1 cells while IL-12 inhibits Th2 cell development.
Evidence of a Th2 inflammatory profile in samples from subjects with asthma led to the Th2 hypothesis for asthma (63). In simple terms for what is an extremely complex system, this hypothesis states that predisposition to a Th2 response allows for the production of cytokines that produce the asthma phenotype. The cellular source for cytokine production may be CD4+ T cells or NKT cells, although other immune cells (e.g., mast cells or eosinophils) may also produce the same types of cytokines. An extension of this proposal (the so-called hygiene hypothesis) has been put forward to account for the increasing frequency of the excessive and persistent Th2 inflammatory phenotype found in the population. The hygiene hypothesis suggests that a combination of cleaner environment and the use of vaccines and antibiotics have resulted in the persistence of a sustained newborn-like immune response that is primarily skewed toward Th2 behavior. Thus, the relatively sterile environment of the newborn prevents the normal maturation of the immune response from an early Th2-prone response (seen at birth) to a Th1 response (seen after infancy).
Although these ideas remain intriguing, other work has repeatedly challenged the hygiene proposal as an oversimplification of the role of the IFN-signaling pathway (64). Moreover, the presence of a dichotomous CD4+ T cell response has not been clearly demonstrated in humans. Indeed, there is some evidence that both Th1 and Th2 cytokines may be overproduced in asthma and that Th1-style signaling is increased in the airway epithelium of subjects with asthma (65–67). Furthermore, pharmacologic agents that have clear benefits, such as glucocorticoids, appear to actually skew the immune response toward a Th2 response, and trials aimed at inhibiting Th2 cytokines or augmenting a Th1 immune response have demonstrated a decrease in eosinophils but no change in airway hyperreactivity or asthma symptoms (68–70).
INNATE IMMUNE PARADIGM
The possibility that asthma may also be influenced by the innate immune system derived from the observation that mucosal epithelial cells could produce inflammatory mediators and cytokines that direct immune cells into the airway tissue. Subsequent work indicated that the adaptive immune response requires additional signals to ensure an appropriate response toward pathogens rather than self or innocuous environmental antigens. The innate immune response appears to provide these additional signals (71). Although definitive proof is still forthcoming, it has been widely accepted that the airway epithelial cell actively orchestrates immunity and inflammation. Support is provided by evidence of epithelial production of immunomodulatory glycoproteins, lipids, and cytokines that provide regulatory signals for immune cell traffic and activation in the airway. Specific classes of airway inflammatory proteins that are generated by the airway epithelial cells in the setting of asthma include cell adhesion molecules (such as ICAM-1), chemoattractant cytokines (such as CCL5), and other cytokines (such as IL-12 p80). The same profile of cellular activation is found during the antiviral response, leading to the suggestion that asthmatic inflammation derives from an attempt to evolve a more robust antiviral program. Evidence for altered IFN signaling in the epithelium in asthma further supports this possibility (67). Some have proposed that the innate immune paradigm for asthma pathogenesis may also include other cells of the innate immune system (e.g., macrophages, NK cells, and possibly smooth muscle cells), so these cell populations could also provide inflammatory signals that contribute to asthmatic airway inflammation (72–74).
Recognition of the role of innate immunity in the development of asthma has also led to a broader attempt to reconcile some of the inconsistencies for the Th2 hypothesis for asthma. Thus, animal models have demonstrated that at least some components of a Th1 immune response are required for the development of a Th2 immune response (75, 76), and components of the Th1 as well as the Th2 immune response are increased in subjects with asthma (65, 66). In addition, respiratory viral infections that classically activate Th1 responses and serve as an acute trigger of asthmatic attacks, now appear to have the capacity to produce a chronic asthma phenotype, at least experimentally in the genetically susceptible host (77, 78). In at least one circumstance, it appears that viruses can use a hit-and-run strategy to permanently alter host immune behavior toward an asthma phenotype (79). Extensions of this concept to studies of asthmagenic viruses (such as respiratory syncytial virus and human metapneumovirus) have raised the possibility of modifying immune signaling to provide an advantage to the host over the virus (80, 81). To incorporate these new observations of airway behavior into previous ones, and especially to include abnormalities in airway epithelial behavior and antiviral response as well as allergic predisposition, additional paradigms are needed and are being developed (73). To be fully satisfactory, these new paradigms must also incorporate genetic susceptibility to the development of asthma.
GENETIC PARADIGM
The genetic predisposition to asthma has been described for at least three centuries and has since been repeatedly analyzed in more formal studies. In recent times, the approach to genetic studies of asthma has relied on population studies to link specific chromosomal regions and in turn specific genes to the asthma phenotype. Early studies focused on specific candidate genes (generally identified from cell and molecular biology experiments), whereas later studies have considered more global analysis such as whole genome scans (at least in experimental systems). These approaches to the analysis of complex diseases, including asthma, have been recently reviewed (9), and have made the point that new thinking is necessary to approach this problem. This change has come about with the recognition that each genetic abnormality may only be contributing a small fraction to the overall asthma phenotype, and these variations must be interpreted in the context of how the genome is inherited in blocks (i.e., as haplotypes). In addition, there are critical gene–gene and gene–environment interactions that must be taken into account in the analysis of the genetic basis of complex disease (9). Nonetheless, while difficult to approach from a biologic and computational standpoint, new genetic tools have allowed for further progress. As primary examples, an expanding library of single nucleotide polymorphisms (SNPs) can be used as informative markers of linkage disequilibrium. In addition, the annotation of the genome for humans and other species relevant to experimental models of asthma (e.g., mice, monkeys, and rats) continues to become more comprehensive and so further aids positional cloning and analysis of asthma genes. Techniques combining genetic mapping with gene expression analysis (e.g., by oligonucleotide and chromosomal microarray) are also being applied.
To date, at least 64 human genes have been identified that associate with asthma (82). However, only a small subset of these genes have reproducibly demonstrated positive associations in separate populations and even fewer have demonstrated a change in protein expression in individuals with asthma or an asthma phenotype in animal studies (83, 84). Nonetheless, some interesting developments have already proven useful. For example, the β2-adrenergic receptor has exhibited genetic and corresponding functional diversity that appears to translate into variable responsiveness to treatment with β-adrenergic receptor agonists (85, 86). In addition, early studies in experimental models have indicated that the complex phenotype of asthma can be segregated into individual traits suitable for genetic mapping (73). Other experimental studies indicate that susceptibility to development of the asthma phenotype can be genetically defined, e.g., in the case of the virus-induced asthma phenotype. The development and use of inbred, crossbred, and congenic strains of mice are critical for these types of studies. The genetic approach will no doubt be further refined and so lead to new insights into asthma pathogenesis, but it has been and will continue to be critical to define the asthma phenotype in terms of specific and quantifiable traits.
SUMMARY
Over the past 100 years, a series of models have been proposed to account for the pathophysiologic abnormalities of asthma (summarized in Figure 1). Consistent with the nature of asthma as a complex disease, the models for asthma pathogenesis have also become increasingly complex. Nonetheless, there has been marked progress in defining cellular and molecular mechanisms of normal and abnormal airway behavior. Research has moved from a descriptive functional approach to one that relies on cellular and molecular biology, immunology, microbiology, and genetics/genomics as well as pathophysiology. These disciplines have moved current efforts to define asthma heterogeneity in quantitative and molecular terms and to determine the precise role of the airway inflammation that likely accounts for disease initiation and progression. Current proposals are attempting to incorporate the influence of the innate and adaptive immune systems as well the role of host genetics and environmental stimuli, particularly viral infection. Considerable progress has been made and is continuing at an accelerating pace. We submit that advances in genetics, genomics, proteomics, and lipidomics will further aid in the identification and characterization of distinct mechanistic pathways for driving the asthma phenotype, and that these will provide better biomarkers for the various populations with asthma as well as more effective asthma prevention and treatment regimens.
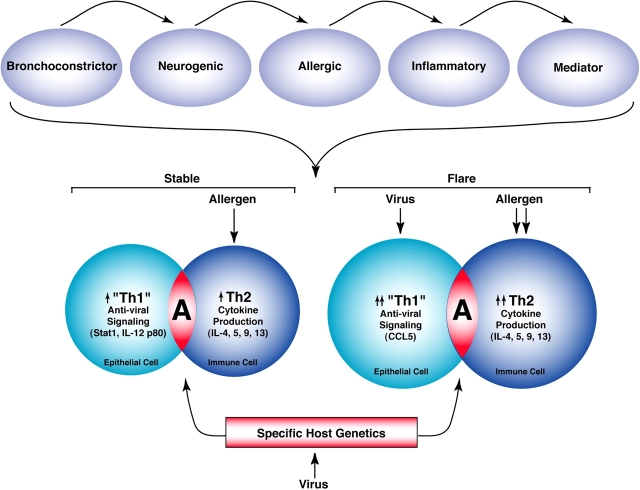
Figure 1.
Evolution of asthma paradigms. Schemes for asthma pathogenesis have ranged from bronchoconstrictor, neurogenic, allergic, inflammatory, and mediator (top panel) as outlined in the text. A current paradigm (bottom panel) attempts to incorporate abnormalities in airway epithelial behavior, antiviral response, and allergic predisposition for the role of the airway immune response in the development of the asthma phenotype. The bottom left panel illustrates how increases in Th1-like antiviral signals (e.g., Stat1 activation and IL-12 p80 expression) in epithelial cells and allergen-driven production of Th2 cytokines (e.g., IL-4, IL-5, IL-9, and IL-13) in immune cells are characteristic of subjects with asthma (designated as an “A”) studied under stable conditions. The bottom right panel illustrates how further increases in epithelial signaling (driven by viral infection) or Th2 cytokine production (driven by further allergen exposure) may develop in subjects with asthma during a flare induced by natural stimuli or by withdrawal from glucocorticoid treatment. In addition, the underlying development of chronically abnormal cellular behavior may depend on respiratory viral infection (and perhaps other environmental stimuli) and a subsequent host response determined by specific genetic programming. The combination of epithelial, viral, and allergic components (as well as the epigenetic characteristics of the process) led to designation of this pathogenesis scheme as an Epi-vir-all paradigm. Modified from Ref. 73.
This work was supported by grants from the National Heart, Lung, and Blood Institute, the Martin Schaeffer Fund, and the Alan A. and Edith L. Wolff Charitable Trust.
Conflict of Interest Statement: M.J.W has no declared conflicts of interest; M.J.H. has no declared conflicts of interest.
References
- 1.Veith I. The yellow emperor's classic of internal medicine. Cambridge, England: Cambridge University Press; 1966.
- 2.Saavedra-Delgado AM, Cohen SG. Huang-Ti, the Yellow Emperor and the Nei Ching: antiquity's earliest reference to asthma. Allergy Proc 1991;12:197–198. [DOI] [PubMed] [Google Scholar]
- 3.Sakula A. A history of asthma. The FitzPatrick lecture 1987. J R Coll Physicians Lond 1988;22:36–44. [PMC free article] [PubMed] [Google Scholar]
- 4.Marketos S, Ballas C. Bronchial asthma in medical literature of Greek antiquity. Hist Sci Med 1982;17:35–39. [PubMed] [Google Scholar]
- 5.Keeney EL. The history of asthma from Hippocrates to Meltzer. J Allergy Clin Immunol 1964;35:215–226. [DOI] [PubMed] [Google Scholar]
- 6.Brodie MD, Dixon TG. The pathology of asthma. Transactions of the Pathological Soc of London 1903;liv:17. [Google Scholar]
- 7.Boushey HA, Holtzman MJ, Sheller JR, Nadel JA. Bronchial hyperreactivity. Am Rev Respir Dis 1980;121:389–413. [DOI] [PubMed] [Google Scholar]
- 8.Solway J, Fredberg JJ. Perhaps airway smooth muscle dysfunction contributes to asthmatic bronchial hyperresponsiveness after all. Am J Respir Cell Mol Biol 1997;17:144–146. [DOI] [PubMed] [Google Scholar]
- 9.Holtzman MJ, Kim EY, Morton JD. Genetic and genomic approaches to complex lung diseases using mouse models. In: Peltz G, editor. Computational genetics and genomics: tools for understanding disease. Totowa, NJ: Humana Press Inc.; 2005. pp. 99–141.
- 10.Bleecker ER. Similarities and differences in asthma and COPD. The Dutch hypothesis. Chest 2004;126:93S–95S. (discussion 159S–161S). [DOI] [PubMed] [Google Scholar]
- 11.Postma DS, Boezen HM. Rationale for the Dutch hypothesis: allergy and airway hyperresponsiveness as genetic factors and their interaction with environment in the development of asthma and COPD. Chest 2004;126:96S–104S. (discussion 159S–161S). [DOI] [PubMed] [Google Scholar]
- 12.Stephens NL, Li W, Jiang H, Unruh H, Ma X. The biophysics of asthmatic airway smooth muscle. Respir Physiol Neurobiol 2003;137:125–140. [DOI] [PubMed] [Google Scholar]
- 13.Roth M, Johnson PR, Borger P, Bihl MP, Rudiger JJ, King GG, Ge Q, Hostettler K, Burgess JK, Black JL, et al. Dysfunctional interaction of C/EBPalpha and the glucocorticoid receptor in asthmatic bronchial smooth-muscle cells. N Engl J Med 2004;351:560–574. [DOI] [PubMed] [Google Scholar]
- 14.Reed CE. The pathogenesis of asthma. Med Clin North Am 1974;58:55–63. [DOI] [PubMed] [Google Scholar]
- 15.Persson CG. On the medical history of xanthines and other remedies for asthma: a tribute to HH Salter. Thorax 1985;40:881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stolkind E. The history of bronchial asthma and allergy. Proc R Soc Med 1933;26:1120–1126. [PMC free article] [PubMed] [Google Scholar]
- 17.West S. Diseases of the organs of respiration. Philadelphia: J. B. Lippincott Company; 1902.
- 18.Powell RD, Hartley PH. On diseases of the lung and pleurae including tuberculosis & mediastinal growths, 5th ed. Philadelphia: P. Blakiston's Son & Co.; 1911. pp. 215–236.
- 19.Simonsson BG, Jacobs FM, Nadel JA. Role of autonomic nervous system and the cough reflex in the increased responsiveness of airways in patients with obstructive airway disease. J Clin Invest 1967;46:1812–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szentivanyi A. The beta adrenergic theory of the atopic abnormality in bronchial asthma. J Allergy 1968;42:203–232. [Google Scholar]
- 21.Maggi CA, Giachetti A, Dey RD, Said SI. Neuropeptides as regulators of airway function: vasoactive intestinal peptide and the tachykinins. Physiol Rev 1995;75:277–322. [DOI] [PubMed] [Google Scholar]
- 22.McDonald DM, Bowden JJ, Baluk P, Bunnett NW. Neurogenic inflammation: a model for studying efferent actions of sensory nerves. Adv Exp Med Biol 1996;410:453–462. [PubMed] [Google Scholar]
- 23.Casale TB, Baraniuk JN. Functional activity of lower airway nerves. In: Busse WW, Holgate ST, editors. Asthma and rhinitis. Cambridge, MA: Blackwell Science; 2000. pp. 866–890.
- 24.Jartti T. Asthma, asthma medication and autonomic nervous system dysfunction. Clin Physiol 2001;21:260–269. [DOI] [PubMed] [Google Scholar]
- 25.Cooke RA, Vander Veer A. Human sensitization. J Immunol 1916;1:201–305. [Google Scholar]
- 26.Wolff-Eisneer A. Das Heufieber: sein Wesen und seine Behandlung. Munchen: Lehmano; 1906.
- 27.Emanuel MB. Histamine and the antiallergic antihistamines: a history of their discoveries. Clin Exp Allergy 1999;29:1–11. (discussion 12). [DOI] [PubMed] [Google Scholar]
- 28.Magendie F. Lectures on blood. Philadelphia: Harrington, Barrington, and Haswell; 1839.
- 29.Portier P. De l'action anaphylactique de certains venins. C R Seances Soc Biol Fil 1902;54:170–172. [Google Scholar]
- 30.Meltzer SJ. Bronchial asthma as a phenomena of anaphylaxis. JAMA 1910;12:1021–1024. [Google Scholar]
- 31.Auer J, Lewis PA. The physiology of the immediate reaction of anaphylaxis in the guinea-pig. J Exp Med 1910;12:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber HL, Koessler KK. The pathology of bronchial asthma. Arch Intern Med 1922;30:689–760. [Google Scholar]
- 33.Cecil RL. A text-book of medicine. Philadelphia: W. B. Saunders Company; 1928. pp. 481–489.
- 34.Walker IC. A clinical study of 400 patients with bronchial asthma. Boston Med Surg J 1918;clxxix:288–300. [Google Scholar]
- 35.Norris GW, Landis HR. Bronchial asthma: diseases of the chest and the principles of physical diagnosis. Philadelphia: W. B. Saunders Company; 1918. pp. 270–276.
- 36.Rackemann FM. A clinical classification of asthma based upon a review of six hundred and forty-eight cases. Am J Med Sci 1921;clxii:802–811. [Google Scholar]
- 37.Prausnitz D, Kustner H. Studien uber die Uberempfindlichkeit. Zentralblatt fur Bakteriologie 1921;86:160–175. [Google Scholar]
- 38.Ishizaka K, Ishizaka T, Hornbrook MM. Physicochemical properties of reaginic antibody. V. Correlation of reaginic activity wth gamma-E-globulin antibody. J Immunol 1966;97:840–853. [PubMed] [Google Scholar]
- 39.Dale HH. Some chemical factors in the control of circulation. Lecture 3. Lancet 1929;i:1285–1290.
- 40.Katz G, Cohen S. Experimental evidence for histamine release in allergy. JAMA 1941;117:1782–1783. [Google Scholar]
- 41.Weiss S, Robb GP, Blumgart HL. The velocity of blood flow in health and disease as measured by the effect of histamine on the minute vessels. Am Heart J 1929;4:664. [Google Scholar]
- 42.Holtzman MJ. Epithelial cell regulation of arachidonic acid oxygenation. In: Farmer SG, Hay DWP, editors. The airway epithelium: physiology, pathophysiology, and pharmacology, lung biology in health and disease. New York: Marcel Dekker; 1991. pp. 65–115.
- 43.Lewis RA, Austen KF, Drazen JM, Clark DA, Marfat A, Corey EJ. Slow reacting substances of anaphylaxis: identification of leukotrienes C-1 and D from human and rat sources. Proc Natl Acad Sci USA 1980;77:3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy RC, Hammarstrom S, Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci USA 1979;76:4275–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Busse W, Lemanske RF. Asthma. N Engl J Med 2001;344:350–362. [DOI] [PubMed] [Google Scholar]
- 46.Holtzman MJ, Aizawa H, Nadel JA, Goetzl EJ. Selective generation of leukotriene B4 by tracheal pithelial cells from dogs. Biochem Biophys Res Commun 1983;114:1071–1076. [DOI] [PubMed] [Google Scholar]
- 47.Djukanovic R, Roche WR, Wilson JW, Beasley CR, Twentyman OP, Howarth RH, Holgate ST. Mucosal inflammation in asthma. Am Rev Respir Dis 1990;142:434–457. [DOI] [PubMed] [Google Scholar]
- 48.Bousquet J, Jeffery PK, Busse WW, Johnson M, Vignola AM. Asthma: from bronchoconstriction to airways inflammation and remodeling. Am J Respir Crit Care Med 2000;161:1720–1745. [DOI] [PubMed] [Google Scholar]
- 49.Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, Bousquet J. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med 1998;157:403–409. [DOI] [PubMed] [Google Scholar]
- 50.De Monchy JG, Kauffman HF, Venge P, Koeter GH, Jansen HM, Sluiter HJ, De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis 1985;131:373–376. [DOI] [PubMed] [Google Scholar]
- 51.Metzger WJ, Zavala D, Richerson HB, Moseley P, Iwamota P, Monick M, Sjoerdsma K, Hunninghake GW. Local allergen challenge and bronchoalveolar lavage of allergic asthmatic lungs. Description of the model and local airway inflammation. Am Rev Respir Dis 1987;135:433–440. [DOI] [PubMed] [Google Scholar]
- 52.Laitinen LA, Laitinen A, Heino M, Haahtela T. Eosinophilic airway inflammation during exacerbation of asthma and its treatment with inhaled corticosteroid. Am Rev Respir Dis 1991;143:423–427. [DOI] [PubMed] [Google Scholar]
- 53.Herxheimer H. The late bronchial reaction in induced asthma. Int Arch Allergy Appl Immunol 1952;3:323–328. [DOI] [PubMed] [Google Scholar]
- 54.Booij-Noord H, Orie NG, De Vries K. Immediate and late bronchial obstructive reactions to inhalation of house dust and protective effects of disodium cromoglycate and prednisolone. J Allergy Clin Immunol 1971;48:344–354. [DOI] [PubMed] [Google Scholar]
- 55.Burge PS, Efthimiou J, Turner-Warwick M, Nelmes PT. Double-blind trials of inhaled beclomethasone diproprionate and fluocortin butyl ester in allergen-induced immediate and late asthmatic reactions. Clin Allergy 1982;12:523–531. [DOI] [PubMed] [Google Scholar]
- 56.Dworski R, Fitzgerald GA, Oates JA, Sheller JR. Effect of oral prednisone on airway inflammatory mediators in atopic asthma. Am J Respir Crit Care Med 1994;149:953–959. [DOI] [PubMed] [Google Scholar]
- 57.Barnes PJ. Inhaled glucocorticoids for asthma. N Engl J Med 1995;332:868–875. [DOI] [PubMed] [Google Scholar]
- 58.Vathenen AS, Knox AJ, Wisniewski A, Tattersfield AE. Time course of change in bronchial reactivity with an inhaled corticosteroid in asthma. Am Rev Respir Dis 1991;143:1317–1321. [DOI] [PubMed] [Google Scholar]
- 59.Haahtela T, Vidgren M, Nyberg A, Korhonen P, Laurikainen K, Silvasti M. A novel multiple dose powder inhaler: salbutamol powder and aerosol give equal bronchodilatation with equal doses. Ann Allergy 1994;72:178–182. [PubMed] [Google Scholar]
- 60.Hart L, Lim S, Adcock I, Barnes PJ, Chung KF. Effects of inhaled corticosteroid therapy on expression and DNA-binding activity of nuclear factor-κB in asthma. Am J Respir Crit Care Med 2000;161:224–231. [DOI] [PubMed] [Google Scholar]
- 61.Castro M, Bloch SR, Jenkerson MV, DeMartino S, Hamilos DL, Cochran RB, Zhang XE, Wang H, Bradley JP, Schechtman KB, et al. Asthma exacerbations after glucocorticoid withdrawal reflects T cell recruitment to the airway. Am J Respir Crit Care Med 2004;169:842–849. [DOI] [PubMed] [Google Scholar]
- 62.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone: I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol 1986;136:2348–2357. [PubMed] [Google Scholar]
- 63.Robinson DS, Hamid Q, Ying S, Tsicopoulos A, Barkans J, Bentley AM, Corrigan C, Durham SR, Kay AB. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992;326:298–304. [DOI] [PubMed] [Google Scholar]
- 64.Holtzman MJ. Where are the gaps in asthma research? A counter-perspective. J Allergy Clin Immunol 2003;111:244–247. [DOI] [PubMed] [Google Scholar]
- 65.Cembrzynska-Nowak M, Szklarz E, Inglot AD, Teodorczyk-Injeyan JA. Elevated release of tumor necrosis factor-α and interferon-γ by bronchoalveolar leukocytes from patients with bronchial asthma. Am Rev Respir Dis 1993;147:291–295. [DOI] [PubMed] [Google Scholar]
- 66.Krug N, Madden J, Redington AE, Lackie P, Djukanovic R, Schauer U, Holgate ST, Frew AJ, Howarth PH. T-cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Am J Respir Cell Mol Biol 1996;14:319–326. [DOI] [PubMed] [Google Scholar]
- 67.Sampath D, Castro M, Look DC, Holzman MJ. Constitutive activation of an epithelial signal transducer and activator of transcription (STAT) pathway on asthma. J Clin Invest 1999;103:1353–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function*. Annu Rev Immunol 2000;18:309–345. [DOI] [PubMed] [Google Scholar]
- 69.Leckie MJ, ten Brinke A, Khan J, Diamant Z, O'Connor BJ, Walls CM, Mathur AK, Cowley HC, Chung KF, Djukanovic R, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000;356:2144–2148. [DOI] [PubMed] [Google Scholar]
- 70.Bryan SA, O'Connor BJ, Matti S, Leckie MJ, Kanabar V, Khan J, Warrington SJ, Renzetti L, Rames A, Bock JA, et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet 2000;356:2149–2153. [DOI] [PubMed] [Google Scholar]
- 71.Medzhitov R, Janeway C Jr. Innate immunity. N Engl J Med 2000;343:338–344. [DOI] [PubMed] [Google Scholar]
- 72.Holtzman MJ, Castro M, Look DC, O'Sullivan M, Walter MJ. Regulation of epithelial-leukocyte interactions and epithelial immune-response genes. In: Busse W, Holgate S, editors. Asthma and Rhinitis. Cambridge, MA: Blackwell Scientific; 2000. pp. 784–800.
- 73.Holtzman MJ, Morton JD, Shornick LP, Tyner JW, O'Sullivan MP, Antao A, Lo M, Castro M, Walter MJ. Immunity, inflammation, and remodeling in the airway epithelial barrier: epithelial-viral-allergic paradigm. Physiol Rev 2002;82:19–46. [DOI] [PubMed] [Google Scholar]
- 74.Panettieri RA Jr. Airway smooth muscle: immunomodulatory cells? Allergy Asthma Proc 2004;25:381–386. [PubMed] [Google Scholar]
- 75.Randolph DA, Carruthers CJ, Szabo SJ, Murphy KM, Chaplin DD. Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J Immunol 1999;162:2375–2383. [PubMed] [Google Scholar]
- 76.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest 1999;103:175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemanske RF Jr. Viruses and asthma: Inception, exacerbation, and possible prevention. J Pediatr 2003;142:S3–S7. (discussion S7–S8). [DOI] [PubMed] [Google Scholar]
- 78.Stein RT, Sherrill D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, Wright AL, Martinez FD. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet 1999;354:541–545. [DOI] [PubMed] [Google Scholar]
- 79.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest 2002;110:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lo MS, Brazas RM, Holtzman MJ. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and type I interferon responsiveness. J Virol 2005; (in press). [DOI] [PMC free article] [PubMed]
- 81.Sumino KC, Agapov E, Pierce RA, Trulock EP, Pfeifer JD, Ritter JH, Gaudreault-Keener M, Storch GA, Holtzman MJ. Detection of severe human metapneumovirus infection by real-time PCR and distinct histopathology. J Infect Dis (In press) [DOI] [PMC free article] [PubMed]
- 82.Weiss ST, Raby BA. Asthma genetics 2003. Hum Mol Genet 2004;13:R83–R89. [DOI] [PubMed] [Google Scholar]
- 83.Cookson W, Moffatt M. Making sense of asthma genes. N Engl J Med 2004;351:1794–1796. [DOI] [PubMed] [Google Scholar]
- 84.Kere J, Laitinen T. Positionally cloned susceptibility genes in allergy and asthma. Curr Opin Immunol 2004;16:689–694. [DOI] [PubMed] [Google Scholar]
- 85.Grayson MH, Holtzman MJ. Asthma. In: Dale DC, editor. ACPMedicine. New York: WebMD Publishing; 2005.
- 86.Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, Deykin A, Fagan JK, Fahy JV, Fish J, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet 2004;364:1505–1512. [DOI] [PubMed] [Google Scholar]