Abstract
Exuberant inflammatory responses are associated with respiratory failure during Pneumocystis pneumonia. Alveolar epithelial cells (AECs) promote Pneumocystis attachment and proliferation, but also contribute prominently to host cytokine-mediated inflammation during pneumonia. Recent investigations indicate that AECs produce macrophage inflammatory protein-2 (MIP-2) and tumor necrosis factor-α (TNF-α) following challenge with Pneumocystis carinii. Nuclear factor-κB (NF-κB) is a ubiquitous transcription factor critical for regulation of proinflammatory cytokine expression. Herein, we assess rat AEC NF-κB responses to challenge with a P. carinii β-glucan cell wall component (PCBG). Prominent nuclear translocation of p65 NF-κB was demonstrated following PCBG challenge. NF-κB activation was in part mediated through Protein Kinase C (PKC) signaling pathways. PCBG challenge of AECs was also shown to induce MIP-2 and TNF-α mRNA production, a response that was ameliorated by NF-κB inhibition. MIP-2 protein expression was also dramatically increased by PCBG challenge, in a manner that was significantly attenuated by both PKC and NF-κB inhibition. The data further demonstrate that AEC chemokine responses were not mediated by the recently described dectin-1 receptor, but instead involved participation of cell surface lactosylceramide. These data support a significant role for AECs in host responses during Pneumocystis pneumonia, and further indicate that β-glucan induces inflammatory cytokine production through NF-κB–dependent mechanisms.
Keywords: pneumocystis, alveolar epithelial cells, nuclear factor-κB, chemokines
Pneumocystis pneumonia remains a significant cause of morbidity and mortality in immunocompromised hosts. Despite available medications, the case mortality for Pneumocystis pneumonia continues to range between 15 and 40%, with greater mortality observed in patients immunocompromised by conditions other than AIDS (1–3). Severe Pneumocystis pneumonia is characterized by intense neutrophilic lung inflammation associated with diffuse alveolar damage, gas exchange impairment, and respiratory failure (4, 5). Notably, the degree of neutrophilic inflammation has been shown to be a stronger predictor of respiratory failure and death than organism burden during infection (1, 6–8). The mechanism by which Pneumocystis organisms induce lung inflammation remains incompletely elucidated, but recent studies implicate Pneumocystis β-1,3-glucan cell wall constituents in strongly promoting lung inflammation (9, 10).
Alveolar epithelial cells (AECs) exert central activities in the pathogenesis of Pneumocystis pneumonia. Ultrastructural studies reveal that Pneumocystis organisms closely associate with the alveolar epithelium of infected human and animal lungs, supporting the hypothesis that binding of Pneumocystis to AECs is essential for the establishment of infection (11, 12). Evidence also suggests that AECs, once considered simply passive gas exchange barriers, also actively participate in host defense against infectious agents (13–16). We recently demonstrated that rat AECs produce macrophage inflammatory protein-2 (MIP-2) in response to Pneumocystis carinii β-glucan (PCBG) challenge (17). MIP-2 represents the rodent homolog of the human C-X-C chemokine interleukin-8, a potent neutrophil chemoattractant (18). We also demonstrated that AECs produce tumor necrosis factor-α (TNF-α), a cytokine known to have numerous proinflammatory activities, following PCBG challenge. Recent investigations further reveal that, on a cell-by-cell basis, AECs produce more of these cytokines than do professional innate immune effector cells such as alveolar macrophages (17). Such observations suggest that MIP-2 and TNF-α produced by AECs substantially contribute to pulmonary inflammation observed during Pneumocystis pneumonia.
Nuclear factor-κB (NF-κB) functions as an important transcriptional regulator of many inflammatory responses. When inactive, this ubiquitous member of the Rel-related transcription factor family exists as a cytosolic heterodimer of p65 (NK-κB1) and p50 (RelA) subunits, associated with the inhibitory I-κB subunit. NF-κB activation results in I-κBα phosphorylation and dissociation from p65/p50 components, allowing the heterodimer to translocate to the nucleus and promote proinflammatory gene transcription (19, 20). Previous studies have confirmed the importance of NF-κB in inflammatory responses of alveolar macrophages to PCBG (9, 10, 21, 22). However, the surface receptor mechanisms initiating AEC activation are distinct from those mediating macrophage activation, and the mechanisms of inflammatory gene activation in AECs remain undefined (17, 21).
In particular, it is not known whether NF-κB participates in regulation of the inflammatory responses of alveolar epithelial cells following challenge with PCBG. A greater understanding of the signaling pathways involved in AEC inflammatory responses may yield potential novel therapeutic targets for Pneumocystis pneumonia. Accordingly, the current study was performed to determine the role of NF-κB in PCBG-induced AEC inflammatory responses, specifically the production of the proinflammatory chemokines MIP-2 and TNF-α. In addition, we assessed the roles of the recently described dectin-1 receptor and Protein Kinase C (PKC) signaling pathways in mediating AEC responses to this major Pneumocystis cell wall component.
MATERIALS AND METHODS
Reagents and Organisms
Unless otherwise noted, all general reagents were from Sigma Chemical Co. (St. Louis, MO). The animal experiments were reviewed and approved by the Mayo Institutional Animal Care and Usage Committee before initiation of these studies. P. carinii was originally obtained through the American Type Culture Collection (ATCC, Bethesda, MD) and maintained in our colony of dexamethasone-treated immunosuppressed Long Evans rats (HSD, Inc., Indianapolis, IN), as we previously reported (11, 21). Monoclonal antibody 2A11, which recognizes the dectin-1 receptor, was generously provided by Dr. Gordon Brown, University of Cape Town, South Africa (23).
PCBG was prepared as we recently described (17). Briefly, P. carinii organisms were isolated from lungs of heavily infected rats, autoclaved, and disrupted by ultrasonication. Glucan was isolated by NaOH digestion and lipid extraction, washed first with 0.1% SDS, and then vigorously washed with distilled physiologic saline to remove the detergent. This isolate was previously characterized to contain a predominantly glucose-rich complex carbohydrate complex, which was degraded by β-1,3-glucanases. Only those β-glucan preparations which consistently displayed < 0.125 units of endotoxin by the Limulus amebocyte lysate method were used in these studies (17).
Alveolar Epithelial Cell Isolation
Rat alveolar epithelial cells (AECs) were isolated as described by Dobbs and colleagues (24). In short, pentobarbital-anesthetized rats (∼ 250 g) were killed by transection of the inferior vena cava. The pulmonary vasculature was perfused with saline. The trachea was isolated and the lungs depleted of alveolar macrophages by multiple lavages. AECs were separated from the basement membrane by incubation with porcine elastase, and the lungs were minced, filtered, and centrifuged. Recovered cells were suspended in serum-free DMEM and incubated for 1 h in Petri dishes coated with rat IgG to remove residual macrophages. The supernatant was collected and centrifuged before suspension of the epithelial cells in DMEM with 10% bovine calf serum with penicillin (50,000 units/liter) and streptomycin (50 mg/liter). AECs were counted using a standard hemocytometer. The AECs were incubated (37°C, 5% CO2) and allowed to adhere to culture plates for at least 48 h. The media was changed after the initial 24 h. After 48 h, the cells had largely lost lamellar inclusion bodies, and were quite spread and attached, displaying morphology more reminiscent of Type I cells than the Type II cell morphology originally observed immediately after isolation. Prior studies have demonstrated that Pneumocystis interacts predominantly with Type I cells, but also to a lesser degree with Type II cells (1, 12).
Fluorescence Microscopy to Detect NF-κB Translocation
To initially demonstrate NF-κB activation following AEC stimulation with PCBG, we evaluated nuclear translocation of immunofluorescently-labeled p65 NF-κB (21). AECs were isolated and cultured on sterile fibronectin-coated 22 × 22 mm glass coverslips in 6-well tissue culture plates, then challenged with PCBG (100 μg/ml) for 1 h before fixation with 2% paraformaldehyde for 10 min at 37°C. After fixation, the cells were permeabilized with methanol for 2 min at 22°C, and rinsed with phosphate-buffered saline (PBS). Nonspecific binding was blocked with 5% goat serum in PBS for 30 min at room temperature. The cells were then incubated with goat anti-mouse p65 (0.5 μg/ml; Santa Cruz Biotechnology, Santa Cruz, CA) for 60 min at room temperature. Coverslips were next washed extensively and incubated for 60 min with a 1:50 dilution of Texas Red fluorophore–conjugated donkey anti-goat polyclonal antibody (Jackson ImmunoResearch Laboratories, New Grove, PA). Nuclear counterstaining was performed with 4′,6-diamidino-2-phenylindole (DAPI; 0.1 μg/ml). Following extensive washing, the coverslips were mounted on slides and examined with fluorescence microscopy (Olympus IX70 microscope, Melville, NY) using appropriate optics (Texas Red: excitation = 540/25 nm; emission = 620/60 nm; DAPI: excitation = 360/40 nm; emission = 460/50 nm).
Electrophoretic Mobility Shift Assay for NF-κB
To further evaluate NF-κB activation, nuclear and cytosolic fractions were prepared from AECs with or without PCBG stimulation (100 μg/ml) (21). At specified times, the cells were scraped and suspended in 1 ml of buffer A (10 mM HEPES, pH 7.9; 1.5 mM MgCl2; 10 mM KCl), freshly supplemented with 0.5 mM dithiothreitol, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 2 μg/ml pepstatin, and 1 μM phenylmethylsulfonyl fluoride. Lysis buffer (buffer A containing 0.1% NP-40) was applied for 10 min, followed by centrifugation (6,500 rpm, 3 min, 4°C), with the supernatants collected as the cytosolic fractions. The remaining pellets were resuspended in 15 μl of buffer B (20 mM HEPES, pH 7.9, 25% vol/vol glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA), supplemented with the above protease inhibitors. Nuclear suspensions were agitated for 30 min at 4°C, and then centrifuged (12,000 × g, 20 min, 4°C) to remove debris. The resulting supernatants (nuclear fractions) were collected and diluted with 30 μl of Buffer C (20 mM HEPES, pH 7.9; 20% vol/vol glycerol; 0.05M KCl; 0.2 mM EDTA) with protease inhibitors. Cytosolic and nuclear fractions were stored at −70°C. Protein concentrations were determined by Coomassie protein assay (Pierce Chemical Co., Davenport, IA) and referenced against bovine serum albumin standards. Electrophoretic mobility shift assays (EMSA) were then performed on nuclear lysates, as described (21). A double-stranded DNA probe that binds NF-κB (5′ AGT TGA GGG GAC TTT CCC AGC 3′; Santa Cruz Biotechnology) was radiolabeled with (γ-32P)-ATP and incubated with 5 μg of nuclear extract for 30 min at 22°C, separated on 6% polyacrylamide gels, and visualized by autoradiography.
The Role of Signaling Kinases in PCBG-Induced NF-κB Activation
To determine the potential roles of mitogen-activated protein kinase (MAPK) and PKC signaling pathways during NF-κB activation in response to PCBG, AECs were pre-incubated in the presence of the upstream MEK1 (MAPK kinase) inhibitor (UO126, 40 μM; Promega, Madison, WI), the p38 MAPK inhibitor (SB 203580, 40 μM; Promega), or the PKC inhibitor (Gö6976 X μM; Calbiochem, Inc., La Jolla, CA) for 45 min before stimulation with PCBG (5 × 106 particles/ml). Concentrations of inhibitors tested were those previously determined to suppress kinase activities, and were not associated with significant cellular toxicity (25–27). Nuclear extracts were collected and NF-κB p50 activity measured using the Mercury TransFactor Kit (Clontech, Palo Alto, CA), a solid phase immunoassay measuring nuclear content of the p50 NF-κB component. Briefly, AECs were washed with PBS and incubated with lysis buffer (10 mM HEPES, pH 7.9, 1.5 mM MgCl, 10 mM KCl, 0.1 M DTT, and a protease inhibitor cocktail) for 15 min on ice. After washings, a narrow-gauge (No. 27) syringe (Tyco Health Care, Mansfield, MA) was used to slowly draw and eject the cell suspension ten times. The suspension was centrifuged (10,000 × g, 20 min), and the pellet resuspended in nuclear extraction buffer (20 mM HEPES, pH 7.9, 1.5 mM MgCl, 0.42 M NaCl, 0.2 mM EDTA, 25% vol/vol glycerol). After disrupting the nuclei with a narrow-gauge syringe, the nuclear suspension was shaken for 30 min at 4°C and then centrifuged (20,000 × g, 5 min). Nuclear extract protein concentrations were determined by Coomassie assay. The nuclear extracts were added to plates coated with oligonucleotides containing the consensus binding sequence for NF-κB p50. Samples were also added to mutant DNA-coated wells as a background nonspecific DNA binding control. Bound transcription factor was detected using a primary antibody directed against NF-κB. After washings, the wells were incubated with anti-rabbit IgG-HRPO–conjugated secondary antibody (1:1,000 dilution), followed by additional washing and addition of 3′,3′,5′,5′ tetramethylbenzidine substrate, with absorbances measured at 655nm.
Northern Analysis of Inflammatory Gene Expression following PCBG Stimulation
We next assessed whether NF-κB activity regulates expression of inflammatory genes following PCBG challenge. To accomplish this, AECs were cultured (3 × 107 cells/well) with or without the NF-κB inhibitor pyrrolidine dithiocarbamate (PDTC) at the indicated concentrations for 2 h before and throughout a subsequent 4-h incubation with PCBG (100 μg/ml). RNA was extracted from each sample (Trizol Reagent; Invitrogen Life Technologies, Inc., Carlsbad, CA), and equal RNA (5.0 μg) separated through 1.2% agarose in the presence of 2.2 M formaldehyde, and transferred to nitrocellulose. To assess expression of prototypic inflammatory genes, a 299-bp TNF-α probe was labeled with [γ-32P] dCTP (Amersham Biosciences, Piscataway, NJ) by a random primer method, added to the hybridization solution (1 × 106 cpm/ml), and incubated with the membranes for 1 h at 68°C. After hybridization, the membranes were washed four times with 2× salt sodium citrate solution (SSC, where 1× solution contained 150 mM NaCl and 15 mM sodium citrate; pH 7.0) with 0.05% SDS at room temperature for 40 min followed by 0.1× SSC with 0.1% SDS solution at 50°C for 40 min, then visualized by autoradiography. Following decay of the TNF-α hybridization signal, a 303-bp MIP-2 DNA probe was generated and rehybridized with the same nitrocellulose-bound samples.
MIP-2 Production following PCBG Stimulation in the Presence of NF-κB Inhibition
We further assessed the effect of NF-κB inhibition on AEC MIP-2 protein production following PCBG stimulation. AECs were incubated with the NF-κB inhibitors tepoxalin (30 μM; Johnson PRI, Raritan, NJ) or PDTC (40 μM) for 30 min before and through a subsequent 6-h challenge with PCBG. These concentrations had no affect on cell viability (21). Supernatants were collected after 6 h of PCBG stimulation, and MIP-2 levels were quantified by enzyme-linked immunosorbent assay (ELISA; Biosource, Camarillo, CA).
The Role of Dectin-1 in AEC Responses to PCBG
Recent investigations indicate that dectin-1 is a major cell surface receptor on macrophages and other cells mediating uptake and cell activation in response to β-glucans (23, 28, 29). To address whether dectin-1 also functions in epithelial responses to glucans, AECs and alveolar macrophages were isolated, and membrane proteins extracted, as described (17). The membrane proteins were separated by SDS-PAGE, transferred, and analyzed with immunoblot using an antibody recognizing the dectin-1 receptor (23). In addition, RNA was extracted (Triazol Reagent) and Northern analysis for dectin-1 was performed. Finally, cultured AECs were preincubated for 20 min with either anti–dectin-1 (Antibody 2A11) or anti-CDw17 antibodies, or nonimmune immunoglobulin (20 μg/ml each; Sigma), and stimulated with the PCBG (5 × 106 particles/ml) for 16 h in the presence of these antibodies at the indicated concentrations. Our prior studies have indicated that lactosylceramide (CDw17) serves as an alternate β-glucan receptor mediating cell activation (17). Supernatants were collected after 16 h and MIP-2 concentrations determined by ELISA. Cell viability was confirmed using the XTT Cell Proliferation Kit II (Roche, Mannheim, Germany).
Statistical Analysis
All data are expressed as the mean ± SEM. Differences between groups were determined using two-tailed Student's t test. Statistical testing was performed using a SPSS/JMP software program (SPSS, Inc., Chicago, IL), with statistical differences considered significant if P < 0.05.
RESULTS
PCBG Induces NF-κB Activation in AECs
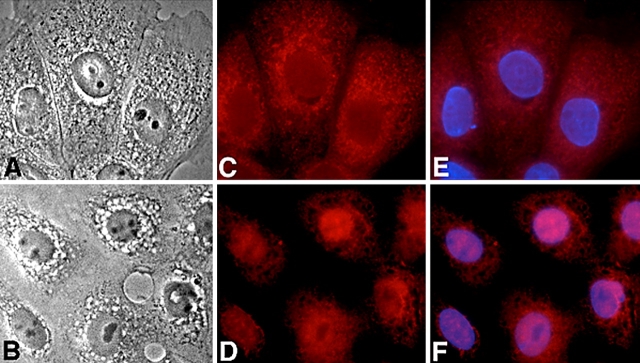
Inflammatory gene activation involves phosphorylation and degradation of cytosolic I-κBα and the subsequent translocation of the liberated NF-κB subunits to the nucleus, thus initiating transcription (21). Stimulation of cultured alveolar epithelial cells with PCBG-induced nuclear translocation of p65 NF-κB over the first hour of cell stimulation (Figure 1). Immunofluorescence reveals that in unstimulated AECs the dominant location of p65 NF-κB is cytosolic, whereas marked translocation to the AEC nucleus occurred following challenge with PCBG. Identical findings were observed for experiments performed in the presence of antibodies to the p50 NF-κB component (data not shown).
Figure 1.
Fluorescence microscopy reveals nuclear translocation of NF-κB in alveolar epithelial cells following challenge with PCBG. (A) Phase contrast microscopy of unstimulated cultured AECs. (B) Phase contrast microscopy of AECs stimulated with PCBG particles over 40 min. (C) Texas Red immunofluorescence of p65 NF-κB component reveals that the transcription factor remains in the cytosolic compartment of unstimulated AECs. (D) Instead, p65 NF-κB is predominantly translocated into the nuclei of AECs stimulated with PCBG for 40 min. (E and F) Superimposed DAPI-counterstained images confirm the nuclear positions.
To further document the time course of NF-κB activation in AECs after stimulation with PCBG, electromobility shift assays were undertaken (Figure 2). NF-κB subunits were observed in AEC nuclear fractions as early as 20 min after the onset of PCBG challenge. Maximal NF-κB nuclear translocation of the P65/p50 subunits was demonstrated between 40 and 50 min of stimulation. Decrements in NF-κB presence in the nuclear fraction were again noted at ∼ 60 min, and continued thereafter.
Figure 2.
Electrophoretic mobility shift assay demonstrates the time course of NF-κB nuclear translocation in PCBG-stimulated AECs. Minimal NF-κB signal is noted in the nuclear lysates of alveolar epithelial cells, which have not been stimulated with PCBG (C = control). In contrast, after PCBG challenge there is a dramatic increase in the nuclear content of p65/p50 NF-κB that is initiated by 20 min and is maximal after 40–50 min. The autoradiographic NF-κB signal begins to diminish 1 h after challenge.
PCBG-Mediated Activation of Alveolar Epithelial Cell NF-κB Is Mediated by PKC
To next address the potential role of signaling kinases in NF-κB activation, cultured AECs were stimulated with PCBG in the presence of the MEK-1 inhibitor UO126, the P38 MAPK inhibitor SB203580, and the PKC inhibitor Gö6976. NF-κB activation was quantified by a solid phase immunoassay measuring the nuclear content of p50 NF-κB (Figure 3). In these studies, only PKC inhibition significantly suppressed NF-κB activation, resulting in approximately a 64.5 ± 1.0% reduction of activity (P = 0.0023 compared with PCBG-stimulated AECs without inhibitor). The inhibitors to MEK-1 and P38 MAPK exerted no significant effect on NF-κB activation.
Figure 3.
PKC inhibition suppresses PCBG-stimulated NF-κB activation in AECs. To evaluate the potential role of signaling kinases in PCBG-induced cell activation, AECs were stimulated with PCBG in the presence of inhibitors to MEK1 (U0126), p38 MAPK (SB203580), or PKC (Gö6976). NF-κB activation was measured by a solid phase assay detecting nuclear translocation of p50 NF-κB. Only the inhibition of PKC activity significantly reduced NF-κB activation in AECs challenged with PCBG (*P < 0.05 compared with AECs stimulated with PCBG in the absence of inhibitor).
PCBG Induces NF-κB–Dependent MIP-2 and TNF-α mRNA Expression in AECs
PCBG is known to induce secretion of inflammatory chemokines and cytokines such as MIP-2 and TNF-α from lung cells. Accordingly, we next evaluated whether NF-κB was required for PCBG inflammatory gene expression in cultured AECs (Figure 4). Northern hybridization demonstrated that AEC significantly increased the expression of both MIP-2 and TNF-α mRNA following challenge with PCBG. Furthermore, this enhanced inflammatory gene expression was largely reversed by doses of the potent NF-κB inhibitor PDTC as low as 12.5 μM. In the presence of PDTC, the mRNA concentrations of PCBG challenged AECs approximated those of unstimulated cells. Thus, PCBG stimulates MIP-2 and TNF-α gene expression in an NF-κB–dependent manner.
Figure 4.
PCBG induces MIP-2 and TNF-α mRNA production through NF-κB–dependent mechanisms. AECs were challenged with PCBG, with or without treatment by PDTC over 4 h and total RNA isolated and submitted to Northern hybridization. PCBG induced enhanced expression of both MIP-2 and TNF-α mRNA. These levels of cytokine expression were suppressed back to basal levels in the presence of the NF-κB antagonist PDTC.
Intact P. carinii Organisms Also Induce MIP-2 Secretion from AECs though Less Potently than Purified PCBG
To further understand the extent to which intact Pneumocystis organisms stimulate AEC chemokine release, we next challenged AECs with either intact P. carinii (1 × 108) or with purified PCBG particles (200 μg/ml) (Figure 5). These concentrations were based upon our previous studies and preliminary observations (9, 10, 21). After overnight culture, the media were analyzed for MIP-2 by ELISA. Both intact P. carinii organisms and PCBG significant induced the secretion of MIP-2 from the cultured AECs (P < 0.0001 comparing either PC- or PCBG-stimulated AECs to unstimulated controls). However, as we anticipated, the purified PCBG particles exhibited significantly greater potency than the whole P. carinii organisms (P < 0.0028 comparing PCBG to PC stimulated AECs).
Figure 5.
Comparison of intact P. carinii and PCBG stimulation of AECs in the presence or absence of polymixin. AECs (1 × 106) were challenged with either intact P. carinii organisms (1 × 108) or with purified PCBG (200 μg/ml), with or without treatment with polymixin (1 μg/ml) over 18 h. The media were collected and assayed for MIP-2 by ELISA. Both intact P. carinii organisms and PCBG induced significant secretion of MIP-2 from the cultured AECs (*P < 0.05 compared with unstimulated controls). However, as anticipated, the purified PCBG particles were significantly more potent than the whole P. carinii organisms (##P < 0.05 comparing PCBG to PC). Notably, the presence of polymixin did not significantly alter the stimulation resulting from either P. carinii or from PCBG, thus excluding endotoxin as a substantial cause of these responses.
Furthermore, our prior studies characterizing PCBG responses in whole animals and with cultured lung cells have demonstrated that contaminating lipopolysaccharide (LPS) is not present to any detectable extent in our PCBG preparations, and is not responsible for inflammatory responses related to this Pneumocystis cell wall component (9, 10, 21). However, in the current study, to further exclude the possibility of endotoxin contamination, additional assays were conducted in both the presence of absence of polymixin (1 μg/ml) (Figure 5). This concentration has been effective in our earlier studies in excluding low-level concentrations of LPS contamination (9). Notably, the presence of polymixin did not significantly alter the stimulation resulting from either P. carinii or from PCBG. Thus, endotoxin does not appear to be a substantial cause of the observed chemokine responses to either Pneumocystis or PCBG.
AEC MIP-2 Production Stimulated by PCBG Requires NF-κB Activity
Furthermore, as demonstrated in Figure 6, MIP-2 protein secretion was also strongly induced in AECs following PCBG challenge, and this response was significantly attenuated by inhibition of NF-κB activity. MIP-2 responses were strongly and significantly inhibited by both the NF-κB antagonists PDTC and tepoxalin. In addition, inhibition of PKC activity (but not MEK1 or P38 MAP kinases) also substantially suppressed AEC chemokine secretion in response to Pneumocystis cell wall β-glucans. Taken together, these data indicate that AEC activation in response to PCBG involves participation of PKC signaling pathways with resulting activation of NF-κB leading to chemokine generation.
Figure 6.
PCBG stimulation of MIP-2 protein production is attenuated by inhibitors of NF-κB. AECs were stimulated with PCBG for 6 h and MIP-2 protein production measured by ELISA in the presence or absence of protein kinase inhibitors and NF-κB antagonists. While incubation of AECs with antagonists of MEK1 (U0126) and P38 (SB203580) had no discernable impact on MIP-2 production, inhibition of PKC (Gö6976) significantly suppressed generation of this chemokine. In addition, both the NF-κB inhibitors tepoxalin and PDTC significantly reduced β-glucan stimulation of MIP-2 release (*P < 0.05 compared with control).
PCBG Responses in AECs Are Not Mediated by Dectin-1 but Instead Require Lactosylceramide
The dectin-1 surface receptor is expressed on macrophages and other cells and promotes uptake of yeast and zymosan particles, a glucan-rich cell wall preparation. In addition, dectin-1 has been implicated in the initiation of cell inflammatory activation (28, 29). Earlier studies have not specifically evaluated the presence of dectin-1 on AECs. Accordingly, we sought to identify dectin-1 receptors on AECs and to evaluate their potential role in inflammatory activation in response to PCBG. However, both immunoblot analysis and Northern analysis failed to detect either dectin-1 protein or mRNA in our AEC cultures (Figure 7). Instead, dectin-1 was easily demonstrated in alveolar macrophage preparations analyzed in parallel. As anticipated from the preceding experiments, anti–dectin-1 antibody did not significantly inhibit MIP-2 production following stimulation with PCBG, further indicating that AECs use an alternate β-glucan receptor (Figure 8). We recently published our findings that lactosylceramide functions as such an alternate β-glucan receptor (17). In contrast to anti–dectin-1, incubation of AECs with antibody recognizing lactosylceramide (anti-CDw17), substantially inhibited AEC MIP-2 generation in response to PCBG, confirming our earlier observations (17).
Figure 7.
Alveolar epithelial cells lack the dectin-1 β-glucan receptor. Primary alveolar epithelial cells and alveolar macrophages were isolated and membrane proteins and RNA extracted. A. Northern analysis revealed no detectable dectin-1 mRNA in the AEC preparations. However, dectin-1 message was easily detected in alveolar macrophages. B. The isolated membrane proteins were further separated by SDS-PAGE, transferred and analyzed with immunoblot using an antibody recognizing the dectin-1 β-glucan receptor (2A11). While, alveolar macrophages exhibited dectin-1, alveolar epithelial cells were devoid of this receptor.
Figure 8.
Effect of antibodies to dectin-1 and lactosylceramide on MIP-2 release by alveolar epithelial cells incubated with PCBG. Isolated AECs were incubated with media alone, nonimmune mouse immunoglobulin, anti–dectin-1 (2A11), or anti-lactosylceramide (anti-CDw17) (200 μg/ml each) for 30 min before and throughout a subsequent 24 h challenge with PCBG (5 × 106 particles/ml). MIP-2 generation under these conditions was quantified by ELISA. Anti-dectin exhibited no significant suppression in MIP-2 release. However, consistent with our previous observations, anti-lactosylceramide (anti-CDw17) significantly suppressed MIP-2 release from AECs challenged with PCBG (17). Data points are expressed as mean ± SEM (*P < 0.05, compared with control AECs incubated with PCBG alone).
DISCUSSION
Pneumocystis pneumonia remains an important life-threatening infection of immunocompromised hosts, resulting in high rates of respiratory failure and death (1, 4). Severe Pneumocystis pneumonia in humans is characterized by intense neutrophilic lung inflammation associated with gas exchange impairment, diffuse alveolar damage, and respiratory failure (5, 6, 8). Additional studies further implicate CD8 lymphocytes in also mediating deleterious lung inflammation during Pneumocystis infection (30). Pneumocystis cell wall β-glucans strongly stimulate such lung inflammation by promoting cytokine and chemokine generation from lung cells (9, 17, 21, 31). In earlier studies, we demonstrated that alveolar epithelial cells freshly isolated from Pneumocystis glucan–challenged rats exhibit enhanced mRNA expression for MIP-2 and related chemokines (17). In addition, we have also shown that isolated Pneumocystis β-glucan instilled into the lungs of immune-competent mice strongly induces both neutrophil recruitment and cytokine secretion recovered in the bronchoalveolar lavage (9). Previous studies strongly implicate that host lung infiltration with inflammatory cells, including neutrophils and lymphocytes, rather than direct toxic effects of the organisms is largely responsible for respiratory impairment during this infection (1, 5, 7, 8).
MIP-2 and TNF-α are two such mediators that potently stimulate lung recruitment of immune effector cells. AECs produce significant quantities of MIP-2 and TNF-α in response to PCBG, to a greater extent than even professional immune effector cells such as alveolar macrophages (17, 32). The present study further demonstrates that AEC generation of chemokines in response to PCBGs relies on NF-κB–dependent mechanisms. AEC activation of NF-κB signaling occurs in part through PKC signaling pathways and appears to not require the recently described dectin-1 receptor. Instead, our findings indicate that lactosylceramide, an alternate β-glucan receptor, is important in β-glucan activation of alveolar epithelial cells (17).
NF-κB broadly regulates the expression of multiple genes encoding inflammatory proteins including MIP-2 and TNF-α, proteins in apoptotic pathways, and components of the cell cycle regulatory machinery (33–35). NF-κB exists as an inactive cytosolic complex bound to its inhibitory subunit I-κBα. Following cell activation, I-κBα is phosphorylated and targeted for proteosomal degradation, thereby releasing the DNA binding NF-κB p50/p65 components. The liberated heterodimer translocates to the nucleus and initiates transcription of genes by binding to NF-κB–binding motifs in promoter regions. The promoter region of the MIP-2 gene has recently been shown to contain consensus-binding sites for NF-κB (18). Other studies similarly demonstrate that NF-κB mediates increased expression of MIP-2 mRNA when alveolar epithelial cells are challenged with LPS, vanadium, crocidolite, or overdistension (33, 36, 37).
Nonspecific anti-inflammatory therapies, including adjunctive corticosteroids, have significantly decreased the mortality of Pneumocystis pneumonia (38). New approaches by which lung inflammation might be selectively modulated should prove beneficial to patients with severe Pneumocystis infection. Tepoxalin, a di-aryl-substituted pyrazole, and PDTC are two potent inhibitors of NF-κB activation useful for in vitro studies. Both are thought to prevent the initial targeting and degradation of I-κBα (39–41). The marked attenuation of epithelial cell chemokine production after treatment with tepoxalin and PDTC strongly supports an integral role of NF-κB signaling pathways in mediating epithelial inflammatory responses to PCBGs. Although anti–NF-κB agents are not yet available for clinical testing, these in vitro studies provide useful clues that might eventually be examined in future management of this infection.
Signaling kinases including MAPK and PKC have been implicated in initiating NF-κB activation following various stimuli (42–44). For instance, NF-κB activation has been shown to be associated with the p38 and p42/44 MAPK pathways in responses to LPS (45, 46). Other studies have implicated PKC activity in mediating NF-κB activation following mechanical deformation of cells as well as other stimuli (47). Our data suggest that inhibition of either p38 or MEK1 in AECs does not significant attenuate NF-κB translocation or MIP-2 protein production, indicating that p38 or P42/44 MAPK pathways are not likely significantly involved in AEC β-glucan–mediated responses. In contrast, however, suppression of PKC activity significantly inhibited AEC NF-κB activation and chemokine responses. Thus, selective signaling kinases participate in epithelial responses to the Pneumocystis cell wall components.
Prior studies have defined a number of potential mechanisms through which Pneumocystis interact with cells of the lower respiratory tract. For instance, alveolar macrophages have been shown to interact directly with the mannoprotein gpA on the surface of the organism through macrophage mannose receptors (12, 22). In addition, extracellular matrix proteins including fibronectin, vitronectin, and surfactant proteins A and D coated on the surface of organisms also facilitate interactions of Pneumocystis with alveolar macrophages (1, 12, 22). Interactions of β-glucans on Pneumocystis with macrophages have further been proposed to occur through a variety of mechanisms, including via the integrin CD11b/CD18 receptor (12, 22). The mechanisms by which epithelial cells interact with Pneumocystis are somewhat less well defined. However, once again, interactions of Pneumocystis with the extracellular matrix fibronectin and vitronectin are known to facilitate binding of the organism to epithelial cells through integrin-dependent mechanisms.
Recent investigations have defined important roles for dectin-1 as a major receptor on macrophages and dendritic cells mediating uptake of fungal organisms and zymosan (23, 28, 29). Additional studies indicate that dectin-1 is active in macrophage uptake of Pneumocystis as well as associated inflammatory responses (29). However, the current investigations demonstrate that, distinct from alveolar macrophages, AECs lack dectin-1, yet are fully capable of robust inflammatory responses to fungal cell wall β-glucans, through the activity of lactosylceramide (17).
Taken together, these investigations demonstrate that alveolar epithelial cell chemokine responses to PCBGs are mediated through NF-κB–dependent mechanisms. Initiation of β-glucan responses in AECs does not require the dectin-1, but rather involves participation of the alternative lactosylceramide β-glucan receptor. Furthermore, this signaling of NF-κB activation also involves PKC activity. Additional understanding of lung inflammatory response to Pneumocystis offers hope for novel pharmacologic interventions in this disease, which persists with unacceptably high mortality.
Acknowledgments
The authors thank Mr. Joseph E. Standing for his invaluable assistance in isolating Pneumocystis carinii β-glucan, as well as Drs. Zvezdana Vuk-Pavlovic and Robert Vassallo for many helpful discussions. Dr. Vishwajeet Puri assisted with the initial fluorescence microscopic studies. The authors further recognize Dr. Gordon Brown (University of Cape Town, South Africa) for his generous gift of antibody 2A11 recognizing the dectin-1 receptor.
These studies were supported by NIH Grants R01 HL55934 and R01 HL62150 to A.H.L.
Conflict of Interest Statement: S.E.E. has no declared conflicts of interest; P.Y.H. has no declared conflicts of interest; F.M. has no declared conflicts of interest; T.J.K. has no declared conflicts of interest; Z.V.P. has no declared conflicts of interest; and A.H.L. has no declared conflicts of interest.
References
- 1.Thomas CF Jr, Limper AH. Pneumocystis pneumonia. N Engl J Med 2004;350:2487–2498. [DOI] [PubMed] [Google Scholar]
- 2.Mansharamani NG, Garland R, Delaney D, Koziel H. Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 2000;118:704–711. [DOI] [PubMed] [Google Scholar]
- 3.Curtis RJ, Yarnold PR, Schwartz DN, Weinstein RA, Bennett CL. Improvements in outcomes of acute respiratory failure for patients with human immunodeficiency virus–related Pneumocystis carinii pneumonia. Am J Respir Crit Care Med 2000;162:393–398. [DOI] [PubMed] [Google Scholar]
- 4.Beck JM, Rosen MJ, Peavy HH. Pulmonary complications of HIV infection. Report of the Fourth NHLBI Workshop. Am J Respir Crit Care Med 2001;164:2120–2126. [DOI] [PubMed] [Google Scholar]
- 5.Wright TW, Gigliotti F, Finkelstein JN, McBride JT, An CL, Harmsen AG. Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J Clin Invest 1999;104:1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadaghdar H, Huang ZB, Eden E. Correlation of bronchoalveolar lavage findings to severity of Pneumocystis carinii pneumonia in AIDS: evidence for the development of high-permeability pulmonary edema. Chest 1992;102:63–69. [DOI] [PubMed] [Google Scholar]
- 7.Mason GR, Hashimoto CH, Dickman PS, Foutty LF, Cobb CJ. Prognostic implications of bronchoalveolar lavage neutrophilia in patients with Pneumocystis carinii pneumonia and AIDS. Am Rev Respir Dis 1989;139:1336–1342. [DOI] [PubMed] [Google Scholar]
- 8.Limper AH, Offord KP, Smith TF, Martin WJ II. Pneumocystis carinii pneumonia: differences in lung parasite number and inflammation in patients with and without AIDS. Am Rev Respir Dis 1989;140:1204–1209. [DOI] [PubMed] [Google Scholar]
- 9.Vassallo R, Standing JE, Limper AH. Isolated Pneumocystis carinii cell wall glucan provokes lower respiratory tract inflammatory responses. J Immunol 2000;164:3755–3763. [DOI] [PubMed] [Google Scholar]
- 10.Vassallo R, Standing J, Limper AH. Beta-glucan from Pneumocystis carinii stimulates TNF alpha release from alveolar macrophages. J Eukaryot Microbiol 1999;46:145S. [PubMed] [Google Scholar]
- 11.Limper AH, Edens M, Anders RA, Leof EB. Pneumocystis carinii inhibits cyclin-dependent kinase activity in lung epithelial cells. J Clin Invest 1998;101:1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limper AH. Parasitic adherence and host responses in the development of Pneumocystis carinii pneumonia. Semin Respir Infect 1991;6:19–26. [PubMed] [Google Scholar]
- 13.Xavier AM, Isowa N, Cai L, Dziak E, Opas M, McRitchie DI, Slutsky AS, Keshavjee SH, Liu M. Tumor necrosis factor-α mediates lipopolysaccharide-induced macrophage inflammatory protein-2 release from alveolar epithelial cells: autoregulation in host defense. Am J Respir Cell Mol Biol 1999;21:510–520. [DOI] [PubMed] [Google Scholar]
- 14.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP III, Toews GB, Westwick J, Strieter RM. Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest 1990;86:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright TW, Johnston CJ, Harmsen AG, Finkelstein JN. Analysis of cytokine mRNA profiles in the lungs of Pneumocystis carinii–infected mice. Am J Respir Cell Mol Biol 1997;17:491–500. [DOI] [PubMed] [Google Scholar]
- 16.Wright TW, Johnston CJ, Harmsen AG, Finkelstein JN. Chemokine gene expression during Pneumocystis carinii–driven pulmonary inflammation. Infect Immun 1999;67:3452–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn PY, Evans SE, Kottom TJ, Standing JE, Pagano RE, Limper AH. Pneumocystis carinii cell wall beta-glucan induces release of macrophage inflammatory protein-2 from alveolar epithelial cells via a lactosylceramide-mediated mechanism. J Biol Chem 2003;278:2043–2050. [DOI] [PubMed] [Google Scholar]
- 18.Widmer U, Manogue KR, Cerami A, Sherry B. Genomic cloning and promoter analysis of macrophage inflammatory protein (MIP)-2, MIP-1 alpha, and MIP-1 beta, members of the chemokine superfamily of proinflammatory cytokines. J Immunol 1993;150:4996–5012. [PubMed] [Google Scholar]
- 19.Zingarelli B, Sheehan M, Wong HR. Nuclear factor-kappaB as a therapeutic target in critical care medicine. Crit Care Med 2003;31:S105–S111. [DOI] [PubMed] [Google Scholar]
- 20.Haddad JJ, Land SC. Nuclear factor-kappa b blockade attenuates but does not abrogate lipopolysaccharide-dependent tumor necrosis factor-alpha biosynthesis in alveolar epithelial cells. Biochem Biophys Res Commun 2001;285:267–272. [DOI] [PubMed] [Google Scholar]
- 21.Lebron F, Vassallo R, Puri V, Limper AH. Pneumocystis carinii cell wall beta-glucans initiate macrophage inflammatory responses through NF-kappa B activation. J Biol Chem 2003;278:25001–25008. [DOI] [PubMed] [Google Scholar]
- 22.Vassallo R, Thomas CF Jr, Vuk-Pavlovic Z, Limper AH. Alveolar macrophage interactions with Pneumocystis carinii. J Lab Clin Med 1999;133:535–540. [DOI] [PubMed] [Google Scholar]
- 23.Brown GD, Taylor PR, Reid DM, Willment JA, Williams DL, Martinez-Pomares L, Wong SY, Gordon S. Dectin-1 is a major beta-glucan receptor on macrophages. J Exp Med 2002;196:407–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dobbs LG, Gonzalez R, Williams MC. An improved method for isolating type II cells in high yield and purity. Am Rev Respir Dis 1986;134:141–145. [DOI] [PubMed] [Google Scholar]
- 25.Lin H, Chen C, Li X, Chen BD. Activation of the MEK/MAPK pathway is involved in bryostatin1-induced monocyte differentiation and up-regulation of X-linked inhibitor of apoptosis protein. Exp Cell Res 2002;272:192–198. [DOI] [PubMed] [Google Scholar]
- 26.Sweeney G, Somwar R, Ramlal T, Volchuk A, Ueyama A, Klip A. An inhibitor of p38 mitogen-activated protein kinase prevents insulin-stimulated glucose transport but not glucose transporter translocation in 3T3–L1 adipocytes and L6 myotubes. J Biol Chem 1999;274:10071–10078. [DOI] [PubMed] [Google Scholar]
- 27.Greco S, Muscella A, Elia MG, Salvatore P, Storelli C, Mazzotta A, Manca C, Marsigliante S. Angiotensin II activates extracellular signal regulated kinases via protein kinase C and epidermal growth factor receptor in breast cancer cells. J Cell Physiol 2003;196:370–377. [DOI] [PubMed] [Google Scholar]
- 28.Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L, Gordon S, Wong SY. The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol 2002;169:3876–3882. [DOI] [PubMed] [Google Scholar]
- 29.Steele C, Marrero L, Swain S, Harmsen AG, Zheng M, Brown GD, Gordon S, Shellito JE, Kolls JK. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor. J Exp Med 2003;198:1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright TW, Pryhuber GS, Chess PR, Wang Z, Notter RH, Gigliotti F. TNF receptor signaling contributes to chemokine secretion, inflammation, and respiratory deficits during Pneumocystis pneumonia. J Immunol 2004;172:2511–2521. [DOI] [PubMed] [Google Scholar]
- 31.Benfield TL, Lundgren B, Shelhamer JH, Lundgren JD. Pneumocystis carinii major surface glycoprotein induces interleukin-8 and monocyte chemoattractant protein-1 release from a human alveolar epithelial cell line. Eur J Clin Invest 1999;29:717–722. [DOI] [PubMed] [Google Scholar]
- 32.Thompson AB, Robbins RA, Romberger DJ, et al. Immunological functions of the pulmonary epithelium. Eur Respir J 1995;8:127–149. [DOI] [PubMed] [Google Scholar]
- 33.Driscoll KE, Carter JM, Howard BW, Hassenbein D, Janssen YM, Mossman BT. Crocidolite activates NF-kappa B and MIP-2 gene expression in rat alveolar epithelial cells: role of mitochondrial-derived oxidants. Environ Health Perspect 1998;106:1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun SC, Xiao G. Deregulation of NF-kappa B and its upstream kinases in cancer. Cancer Metastasis Rev 2003;22:405–422. [DOI] [PubMed] [Google Scholar]
- 35.Vermeulen K, Berneman ZN, Van Bockstaele DR. Cell cycle and apoptosis. Cell Prolif 2003;36:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chong IW, Shi MM, Love JA, Christiani DC, Paulauskis JD. Regulation of chemokine mRNA expression in a rat model of vanadium- induced pulmonary inflammation. Inflammation 2000;24:505–517. [DOI] [PubMed] [Google Scholar]
- 37.Vlahakis NE, Schroeder MA, Limper AH, Hubmayr RD. Stretch induces cytokine release by alveolar epithelial cells in vitro. Am J Physiol 1999;277:L167–L173. [DOI] [PubMed] [Google Scholar]
- 38.Bozzette SA, Sattler FR, Chiu J, Wu AW, Gluckstein D, Kemper C, Bartok A, Niosi J, Abramson I, Coffman J, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. N Engl J Med 1990;323:1451–1457. [DOI] [PubMed] [Google Scholar]
- 39.Fiebich BL, Hofer TJ, Lieb K, Huell M, Butcher RD, Schumann G, Schulze-Osthoff K, Bauer J. The non-steroidal anti-inflammatory drug tepoxalin inhibits interleukin- 6 and alpha1-anti-chymotrypsin synthesis in astrocytes by preventing degradation of IkappaB-alpha. Neuropharmacology 1999;38:1325–1333. [DOI] [PubMed] [Google Scholar]
- 40.Liu SF, Ye X, Malik AB. Inhibition of NF-kappaB activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation 1999;100:1330–1337. [DOI] [PubMed] [Google Scholar]
- 41.Nathens AB, Bitar R, Davreux C, Bujard M, Marshall JC, Dackiw AP, Watson RW, Rotstein OD. Pyrrolidine dithiocarbamate attenuates endotoxin-induced acute lung injury. Am J Respir Cell Mol Biol 1997;17:608–616. [DOI] [PubMed] [Google Scholar]
- 42.Gingery A, Bradley E, Shaw A, Oursler MJ. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem 2003;89:165–179. [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharyya A, Pathak S, Datta S, Chattopadhyay S, Basu J, Kundu M. Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem J 2002;368:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer B, Krumbock N, Fresser F, Hochholdinger F, Spitaler M, Simm A, Uberall F, Schraven B, Baier G. Complex formation and cooperation of protein kinase C theta and Akt1/protein kinase B alpha in the NF-kappa B transactivation cascade in Jurkat T cells. J Biol Chem 2001;276:31627–31634. [DOI] [PubMed] [Google Scholar]
- 45.Coxon PY, Rane MJ, Uriarte S, Powell DW, Singh S, Butt W, Chen Q, McLeish KR. MAPK-activated protein kinase-2 participates in p38 MAPK-dependent and ERK-dependent functions in human neutrophils. Cell Signal 2003;15:993–1001. [DOI] [PubMed] [Google Scholar]
- 46.Shi L, Kishore R, McMullen MR, Nagy LE. Lipopolysaccharide stimulation of ERK1/2 increases TNF-alpha production via Egr-1. Am J Physiol Cell Physiol 2002;282:C1205–C1211. [DOI] [PubMed] [Google Scholar]
- 47.Kumar A, Lnu S, Malya R, Barron D, Moore J, Corry DB, Boriek AM. Mechanical stretch activates nuclear factor-kappaB, activator protein-1, and mitogen-activated protein kinases in lung parenchyma: implications in asthma. FASEB J 2003;17:1800–1811. [DOI] [PubMed] [Google Scholar]