Abstract
The lung is continuously exposed to bacteria and their products, and has developed a complex defense mechanism, including neutrophil recruitment. In mice, keratinocyte cell–derived chemokine and macrophage inflammatory protein-2 are the major chemokines for neutrophil recruitment into the lung. We have previously described a role for C-X-C chemokine (CXCL5) in neutrophil trafficking during lipopolysaccharide (LPS)-induced lung inflammation in mice. The aims of the present study were to identify the cellular origin of CXCL5 and to determine the signaling cascades that regulate its expression in the lung during LPS-induced inflammation and in isolated LPS-stimulated CXCL5-expressing cells. Our immunohistochemical analysis indicates that alveolar epithelial type II (AEII) cells are the primary source of CXCL5 in the rodent lung. These in vivo observations were confirmed with primary AEII cells. In addition, our data indicate that the Toll-like receptor 4 (TLR4) signaling cascade involving TLR4, myeloid differentiation factor 88, and Toll–IL-1R domain–containing adapter protein is required to induce CXCL5 expression in the lung. Furthermore, p38 and c-Jun N-terminal kinases are involved in lung CXCL5 expression. Similarly, TLR4, and p38 and c-Jun N-terminal kinases, are associated with LPS-induced CXCL5 expression in AEII cells. These novel observations demonstrate that activation of AEII cells via TLR4-dependent signaling is important for the production of CXCL5 in the lung exposed to LPS.
Keywords: lipopolysaccharide, CXCL5, LIX, lung inflammation, mouse model
Chemokines are small protein molecules that control the inflammatory responses via a plethora of biological functions, including recruitment of specific leukocyte population to sites of inflammation during microbial assault (1, 2). They are categorized into four groups based on the composition of a cysteine motif positioned near the N terminus: C, CC, CXC, and CX3C. The CXC family chemokines have four cysteine residues at the N-terminal in which the first two are separated by a nonconserved amino acid. These groups are further categorized by the presence of the ELR motif (glutamic acid-leucine-arginine) immediately before the CXC sequence. These ELR+CXC chemokines are potent neutrophil chemoattractants and seven of this class have been identified so far in humans: interleukin (IL)-8; neutrophil-activating peptide 2 (NAP-2); growth-related oncogenes (GRO)-α, β, and γ; epithelium-derived neutrophil-activating peptide 78 (ENA-78); and granulocyte chemotactic protein 2 (GCP-2). Among those chemokines, IL-8 is considered to be the most potent neutrophil chemoattractant in humans (1, 2).
In rodents, a homolog of human IL-8 has not been identified. The absence of IL-8 homolog in rodents provides a useful model in rodents to investigate the individual role(s) of other ELR+ chemokines. In this context, keratinocyte cell–derived chemokine (KC) (3, 4), macrophage inflammatory protein (MIP)-2 (5, 6), and lungkine (7, 8) have been discovered as important neutrophil chemoattractants in mice during lung inflammation induced by microbes and/or their products. Most chemokines that are associated with neutrophil trafficking in the lung are secreted in massive quantities by the myeloid cells, such as macrophages and neutrophils (3–6). However, lungkine differs from other murine neutrophil chemoattractants because its secretion is restricted to bronchial epithelial cells (7, 8). In rats, the dominant ELR+ chemokines are referred to as CINC-1 (equivalent to KC and GROα), CINC-2α, CINC-2β, and CINC-3 (equivalent to MIP-2). Lungkine has not been identified in rats.
In an attempt to identify genes upregulated in the lungs of mice during the acute inflammatory process, we performed RNA profiling in mouse lungs between 1 and 24 h after lipopolysaccharide (LPS) challenge. One of the genes that was upregulated in LPS-treated microarrays was the ELR+ CXC chemokine (CXCL5 or LIX, LPS-Induced CXC chemokine) (9) compared with saline controls. CXCL5 is considered the sole rodent homolog of human ENA-78 and GCP-2 (10). In earlier studies, CXCL5 has been shown as a chemotactic agent to isolated murine neutrophils in vitro and in vivo after intradermal injection of CXCL5 (11). More recent in vivo studies, however, have described that CXCL5 can elicit neutrophil accumulation in specific organs. These studies demonstrate that resident cell– (cardiomyocyte), but not inflammatory cell–derived CXCL5, plays an important role in neutrophil influx in myocardium in a rat model of ischemia-reperfusion injury (12) and in a murine model of peritonitis (13). In our earlier studies, we have shown that CXCL5 causes neutrophil accumulation in the lung using recombinant CXCL5 and using an anti-CXCL5 Ab during LPS-induced pulmonary inflammation (9). This evidence suggest that locally secreted cytokines by resident (nonmyeloid) structural cells in the organ may play pivotal roles during inflammation that leads to neutrophil accumulation. However, the cellular origin of CXCL5 in the lung has not been identified. In addition, the signaling cascades that regulate the expression of CXCL5 in the lung and in CXCL5 expressing cells in response to LPS have not been examined previously.
In this investigation, we determined the cellular origin of CXCL5 in the lung and examined the signaling cascades that regulate CXCL5 expression during LPS-induced lung inflammation in a mouse model as well as at the cellular level using CXCL5 secreting rat cells after LPS stimulation. Our data conclude that CXCL5 expression during lung inflammation is predominantly produced by alveolar epithelial type II (AEII) cells, and that the expression of CXCL5 in the lung is regulated by the Toll-like receptor 4 (TLR4) signaling pathway involving TLR4, myeloid differentiation factor 88 (MyD88), and Toll–IL-1R domain–containing adapter protein (TIRAP), and p38 and c-Jun N-terminal (JNK) kinases. In a similar manner, TLR4, and p38 and JNK kinases, regulate CXCL5 expression in isolated primary AEII cells. Taken together, these findings suggest that alveolar epithelium may participate in the innate immune response in the lung through the production of a neutrophil chemoattractant, when they are exposed to gram-negative bacteria and their products.
MATERIALS AND METHODS
Reagents
Endotoxin-free plastic and glasswares were used in all experiments. Purified LPS and myeloperoxidase (MPO) assay reagents were obtained from Sigma (St. Louis, MO). Chemicals, such as SP600125 were purchased from Calbiochem (La Jolla, CA). M39 was obtained from Merck (Rahway, NJ). Protease inhibitor cocktail was obtained from Roche Applied Science (Indianapolis, IN) and tissue fixative was purchased from Streck Laboratories (Omaha, NE). Abs to CXCL5 and their isotype controls were obtained from R&D Systems (Minneapolis, MN), and an Ab to prosurfactant protein C was obtained from Chemicon (Temecula, CA). Primers for PCR were purchased from IDT technologies (Coralville, IA). All real-time PCR reagents and the rat GAPDH primer were obtained from Applied Biosystems (Foster City, CA). An Ab against TLR4 was obtained from Santa Cruz and TIRAP was purchased from Abcam Inc. (Cambridge, MA). An Ab against MyD88 was obtained from Imgenex (San Diego, CA), and all of the secondary Abs were purchased from Jackson Immunoresearch (West Grove, PA). ELISA reagents were obtained from R&D Systems.
Animals
MyD88 gene–disrupted (MyD88−/−) and TLR4 gene–deficient (TLR4−/−) mice were backcrossed 12 times with C57Bl/6, yielding mice that are genetically > 99.9% C57Bl/6 and were obtained from Shizuo Akira (Osaka University, Osaka, Japan). Therefore, C57Bl/6 mice were used as controls for MyD88−/− and TLR4−/− mice. TIRAP gene–disrupted mice and their littermate controls were obtained by breeding between TIRAP+/− males and females as they were in 129 × C57Bl/6 background (14). The Institutional Animal Care and Use Committee granted approval for all studies. Eight- and 10-wk-old mice ranging from 18–28 g were used in all experiments.
LPS Challenge
The rodent model to induce lung inflammation has been described in our previous publications (9, 15, 16). Briefly, mice and rats were exposed to 300 μg/ml of Escherichia coli–derived LPS (O111:B4) in 0.9% saline by aerosolization for 20 min, because this concentration of LPS-induced lung inflammation in a mouse model mimics several key features of human acute lung injury (9, 15, 16). Control groups of mice were treated according to exactly the same protocol except they received 0.9% saline instead of LPS. At 2, 8, and 24 h after LPS or saline inhalation, mice and rats were killed using a lethal dose of pentobarbital via intraperitoneal injection. These time points were selected because LPS-induced cellular responses and pathologic features were remarkably distinct at these designated time points as described earlier (9, 15, 16). In another set of experiments, mice were pretreated with 3 mg/kg of the P38 inhibitor (M39) orally in polyethylene glycol (PEG-400) 2 h before LPS challenge as described previously (16). In the other set of experiments, animals were given the JNK inhibitor (SP600125) in PPCES vehicle (30% PEG-400/20% polypropylene glycol/15% Cremophor EL/5% ethanol/30% saline) final volume 5 μl/g, 3 h before LPS as described earlier (17).
Bronchoalveolar Lavage Fluid Collection
Bronchoalveolar lavage fluid (BALF) was immediately recovered from killed mice by instillation and recovery of 3.0 ml PBS via the tracheal catheter (9, 15, 16). The total cells were enumerated by counting with a hemocytometer. Differential cell count was performed by standard light microscopy based on staining with Diff-Quik (American Scientific Products, McGrew Park, IL). The remaining BALF was centrifuged and the supernatant was collected for CXCL5 measurement. CXCL5 quantitation was performed by ELISA using undiluted BALF according to the manufacturer's recommendations.
Lung Harvesting
At the designated time points, the whole lungs were removed from mice and were immediately snap-frozen, followed by storage at −70°C. They were homogenized in 2 ml of PBS supplemented with 0.1% Triton X-100 and complete protease inhibitor (1 tablet/50 ml media), and the resulting homogenates were centrifuged at 12, 000 × g for 20 min (9, 16). The supernatants were harvested, passed through a 0.22-μm filter, and used immediately for measurement of CXCL5 protein by ELISA at a dilution of 1:200.
Immunohistochemical Analysis
After killing, mouse and rat lungs were perfused and fixed using streck tissue fixative (Streck Laboratories) overnight at room temperature (9). Fixed lungs were mounted in paraffin, and 5 μM sections were made and affixed to microscopic slides. For immunohistochemistry, the sections were deparaffinized with xylene and rehydrated via a series of decreasing concentrations of ethanol. These sections were treated with 5% hydrogen peroxide in tris-buffered saline (pH 7.4) followed by treating with serum to block nonspecific staining. The lung sections were then incubated with anti-rat CXCL5 antibody (Ab). The binding to CXCL5 was amplified using a Vectastatin kit (Vector Laboratories, Burlingame, CA) and detected with diaminobenzidine subsrate (Promega Laboratories, Madison, WI). Sections were scanned using an Olympus BX51 upright microscope. Another section from the same lung lobe was stained with an Ab against prosurfactant protein C (proSP-C), a molecule specifically expressed in AEII cells (18, 19).
Semiquantitative PCR Analysis of Lungs
At 2, 8, and 24 h after challenge, total RNA was extracted from the lungs, and RT-PCR was performed for CXCL5. The sequences of the primers were: CXCL5, 5′-CTCAGTCATAGCCGCAACCGAGC-3′ and 5′-CGCTTCTTTCCACTGCGAGTGC-3′ (260 bp); and GAPDH, 5′-ATG-GAT- GAC-GAT-ATC-GCT-C-3′ and 5′-GAT-TCC-ATA-CCC-AGG-AAG-G-3′ (550 bp). A total of 8 μl were separated on a 1% agarose gel containing ethidium bromide, and bands were visualized and photographed using an ultraviolet transilluminator.
Culture of Rat AEII and Type I–Like Cells and LPS Stimulation
AEII cells were isolated and cultured from specific pathogen–free male Sprague-Dawley rats (Harlan, Indianapolis, IN). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) with 5% rat serum as previously described (18, 19). The purity of AEII cells was confirmed as > 96% using immunofluorescence microscopy with antibodies against cytokeratin and pro-surfactant C as respective markers for epithelial cells and AEII cells (18, 19). The cells were then stimulated with varying concentration of ultra pure LPS (E. coli LPS: O111:B5) for 18 h and proceeded for immunocytochemical analysis, or the supernatants were harvested for CXCL5 protein determination. In another set of experiments, an Ab against the extracellular domain of TLR4 or goat IgG (isotype control) was preincubated with cells at a final concentration of 40 μg to both the apical and basolateral sides 1 h before LPS stimulation. This is the minimal concentration that abolishes LPS-induced cytokine expression, including CXCL5 (S. Jeyaseelan and G. S. Worthen, unpublished data). To inhibit p38 and JNK kinases, cells were incubated with 10 μM and 5 μM respective concentrations of M39 (p38 kinase inhibitor) and SP600125 (JNK kinase inhibitor), because these inhibitors are highly selective at these concentrations as we described in the past (16). AEII cells were dedifferentiated to become type I–like cells in DMEM supplemented with 5% fetal bovine serum in the absence of keratinocyte-derived growth factor (KGF) as previously described (18, 19).
Isolation of Murine Neutrophils and LPS Treatment
Mature murine bone marrow–derived neutrophils were isolated from mouse femurs as described in our previous publication (20). In our previous studies, we have also shown that the marrow neutrophils (> 95% purity) have equivalent functional responses and recirculation patterns as compared with peripheral murine neutrophils (20). A total of 5 × 106 mature neutrophils were stimulated with 30 ng of LPS and incubated for 4 h at 37°C. After the incubation, the supernatant was centrifuged and collected for CXCL5 protein determination by ELISA.
Culture of Rat Alveolar Macrophages and LPS Stimulation
Culture of alveolar macrophages (AMs) were isolated and were grown at a density of 2 × 106 cells/ml/well on a 6-well plate for 3 d using RPMI 1640 media containing 10% fetal bovine serum and were stimulated with 30 ng of LPS. After 18 h incubation, the supernatants were harvested and used to detect the CXCL5 protein by ELISA.
Immunocytochemistry of AEII Cells
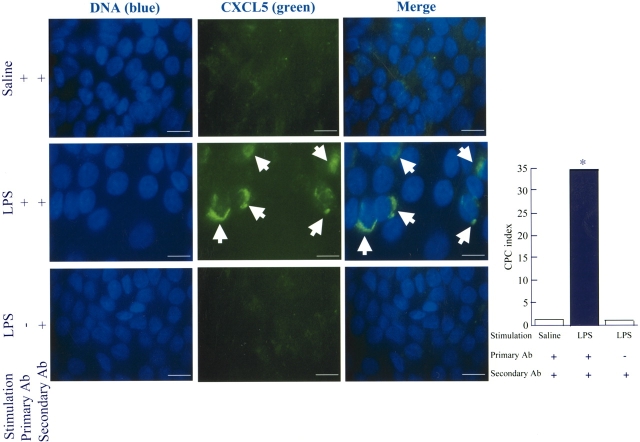
Immunocytochemistry was performed as described earlier (21) with minor modifications. Briefly, AEII cells were grown in DMEM with 5% rat serum. The cells were fixed with alcohol and were immunostained for murine CXCL5 using goat anti-rat CXCL5 IgG (R&D Systems) or isotype-matched control Ab followed by FITC-labeled rabbit anti-goat IgG (Jackson Immunoresearch). CXCL5-producing cell (CPC) index was calculated as immunofluorescence-positive cells/total cells × 100. A total of 3,000 cells in 15 microscopic fields at a magnification of ×100 were enumerated for the calculation.
Western Blot Analysis of AEII Cells
AEII cell lysates were separated by SDS-PAGE using precast Tris-glycine gels with an 18% acrylamide gradient (Bio-Rad, Hercules, CA). Proteins were then transferred to nitrocellulose membranes for 4 h at 85 V at 4°C, and the membrane was blocked with TBS containing 4% nonfat dry milk (NFM) for 1 h. The blot was incubated with goat anti-CXCL5 Ab (diluted 1/200, in TBS containing 4% NFM/0.05% Tween). The blot was then washed three times with PBS and developed using donkey anti-goat-HRP–conjugated secondary Ab (Jackson Immunoresearch) at a 1/4,000 dilution in TBS/Tween and developed with ECL plus Western blot detection system (Amersham Pharmacia Biotech, Piscataway, NJ). The same procedure was employed for the detection of anti-TLR4 (1/200 dilution of 200 μg/ml), -MyD88 (1/200 dilution of 500 μg/ml), and -TIRAP (1/200 dilution of 200 μg/ml) Ab. Appropriate secondary antibodies at a dilution of 1:5,000 concentrations were used.
PCR Analysis of AEII Cells
Real-time.
The method to perform real-time PCR in AEII cells has been previously described (19). Briefly, 4 h after LPS stimulation, the cells were directly lysed into 4 M guanidinium isothiocyanate, 0.5% N-laurelsarcosine, and 0.1 M β-mercaptoethanol in 25 mm sodium citrate buffer. Total RNA was isolated by centrifugation through a 5.7-M CsCl cushion at 150,000 × g for 18 h. The RNA was treated with Rnase-free Dnase I according to the manufacturer's protocol (Ambion Inc., Austin, TX). The primer and probe sets were designed for rat CXC chemokines CXCL5 cDNA using the following sequences: CXCL5, 5′-CCGGTCCTGCTCGTCATT-3′ and 5′-CGATGCTGTGGCAGTGTGA-3′; probe 5′-CCCTGCTGGCATTTCTGCTGCTG-3′. The commercially available rat GAPDH kit was used as an internal control (Applied Biosystems). Real-time PCR reactions were performed with Taqman universal PCR master mix on an ABI Prism 7,700 sequence Detection System (Applied Biosystems) as described in our previous publication (9).
Semiquantitative.
The method to perform semiquantitative RT-PCR to detect TLR4, MyD88, and TIRAP was performed in a manner similar to that previously demonstrated (22). In this set of experiments, AEII cells were used using the following primer sequences: TLR4, 5′-TGGATACGTTTCCTTATAAG-3′ and 5′-GAAATGGAGGCACCCCTTC-3′ (311 bp); MyD88, 5′-GGAGCTTTTCGACGCCTTCA-3′ and 5′-GGACTTGGTGCAAGGGTTGG-3′ (380 bp); TIRAP, 5′-GTGGCCGTTGGAGCAAAGAC-3′ and 5′-TGGCCTCTGCCATCCACATA-3′ (370 bp); β-actin, 5′-ATCATGTTTGAGACCTTCAACA-3′ and 5′-CATCTCTTGCTCGAAGTCCA-3′ (300 bp).
Statistical Methods
For comparison between LPS- and saline-treated groups, one-way ANOVA was used (a P value < 0.05 was considered significant). We used Kaleidograph (version 6.0) (Synergy Software, Reading, PA) to make these calculations. Data expressed as mean ± SD.
RESULTS
Neutralization of CXCL5 Attenuates Neutrophil Sequestration in the Lung in a Concentration-Dependent Fashion In Vivo
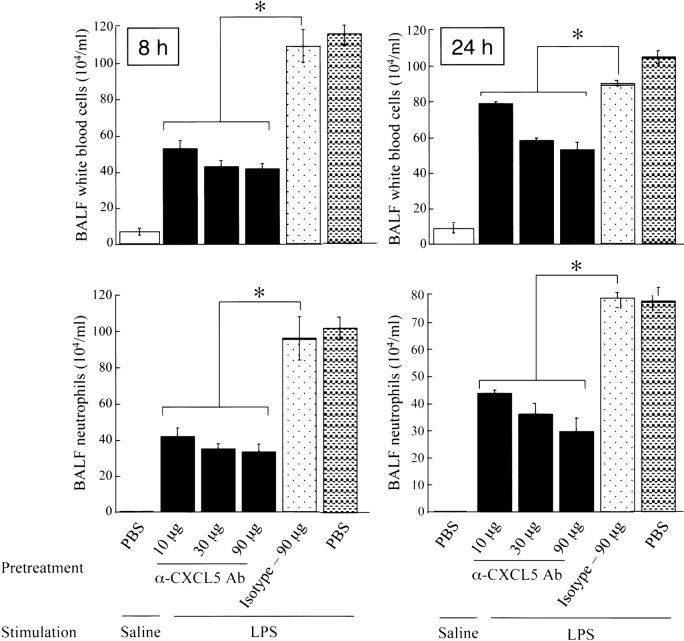
In our previous studies, we have shown that aerosolization of C57Bl/6 mice with 300 μg/ml LPS for 20 min induced inflammatory features at 8 and 24 h, whereas cytokine and chemokine expression peaks at 2 h (9). Therefore, we used 8 and 24 h after LPS challenge for the demonstration of neutrophil accumulation, while 2 h after LPS was the time point selected to measure CXCL5 expression in the current investigation. Although our earlier study indicates that an anti-CXCL5 Ab attenuates neutrophil accumulation in the lung during LPS-induced inflammation at 30 μg (9), in the present study we have used CXCL5 blocking Ab over a range of concentrations. Administration of a CXCL5 blocking Ab 2 h before LPS challenge attenuated LPS-induced WBC increase and neutrophil sequestration in lungs at 8 and 24 h in a concentration-dependent manner (Figure 1), whereas no attenuation was observed with the pretreatment of isotype-matched control Ab (Figure 1). Furthermore, Anti-CXCL5 Ab or isotype-matched control Ab without LPS challenge at the maximal concentration (90 μg) did not influence total WBC or neutrophil counts either at 8 or 24 h (data not shown). These observations suggest that CXCL5 exerts substantial non-redundant effects on neutrophil trafficking in the lung during LPS-induced inflammation.
Figure 1.
Neutralization of CXCL5 in the lung attenuates neutrophil accumulation in a concentration-dependent manner. Mice were pretreated with 10, 30, or 90 μg of CXCL5 blocking Ab or 90 μg of isotype-matched control Ab 2 h before LPS challenge. BALF and lungs were collected at 2, 8, and 24 h after LPS treatment, and total WBC and neutrophils in BALF were determined. One-way ANOVA used to establish the statistical significance (indicated by asterisks) between LPS-treated lungs and saline-treated mice (n = 6 animals/group).
CXCL5 Is Predominantly Induced in AEII Cells in the Lung
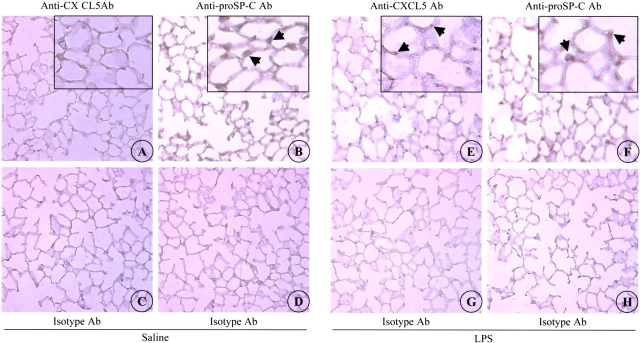
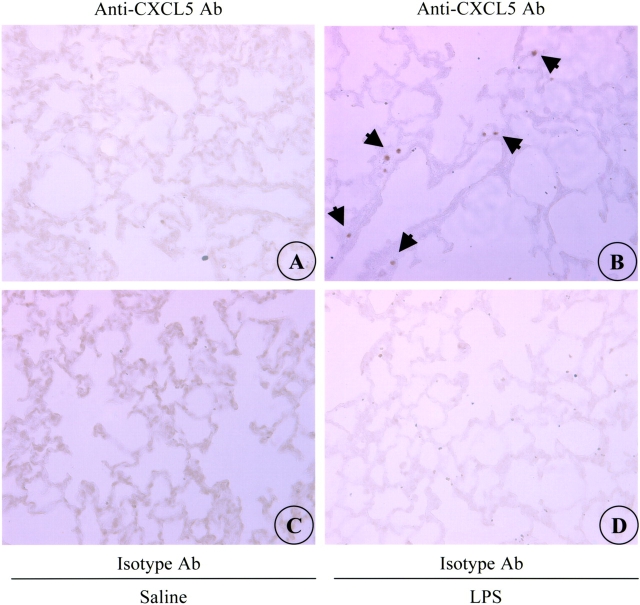
To determine the cells that expressed CXCL5 protein in the lung in vivo, we performed immunohistochemical analysis in normal mouse and rat lung and in lungs of LPS-aerosolized mouse. Twenty-four hours after challenge, CXCL5 immunoreactivity was not detected in saline-aerosolized (normal) lung (Figure 2A), whereas it was detected in lungs of LPS-aerosolized animals (Figure 2E). The specific signal was noted in the alveolus of the lung and was not observed in bronchial epithelial cells, alveolar epithelial type I-like (AEI) cells, neutrophils or macrophages (Figure 2E). Positively labeled cells appear to be AEII cells based on their location in the alveolus and proSP-C staining (Figure 2F). We have included several controls to ascertain that this immunoreactivity was specific for CXCL5: (1) the absence of immunoreactivity seen in sections where isotype-matched control serum was used (Figures 2C and 2G); and (2) no reactivity noted when primary anti-CXCL5 Ab was not included in the immunohistochemistry procedure (data not shown). In a similar manner, immunoreactivity was noted only in LPS-aerosolized rat lungs at 24 h, and the specific reactivity was observed in AEII cells based on their location (Figures 3A–3B). No immunoreactivity was observed in LPS-challenged or saline-challenged lungs when isotype-matched control Ab was used (Figures 3C–3D). These data demonstrate that AEII cells are the primary source of CXCL5 in the lungs of mice and rats.
Figure 2.
CXCL5 expression in mouse lung tissue. Immunoreactivity to CXCL5 is shown in the saline-aerosolized lung (A), and the LPS-aerosolized lung (E) at 24 h. Immunoreactivity to the isotype-matched control Ab is shown in the saline-aerosolized lung (C), and the LPS-aerosolized lung (G). Original magnification: ×200. No specific CXCL5 staining was observed in saline-aerosolized lung (A). Alveolar epithelial cells (arrows) are immunostained with the anti-CXCL5 Ab in LPS-aerosolized lung (E). Immunoreactivity to proSP-C was noted in the AEII cells of saline- (B) and LPS-aerosolized lung (F). Images A, B, E, and F are also shown in insets at a higher magnification. No proSP-C staining is observed in sections stained with isotype control Ab (D and H). This is a representative photomicrograph of five independent experiments.
Figure 3.
CXCL5 expression in rat lungs. While no immunostaining was noted in saline-aerosolized lung, (A) AEII cells are immunostained with anti-CXCL5 Ab in LPS-aerosolized lung (B). No immunoreactivity to isotype-matched control Ab was observed in saline- (C) or LPS-treated lungs (D). This is a representative photomicrograph of six experiments with identical results.
CXCL5 Gene Expression and Secretion Is Induced in AEII Cells In Vitro
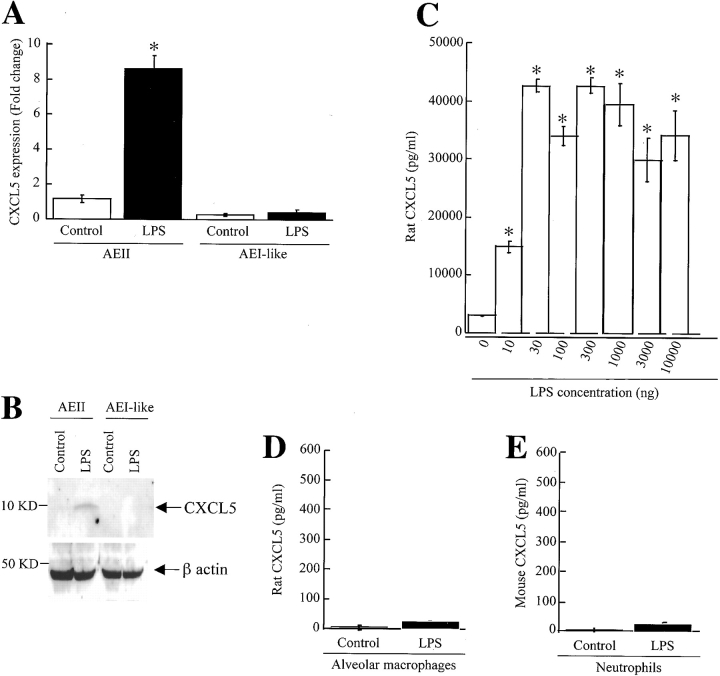
To confirm that AEII cells are able to express CXCL5 at the transcriptional and translational levels, we measured the levels of CXCL5 mRNA and protein in primary culture. We used cultured AEII cells of rat origin instead of mouse origin because (1) immunostaining observations reveal the same cellular origin of CXCL5 (Figure 3), and (2) they are well characterized and recovered in sufficient yield to perform all of our experiments. A very low level of CXCL5 mRNA was detected in unstimulated AEII- or AEI-like cells stimulated with LPS (Figure 4A). On the other hand, an 8.7-fold increase of CXCL5 was noted in AEII cells to 30 ng of LPS at 2 h (Figure 4A). We also measured immunoreactive CXCL5 in the cells and supernatant of rat AEII cells using Western blot and ELISA, respectively (Figures 4B and 4C). We did not notice significant CXCL5 protein expression in unstimulated AEII cells or LPS-stimulated AEI-like cells (Figure 4B). However, AEII cells secrete a large amount of CXCL5 in response to LPS in a concentration-dependent manner, with maximal expression at 30 ng of LPS (Figure 4C). Therefore, we used 30 ng of LPS in all of our subsequent experiments. To validate our immunohistochemical observations that myeloid cells do not express CXCL5, we used isolated murine neutrophils and rat alveolar macrophages. We did not observe CXCL5 protein secretion in isolated primary alveolar macrophages (Figure 4D) or neutrophils in response to LPS (Figure 4E). These results show that isolated AEII cells, but not myeloid cells, predominantly express CXCL5. To further demonstrate that AEII cells are the predominant source of CXCL5, we performed immunocytochemical analysis in AEII cells 18 h after LPS stimulation to validate the observation that AEII cells do produce CXCL5. We did not detect CXCL5 secretion in unstimulated AEII cells (Figure 5). By contrast, 35% of the cultured AEII cells produce CXCL5 (Figure 5). In our control experiments where isotype control Ab was used instead of primary Ab, no immunoreactivity was observed in AEII cells (Figure 5).
Figure 4.
CXCL5 expression by AEII cells in primary culture in response to LPS. Rat AEII cells, AEI-like cells, rat alveolar macrophages, and murine neutrophils were stimulated with 30 ng of LPS. Real-time quantitative PCR was performed in cell lysates 2 h after LPS stimulation or unstimulated (control) and fold change was calculated as described in MATERIALS AND METHODS (A). Cell-associated CXCL5 after stimulation was determined by Western blot (B) and the CXCL5 levels in supernatant was tested 18 h after stimulation (C–E). These results are representative of five separate experiments.
Figure 5.
Detection of cell-associated CXCL5 by immunocytochemistry in AEII cells. AEII cells were stimulated with LPS or unstimulated for 18 h before immunochemical staining with anti-CXCL5 or isotype-matched control Ab. Original magnification: ×1,000. CXCL5-producing cell (CPC) index was calculated as [immunofluorescence positive cells/total cells] × 100. A total of 3,000 cells were enumerated and percent positive cells were calculated. This is a representative result of four independent experiments.
CXCL5 Gene Expression and Secretion in Lung Is Regulated by the TLR4 Signaling Cascade In Vivo
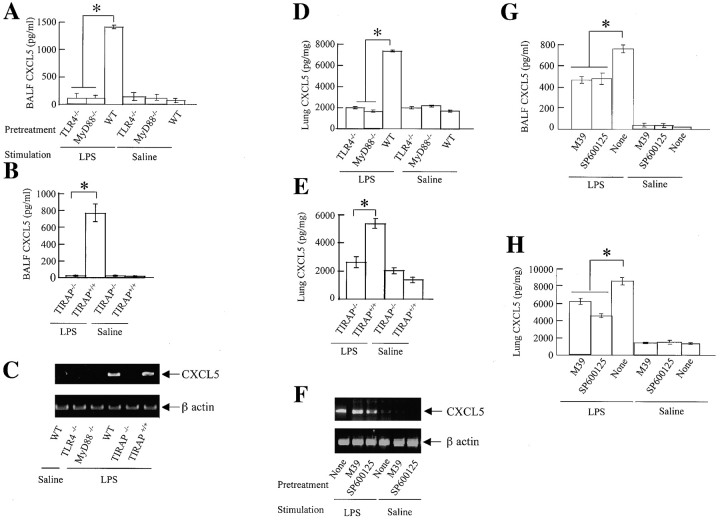
To gain mechanical insights of LPS-induced CXCL5 expression in the lung at the molecular level, we examined the role of TLR4-dependent signaling in the expression of CXCL5 in the lung after LPS challenge. We particularly wished to delineate the role of the pattern recognition receptor TLR4, and its adaptor molecules MyD88 and TIRAP in the TLR4 signaling cascade using TLR4−/−, MyD88−/−, and TIRAP−/− mice. In comparison with their wild-type (WT) mice, TLR4−/−, MyD88−/−, and TIRAP−/− mice showed very low protein expression of CXCL5 in BALF (Figures 6A and 6B) and lung homogenates (Figures 6C–6D) in response to LPS.
Figure 6.
CXCL5 expression in the lung in response to LPS is regulated via the TLR4 signaling cascade. Mice were exposed to 300 μg of nebulized LPS for 20 min and the BALF and lungs were harvested at 2 h for CXCL5 detection. Minimal CXCL5 expression was detected in TLR4−/−, MyD88−/−, and TIRAP−/− mice (A–E). Mice pretreated with the p38 inhibitor (M39) or the JNK inhibitor (SP600125) show attenuated expression of CXCL5 in BALF and lung homogenates (F–H). A total of six mice were tested in each group, and significance was calculated by a one-way ANOVA. Values that are significantly different between groups (P < 0.05) are indicated by asterisks.
Because TLR4-dependent signaling activates JNK and p38 kinases downstream, we also examined their role in the expression of CXCL5 in the lung using inhibitors for JNK and p38 kinases 2 h before LPS challenge in mice. Inhibitors of both JNK and p38 kinases significantly attenuated CXCL5 expression in the lung (Figures 6E–6H). These observations indicate that the TLR4-dependent signaling is important in CXCL5 expression in the lung.
CXCL5 Secretion in AEII Cells Is Also Regulated by the TLR4 Signaling Cascade In Vitro
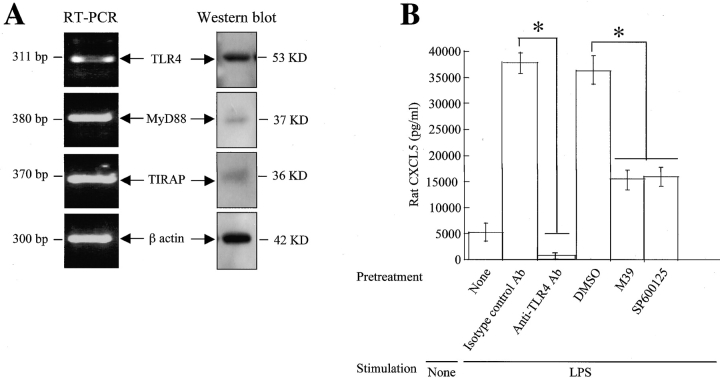
To demonstrate whether the TLR4 signaling cascade is required for mediating CXCL5 expression in isolated primary AEII cells in response to LPS, we performed experiments at the transcriptional and translational levels. Our RT-PCR and Western blot data demonstrate the presence of TLR4, MyD88 and TIRAP in isolated primary AEII cells both at mRNA and protein levels (Figure 7A). AEII cells were pretreated with 40 μg of anti-TLR4 Ab, and 10 μM of p38 (M39) and 5 μM of JNK kinase (SP600125) inhibitors 2 h before 30 ng of LPS stimulation. Anti-TLR4 Ab abolished LPS-induced CXCL5 expression from AEII cells (Figure 7B). By contrast, isotype-matched control to each Ab had no inhibitory effect on CXCL5 protein secretion induced by LPS (Figure 7B). Furthermore, both p38 and JNK kinase inhibitors attenuated LPS-induced CXCL5 protein secretion (Figure 7B). The vehicle (DMSO) had no inhibition in LPS-induced CXCL5 protein level (Figure 7B). Collectively, these data demonstrate that LPS-induced expression of CXCL5 by AEII cells requires activation of the TLR4 signaling cascade.
Figure 7.
CXCL5 expression in AEII cells in primary culture in response to LPS is regulated via the TLR4 signaling cascade. Unstimulated primary AEII cells were used to demonstrate the presence of molecules in the TLR4-dependent cascade, including TLR4, MyD88, and TIRAP at the transcriptional and translational levels (A). AEII cells were pretreated with anti-TLR4, isotype-matched control Ab, DMSO, M39 (p38 kinase inhibitor), and SP600125 (JNK kinase inhibitor) before stimulation with LPS. Unstimulated cells was used as a control. After 18 h incubation, the supernatant was harvested and CXCL5 detection was performed (B). (n = 4). Values that are significantly different between groups (P < 0.05) are indicated by asterisks.
DISCUSSION
Due to breathing of bacteria and their products, including LPS, from the commensals in the upper respiratory tract and the environment, the lung is continuously exposed to inflammatory particles. To deal with these bacteria and their products, the lung has developed a multifaceted defense mechanism. One of the important mechanisms that augments the initial innate immune defense in the lung is the vigorous recruitment of myeloid cells. On the other hand, excessive accumulation of myeloid cells in response to bacteria and/or their products can contribute to lung injury. A better understanding of mechanisms underlying the control of neutrophil influx is crucial to designing improved treatment and prevention strategies to attenuate pulmonary inflammation. The current investigation was undertaken to determine the cellular source of CXCL5 in the lung and to examine the signaling underlying CXCL5 expression in the lung in response to LPS and in isolated LPS-stimulated CXCL5 expressing cells that resulted in several new findings. First, AEII cells, but not AE-I–like cells, are the predominant source of CXCL5 in the lung during inflammation caused by E. coli–derived LPS in vivo. In addition, the CXCL5 expression is regulated by the TLR4-dependent signaling involved in TLR4, MyD88, TIRAP, and p38 and JNK kinases. Furthermore, the in vivo observations are supported by direct observations in AEII cells in primary culture.
During lung inflammation, neutrophil sequestration is a critical event, and this process involves multiple steps, including the production of chemokines, upregulation of cell adhesion molecules, and enhanced cell-to-cell interactions. Secretion of chemokines is the first and critical step for neutrophil sequestration in the lung. Previous investigations have shown that KC (3, 4), MIP-2 (5, 6), lungkine (7, 8), and CXCL5 (9) are the most important chemokines to cause neutrophil accumulation in the murine lung. CXCL5 expression was also observed during pulmonary inflammation induced by gram-negative pathogens, Legionella pneumophila (22), Pseudomonas aeruginosa (23), Klebsiella pneumoniae (24), and Bordetella bronchoseptica (25). In the present study, we determined the cellular origin of CXCL5 in lung inflammation. CXCL5 seems particularly important in regulating lung inflammation because: (1) CXCL5 protein levels in BALF persist until 24 h, whereas KC or MIP-2 levels are elevated in BALF only up to 8 h (9); and (2) CXCL5 protein is induced at higher protein levels in BALF and the lungs compared with KC or MIP-2 (9). These observations suggest that CXCL5 may have a different cellular origin than KC and MIP-2, which are secreted primarily by macrophages and neutrophils (3–6). Our immunohistochemistry results suggest that AEII cells are the main source of CXCL5 in the mouse lung after LPS challenge. In this context, earlier investigations on heart indicate that cardiomyocytes, but not myeloid cells, secrete CXCL5 during ischemia/reperfusion injury model in rats (12) and in a mouse model of peritonitis (13). It is important to note that CXCL5 expression is unlikely to be organ- or cell type–specific. CXCL5 can be induced in satellite cells in response to skeletal muscle injury (26) and in rat spinal cord cells in response to contusion injury (27). These findings suggest that the resident cells in several organs secrete CXCL5 to recruit neutrophils efficiently and to initiate the inflammatory response.
The relative contribution of pulmonary myeloid cells (macrophages and neutrophils) versus resident tissue cells in their contribution to neutrophil accumulation into the lung from blood vessels is uncertain and likely dependent on the stimulus, since both are exposed to inflammatory components. In earlier studies, it has been proposed that myeloid cells in the lung produce a battery of chemotactic substances such as KC (3, 4) and MIP-2 (5, 6) to target lung resident cells, including epithelial cells and fibroblasts to cause cytokine and chemokine expression. On the other hand, the resident cells can also produce chemotactic agents during lung inflammation. In this context, human bronchial epithelial cells (28) and lung type II–like cell line (A549) (29) can express the most potent neutrophil chemoattractant IL-8 in humans. Furthermore, murine bronchial epithelial cells can secrete a neutrophil chemoattractant in the lung, lungkine (7, 8). Clearly, our data indicate that resident alveolar type II structural cells modulate lung inflammation via CXCL5 synthesis and subsequent neutrophil recruitment.
Toll-like receptors (TLRs) recognize pathogen-associated molecular patterns of microbes and/or their products, and so far 11 TLRs have been identified (30). TLR4 signaling is associated with two major adaptor proteins, MyD88 and TIRAP (14). The regulation of the rodent CXCL5 expression in the lung has not been extensively studied. However, the recent discovery of E. coli–derived LPS signaling via TLR4 led us to investigate the molecular mechanisms underlying the activation of this receptor and its signaling intermediates in CXCL5 expression in the lung. Although this is the first description of TLR4 involvement in LPS-induced CXCL5 expression, a number of other studies support the role of the TLR4 signaling in other cytokines or chemokines. For example, we previously reported that TLR4 activation by LPS is necessary to induce KC, MIP-2, tumor necrosis factor-α, and IL-6 in the lung during LPS-induced lung inflammation (31).
Pretreatment with p38 kinase inhibitors has been demonstrated to attenuate neutrophil accumulation and cytokine responses in several animal models such as arthritis and peritonitis. In a pulmonary inflammation model, we have previously shown that a highly selective p38 inhibitor (M39) can reduce pulmonary neutrophil influx induced by LPS (16, 20). Interestingly, the neutrophil influx was reduced in spite of no significant reduction of KC, MIP-2, or monocyte chemotactic protein-1 levels in BALF and serum (16, 20). Because we demonstrate a dose-dependent inhibition of neutrophil accumulation by anti-CXCL5 Ab together with the reduction of CXCL5 levels in the lung by the p38 inhibitor, this suggests that attenuation of CXCL5 may be another mechanism responsible for the reduction in neutrophil influx by the p38 kinase inhibitor. In the present study, pretreatment with a selective JNK kinase inhibitor also attenuates CXCL5 protein production in the lung. In this regard, Lee and coworkers (32) have shown that the JNK inhibitor (SP600125) can reduce LPS-induced inflammatory lung injury via inhibiting nuclear factor-κB activation (32). Because CXCL5 is a nuclear factor-κB–regulated chemokine (12), it is not unreasonable to assume that CXCL5 induction might also have been attenuated by the JNK inhibitor in their studies.
Although earlier studies have shown that TLR4 contributes an important role in myeloid cells (30), recent evidence suggests that LPS-induced signaling cascades also exist in epithelial cells. TLR4 is expressed in renal, colonic and gingival, intestinal, and alveolar epithelium of humans. However, little is known about the expression of TLR4 in rat AEII cells. Furthermore, the signaling cascades underlying the expression of cytokines and chemokines by primary AEII cells in response to LPS has not been previously examined despite that fact that these cells are a likely target for environmentally inhaled LPS and gram-negative bacteria. This is probably due to lack of availability and perfected procedures to isolate them from human lungs. In the current investigation, we demonstrate that primary rat AEII cell shares molecules with myeloid cells in the TLR4-dependent signaling, such as TLR4, MyD88, and TIRAP, as demonstrated both at mRNA and protein levels. In addition, we described that isolated primary rat AEII cells produce CXCL5 after LPS stimulation. In this context, a number of in vitro studies have provided evidence that rodent (mainly rat) AEII cells can produce several proinflammatory cytokines and chemokines, including TNF-α (33), MIP-2 (34), and MCP-1 (21) in response to LPS stimulation, although the signaling cascades in these cells have remained elusive. This is likely due to lack of appropriate reagents for rats as compared with mice or humans. Despite these limitations, we established that LPS-induced expression of CXCL5 by AEII cells is dependent on the activation of TLR4 and p38 and JNK kinases. Current research findings indicate that the TLR4-dependent signaling plays an important role in the induction of other LPS-induced chemokines and cytokines by AEII cells. These observations suggest that LPS signaling cascade in AEII cells is similar to that of myeloid cells to produce cytokines or chemokines. However, future studies are required to further delineate the similarities and differences in signaling between AEII cells and myeloid cells to induce cytokines and chemokines in response to LPS.
Cellular cross talk between myeloid and resident cells is also an important process in the initiation, maintenance, and resolution of an inflammatory process in the lung. In this context, the preferential induction of CXCL5 by AEII cells in the alveolus may be important. Our in vitro findings suggest that CXCL5 could modulate the activation of myeloid cells in the lung via binding and activation of its receptor CXCR2, which is expressed in several cell types in the lung, including neutrophils (35) and alveolar epithelial cells (35).
In conclusion, the present study demonstrates for the first time that LPS-induced expression of CXCL5 in the lung involves activation of AEII cells via the TLR4-dependent signaling, demonstrating the potential in the innate immune response in the lung. In addition, our current study supports the conclusion of our previous report (31) that blocking the TLR4 signaling cascade is a useful strategy to minimize lung injury induced by gram-negative pathogens via attenuating cytokine and chemokine expression and subsequent neutrophil accumulation in the lungs.
Acknowledgments
The authors thank Hong Wei Chu and Michael Fessler for helpful discussions. They thank Kenneth Malcolm for helpful discussions and for his help in immunofluorescence microscopy.
This study was supported by NIH grants P50HL067671-03 and HL-029891, and by a grant from the EPA (X-83084601).
Conflict of Interest Statement: S.J. has no declared conflicts of interest; R.M. has no declared conflicts of interest; S.K.Y. has no declared conflicts of interest; M.Y. has no declared conflicts of interest; S.A. has no declared conflicts of interest; R.J.M. has no declared conflicts of interest; and G.S.W. has no declared conflicts of interest.
References
- 1.Lukacs NW, Hogaboam C, Campbell E, Kunkel SL. Chemokines: function, regulation and alteration of inflammatory responses. Chem Immunol 1999;72:102–120. [DOI] [PubMed] [Google Scholar]
- 2.Baggiolinim M, Dewaldm B, Moser B. Interleukin-8 and related chemotactic cytokines–CXC and CC chemokines. Adv Immunol 1994;55:97–179. [PubMed] [Google Scholar]
- 3.Bozic CR, Kolakowski LF, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol 1995;154:6048–6057. [PubMed] [Google Scholar]
- 4.Frevert CW, Huang S, Danaee H, Paulauskis JD, Kobzik L. Functional characterization of the rat chemokine KC and its importance in neutrophil recruitment in a rat model of pulmonary inflammation. J Immunol 1995;154:335–344. [PubMed] [Google Scholar]
- 5.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci USA 1989;86:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driscoll KE, Hassenbein DG, Howard BW, Isfort RJ, Cody D, Tindal MH, Suchanek M, Carter JM. Cloning, expression, and functional characterization of rat MIP-2: a neutrophil chemoattractant and epithelial cell mitogen. J Leukoc Biol 1995;58:359–364. [DOI] [PubMed] [Google Scholar]
- 7.Rossi DL, Hurst SD, Xu Y, Wang W, Menon S, Coffman RL, Zlotnik A. Lungkine, a novel CXC chemokine, specifically expressed by lung bronchoepithelial cells. J Immunol 1999;162:5490–5497. [PubMed] [Google Scholar]
- 8.Chen SC, Mehrad B, Deng JC, Vassileva G, Manfra DJ, Cook DN, Wiekowski MT, Zlotnik A, Standiford TJ, Lira SA. Impaired pulmonary host defense in mice lacking expression of the CXC chemokine lungkine. J Immunol 2001;166:3362–3368. [DOI] [PubMed] [Google Scholar]
- 9.Jeyaseelan S, Chu HW, Young SK, Worthen GS. Transcriptional profiling of lipopolysaccharide-induced acute lung injury. Infect Immun 2004;72:7247–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith JB, Wadleigh DJ, Xia YR, Mar RA, Herschman HR, Lusis AJ. Cloning and genomic localization of the murine LPS-induced CXC chemokine (LIX) gene, Scyb5. Immunogenetics 2002;54:599–603. [DOI] [PubMed] [Google Scholar]
- 11.Wuyts A, D'Haese A, Cremers V, Menten P, Lenaerts JP, De Loof A, Heremans H, Proost P, Van Damme J. NH2- and COOH-terminal truncations of murine granulocyte chemotactic protein-2 augment the in vitro and in vivo neutrophil chemotactic potency. J Immunol 1999;163:6155–6163. [PubMed] [Google Scholar]
- 12.Chandrasekar B, Smith JB, Freeman GL. Ischemia-reperfusion of rat myocardium activates nuclear factor-KappaB and induces neutrophil infiltration via lipopolysaccharide-induced CXC chemokine. Circulation 2001;103:2296–2302. [DOI] [PubMed] [Google Scholar]
- 13.Madorin WS, Rui T, Sugimoto N, Handa O, Cepinskas G, Kvietys PR. Cardiac myocytes activated by septic plasma promote neutrophil transendothelial migration: role of platelet-activating factor and the chemokines LIX and KC. Circ Res 2004;94:944–951. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto M, Sato S, Hemmi H, Sanjo H, Uematsu S, Kaisho T, Hoshino K, Takeuchi O, Kobayashi M, Fujita T, et al. Essential role for TIRAP in activation of the signalling cascade shared by TLR2 and TLR4. Nature 2002;420:324–329. [DOI] [PubMed] [Google Scholar]
- 15.Fessler MB, Young SK, Jeyaseelan S, Lieber JG, Arndt PG, Nick JA, Worthen GS. A role for hydroxy-methylglutaryl coenzyme A reductase in pulmonary inflammation and host defense. Am J Respir Crit Care Med 2005;171:606–615. [DOI] [PubMed] [Google Scholar]
- 16.Nick JA, Young SK, Arndt PG, Lieber JG, Suratt BT, Poch KR, Avdi NJ, Malcolm KC, Taube C, Henson PM, et al. Selective suppression of neutrophil accumulation in ongoing pulmonary inflammation by systemic inhibition of p38 mitogen-activated protein kinase. J Immunol 2002;169:5260–5269. [DOI] [PubMed] [Google Scholar]
- 17.Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci USA 2001;98:13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason RJ, Lewis MC, Edeen KE, McCormick-Shannon K, Nielsen LD, Shannon JM. Maintenance of surfactant protein A and D secretion by rat alveolar type II cells in vitro. Am J Physiol Lung Cell Mol Physiol 2002;282:L249–L258. [DOI] [PubMed] [Google Scholar]
- 19.Mason RJ, Pan T, Edeen KE, Nielsen LD, Zhang F, Longphre M, Eckart MR, Neben S. Keratinocyte growth factor and the transcription factors C/EBP alpha, C/EBP delta, and SREBP-1c regulate fatty acid synthesis in alveolar type II cells. J Clin Invest 2003;112:244–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nick JA, Young SK, Brown KK, Avdi NJ, Arndt PG, Suratt BT, Janes MS, Henson PM, Worthen GS. Role of p38 mitogen-activated protein kinase in a murine model of pulmonary inflammation. J Immunol 2000;164:2151–2159. [DOI] [PubMed] [Google Scholar]
- 21.Paine R III, Rolfe MW, Standiford TJ, Burdick MD, Rollins BJ, Strieter RM. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J Immunol 1993;150:4561–4570. [PubMed] [Google Scholar]
- 22.Tateda K, Moore TA, Newstead MW, Tsai WC, Zeng X, Deng JC, Chen G, Reddy R, Yamaguchi K, Standiford TJ. Chemokine-dependent neutrophil recruitment in a murine model of Legionella pneumonia: potential role of neutrophils as immunoregulatory cells. Infect Immun 2001;69:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai WC, Strieter RM, Mehrad B, Newstead MW, Zeng X, Standiford TJ. CXC chemokine receptor CXCR2 is essential for protective innate host response in murine Pseudomonas aeruginosa pneumonia. Infect Immun 2000;68:4289–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schurr JR, Young E, Byrne P, Steele C, Shellito JE, Kolls JK. Central role of toll-like receptor 4 signaling and host defense in experimental pneumonia caused by Gram-negative bacteria. Infect Immun 2005;73:532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Widney DP, Hu Y, Foreman-Wykert AK, Bui KC, Nguyen TT, Lu B, Gerard C, Miller JF, Smith JB. CXCR3 and its ligands participate in the host response to Bordetella bronchiseptica infection of the mouse respiratory tract but are not required for clearance of bacteria from the lung. Infect Immun 2005;73:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sachidanandan C, Sambasivan R, Dhawan J. Tristetraprolin and LPS-inducible CXC chemokine are rapidly induced in presumptive satellite cells in response to skeletal muscle injury. J Cell Sci 2002;115:2701–2712. [DOI] [PubMed] [Google Scholar]
- 27.McTigue DM, Tani M, Krivacic K, Chernosky A, Kelner GS, Maciejewski D, Maki R, Ransohoff RM, Stokes BT. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J Neurosci Res 1998;53:368–376. [DOI] [PubMed] [Google Scholar]
- 28.Gon Y, Hashimoto S, Nakayama T, Matsumoto K, Koura T, Takeshita I, Horie T. N-acetyl-L-cysteine inhibits bleomycin-induced interleukin-8 secretion by bronchial epithelial cells. Respirology 2000;5:309–313. [PubMed] [Google Scholar]
- 29.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP, Toews GB, Westwick J, Strieter RM. Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest 1990;86:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akira S, Takeda K. Toll-like receptor signaling. Nat Rev Immunol 2004;4:499–511. [DOI] [PubMed] [Google Scholar]
- 31.Jeyaseelan S, Chu HW, Young SK, Freeman MW, Worthen GS. Distinct roles of pattern recognition receptors CD14 and toll-like receptor 4 in acute lung injury. Infect Immun 2005;73:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HS, Kim HJ, Moon CS, Chong YH, Kang JL. Inhibition of c-Jun NH2-terminal kinase or extracellular signal-regulated kinase improves lung injury. Respir Res 2004;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McRitchie DI, Isowa N, Edelson JD, Xavier AM, Cai L, Man HY, Wang YT, Keshavjee SH, Slutsky AS, Liu M. Production of tumor necrosis factor alpha by primary cultured rat alveolar epithelial cells. Cytokine 2000;12:644–654. [DOI] [PubMed] [Google Scholar]
- 34.Xavier AM, Isowa N, Cai L, Dziak E, Opas M, McRitchie DI, Slutsky AS, Keshavjee SH, Liu M. Tumor necrosis factor-α mediates lipopolysaccharide-induced macrophage inflammatory protein-2 release from alveolar epithelial cells: autoregulation in host defense. Am J Respir Cell Mol Biol 1999;21:510–520. [DOI] [PubMed] [Google Scholar]
- 35.Goncalves AS, Appelberg R. The involvement of the chemokine receptor CXCR2 in neutrophil recruitment in LPS-induced inflammation and in Mycobacterium avium infection. Scand J Immunol 2002;55:585–591. [DOI] [PubMed] [Google Scholar]