Abstract
Submucosal glands are abundant (∼ 1 gland/mm2) secretory structures in the tracheobronchial airways of the human lung. Because submucosal glands express antibacterial proteins, it has been proposed that they contribute to lung defense. However, this concept is challenged by the fact that mice do not have submucosal glands in their bronchial airways, yet are quite resistant to bacterial lung infection. The contribution of airway submucosal glands to host defense is also debated as a pathophysiologic component of cystic fibrosis lung disease. Here, we asked whether submucosal glands protect airways against bacterial infection. By comparing tracheal xenograft airways with and without glands, we found that the presence of glands enhanced bacterial killing in vivo and by airway secretions in vitro. Moreover, immunodepletion studies suggested that lysozyme is a major antibacterial component secreted by submucosal glands. These studies provide evidence that submucosal glands are a major source of antibacterials critical for maintaining sterile airways.
Keywords: innate immunity, lung, lysozyme, glands, antibacterial proteins
Submucosal glands (SMGs) of the lung secrete fluid, mucus, and bacteriocidal proteins that are thought to help protect the lung against bacterial infection (1). Cystic fibrosis (CF), asthma, and chronic bronchitis are diseases in which SMGs are hypothesized to play an important role in lung pathogenesis. In the context of CF, a gene defect in the cystic fibrosis transmembrane conductance regulator (CFTR) leads to chronic bacterial infections of the lung (2). CFTR is highly expressed in SMGs (3–5) and is thought to control the composition and viscosity of glandular fluids secreted into the airways (6–8). Although a link between submucosal gland function and lung innate immunity has been widely speculated, in vivo evidence has been lacking.
Anatomically, SMGs are composed of a series of interconnecting tubules and ducts localized beneath the airway surface. Secretory products move vectorially from the distal serous and mucous tubules which connect with the airway lumen through collecting and ciliated ducts (9). Lysozyme, an abundant antibacterial factor in the human lung (0.5 mg/ml) (10, 11), is predominantly synthesized by serous cells of SMGs (12, 13). Until recently, in vivo evidence for a biological role of lysozyme in the human airway has been limited. It has been shown that part of the antibacterial activity in human nasal secretions depleted of cationic polypeptides can be restored by adding back lysozyme (14). Given the fact that lysozyme is highly expressed in SMGs of the human airway (15), it has been generally accepted that SMGs play an important role in protecting the human lung from bacterial infection. However, because surface airway epithelial cells appear to also express lysozyme at lower levels (16), it is currently unclear if SMGs production of lysozyme is required for airway innate immunity.
In part, understanding SMG function in the human lung has been hindered by differences in mouse and human lung biology. In contrast to humans, relatively few airway SMGs exist in mouse trachea and are absent throughout the remaining cartilaginous airways (17). In mice, type II alveolar cells have the capacity to produce lysozyme (18). These differences between mice and humans have been speculated to be one contributing factor for explaining why the human CF lung phenotype characterized by spontaneous bacterial infection is not reproduced in CFTR-deficient mice.
In the present study, we adapted airway xenograft models to study the contribution of SMGs in protecting the airway from bacterial infection. Ferret proximal airways with and without SMGs were generated by subcutaneously grafting native (with glands) or reconstituted (without glands) tracheas in the flanks of nu/nu mice. Importantly, the distribution of surface airway epithelial cells types (i.e., ciliated, goblet, nonciliated columnar, basal, and intermediate cells) in these two airway models is indistinguishable (19). Hence, glandular contribution to airway antibacterial activity could be directly assessed. Our studies demonstrated that xenograft airways with SMGs more effectively cleared bacterial infection in vivo and produced substantially greater lysozyme and lactoferrin than airways lacking SMGs. Furthermore, depletion of lysozyme from glandular airway secretions significantly reduced antibacterial activity. These studies provide evidence that SMGs are an important source of innate immunity in the airway.
MATERIALS AND METHODS
Generation of Ferret Xenograft Airways
Xenograft airways with and without glands were generated as previously described (19). Xenograft airways without glands were generated from harvested surface airway epithelial cells from an adult ferret and were seeded onto denuded rat tracheas stripped of endogenous airway epithelia by freeze-thawing and washing three times. Tracheas were ligated to flexible plastic tubing, implanted subcutaneously into the flanks of nu/nu mice, and used for experiments after regeneration of an intact surface epithelia at 5–6 wk after transplant. For generating xenograft airways with glands, proximal segments of freshly excised 3-wk-old ferret tracheas were ligated to flexible tubing, implanted subcutaneously into nu/nu mice, and used for experiment at 3 wk after transplantation.
Antibacterial Luminescence Assay
Antimicrobial luminescence assays were used as previously described (11), and measured antibacterial activity as reflected by a reduction in the energy-dependent luminescence of bacteria expressing the Photorhabdus luminescens luciferase gene. All experiments were performed with xenograft airway secretions harvested from xenografts in 100 μl of iso-osmotic 5% mannitol or human nasal secretions harvested in water. Experimental samples are always referenced to controls receiving an equivalent amount of vehicle (5% mannitol or water for human nasal secretions).
Radial Diffusion Assay
We used a modified radial diffusion assay to investigate bacterial sensitivity to SMG secretions as previously described (21). Briefly, 4 × 106 of Escherichia coli DH5α at mid-log phase was suspended in an underlay gel consisting of low salt agarose in 10 mM sodium phosphate (pH 7.4). Secretions from glandular and nonglandular airways were lyophilized and then reconstituted in an equal volume of 5% mannitol. Three-millimeter-diameter wells were punched into the gel and filled with 330 μg of secretions from xenografts with or without glands or 5% mannitol as a control. The plates were then incubated for 3 h at 37°C. A nutrient-rich gel was then overlaid and the plates were incubated overnight at 37°C. Zones of clearance were manually measured.
In Vivo Bacterial Survival
The antibacterial activity of ferret tracheal xenografts with and without glands was assessed by inoculating 6-wk-old grafts with 1 × 105 cfu of kanamycin-resistant E. coli EC838 (a gift from Dr. David Weiss, University of Iowa). The xenografts were monitored for bacterial survival by irrigation at various time points after inoculation and plating effluents on kanamycin containing agar to quantify cfu. At the end of experiments (14 d), xenograft airway tissue homogenates were similarly quantified for total cfu by plating dilutions of the homogenate.
Electrophoresis of Tracheal Secretions
The complexity of proteins found in xenograft airway secretions was evaluated with Brilliant Blue stained acid-urea polyacrylamide gel electrophoresis (AU-PAGE) and SDS-PAGE gels as previously described (22). In addition, SDS-PAGE Western blots were used to evaluate the abundance of lysozyme and lactoferrin in xenograft airway secretions using antibodies purchased from Neomarkers Inc. (Fremont, CA) and US Biological Inc. (Swampscott, MA), respectively.
Immunodepletion of Lysozyme
Lysozyme immunodepletion was performed using 50 μl of washed protein-A-Dyna beads loaded with anti-lysozyme antibody (25 μl) (Novus Inc., Littleton, CO) or without antibody (mock). Fifty microliters of xenograft airway secretions, or 200 μl of human nasal secretions, were incubated at 4°C with constant rotation for 1 h. After magnetic removal of beads, secretions were immunodepleted a second time. Two serial immunodepletions were sufficient to remove all lysozyme from secretions. Immunodepleted xenograft airway secretions were run on a 15% SDS-PAGE and blotted with an anti-lysozyme antibody (Neomarkers Inc.) or tested for antibacterial activity using the luminescent assay.
RESULTS
To address the importance of SMGs in airway innate immunity, we utilized ferret tracheal xenograft models of reconstituted airways with and without SMGs (19) (Figure 1A). Unlike mice, ferret cartilaginous airways have similar airway cell types and abundance of SMGs as seen in humans (17, 20). Xenograft airways without SMGs were generated from isolated adult ferret tracheal airway epithelial cells that were grafted onto canulated denuded rat tracheal subcutaneous implants of nu/nu mice. As previously described, these xenografts regenerate an intact airway epithelium lacking glands at 5–6 wk after transplant (19). Canulated xenograft trachea with SMGs were generated from freshly excised 3-wk-old ferret tracheas also implanted into nu/nu mice and used for experimentation at 3 wk after transplantation. Importantly, ferret tracheal xenografts with and without SMGs possess an indistinguishable distribution of surface airway epithelial cell types (19). Hence, any difference between the two types of airways can be attributed to the presence of SMGs.
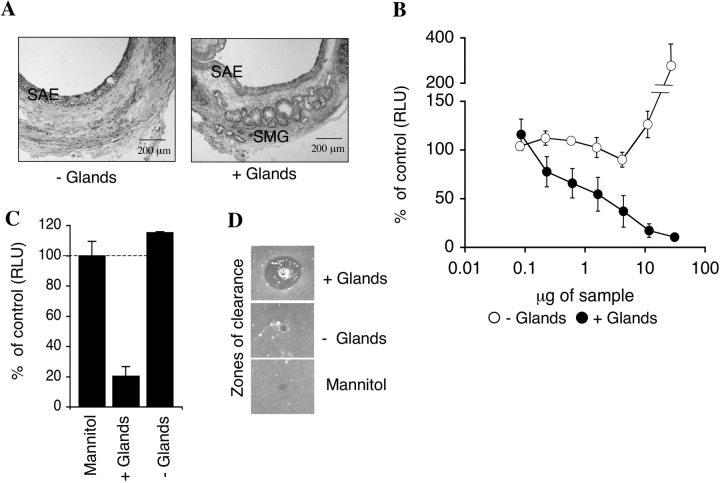
Figure 1.
Antibacterial activity of ferret xenograft airway secretions. (A) Hematoxylin and eosin (H&E)-stained sections of xenograft trachea with and without glands. SAE, surface airway epithelium; SMG, submucosal glands (bar = 200 μm). (B) The antibacterial activity of equal μg quantities of secretions from tracheal xenografts, with (filled circles) or without (open circles) glands was evaluated using an E. coli bioluminscence assay. (C) The antibacterial activity of 4.3 μg of secretions from xenografts with or without glands against P. aeruginosa was evaluated using a bioluminescence assay. Values in B and C represent the percent of control (5% mannitol) (Mean ± SEM, n = 4 independent samples from 4 different xenografts). (D) The antibacterial activity of 330 μg of tracheal xenograft secretions with and without glands as compared with 5% mannitol (vehicle control) was evaluated using a radial diffusion assay.
Using a luciferase-based bacterial growth assay (11), secretions harvested from glandular airways show significant antibacterial activity against E. coli (Figure 1B) and Pseudomonas aeruginosa (PAO1) (Figure 1C). In contrast, antibacterial activity was not detected in secretions from nonglandular airways (Figures 1B and 1C). This SMG-associated antibacterial activity was also confirmed using radial diffusion assays (21) (Figure 1D) and cfu assays (data not shown). Interestingly, high μg concentrations of nonglandular airway secretions led to increased growth of bacteria, a finding not observed with glandular airway secretions (Figure 1B). Cumulatively, these data demonstrate that secretions from glandular airways have a higher capacity to kill bacteria than do nonglandular airways. Furthermore, they suggest that glandular secretions may counteract growth-promoting activity of surface airway epithelial secretions.
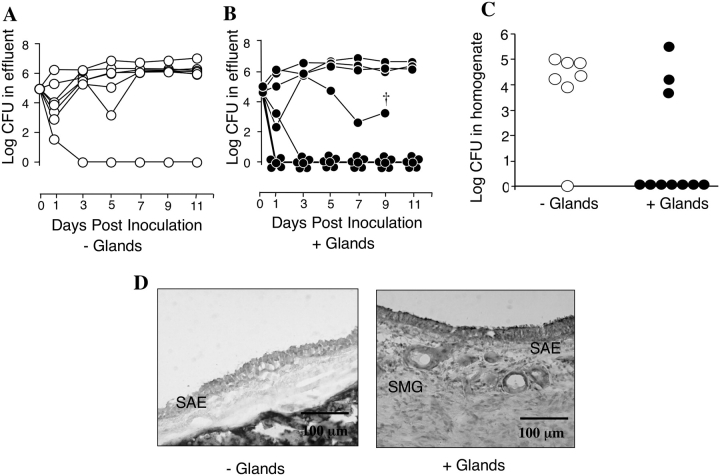
To investigate whether the presence of SMGs enhances the ability of airways to clear bacterial infections, 1 × 105 cfu of E. coli were directly inoculated into the lumen of xenograft airways with and without SMGs. Following infection, xenografts were monitored for in vivo bacterial survival at various time points by quantifying cfu in the effluents (Figures 2A and 2B). The rate of in vivo bacterial clearance was higher in airways with glands as compared with airways without glands. After 14 d, the xenografts were harvested and bacterial survival in tracheal tissues was assessed using a cfu assay. The median bacterial survival in xenografts without glands was significantly greater (P < 0.05) than xenografts with glands (Figure 2C). Histologic analysis of tracheal xenografts at 14 d after infection demonstrated that colonized and noncolonized xenografts both maintained an intact airway epithelium throughout the experiment (Figure 2D). However, neutrophil infiltration was more predominant in grafts that maintained a persistent infection. In summary, these findings provide strong evidence that SMGs protect the airway from bacterial infection.
Figure 2.
In vivo survival of bacteria in xenograft airways. (A and B) In vivo survival of bacteria in xenograft airways as measured in the effluent at the indicated time points following in vivo inoculation of 1 × 105 cfu of E. coli. Each xenograft is plotted as a separate line. † This xenograft was not able to be perfused after this time point, but upon harvest of the graft was found to be free of bacteria. (C) In vivo survival of E. coli in xenograft tissue homogenates at 14 d after inoculation. Medians are significantly different using a nonparametric Mann Whitney two-tailed assay (P < 0.05), n = 7 independent xenografts without glands (open circles) and n = 10 independent xenografts with glands (filled circles). The same xenografts shown in A and B are shown in C. (D) H&E-stained sections of representative tracheal xenografts without (left panel) with glands (right panel) at 14 d after bacterial inoculation. The xenograft without glands retained bacterial colonization at the time of harvest, whereas the glandular xenograft did not. SAE, surface airway epithelium; SMG, submucosal glands (bar = 100 μm).
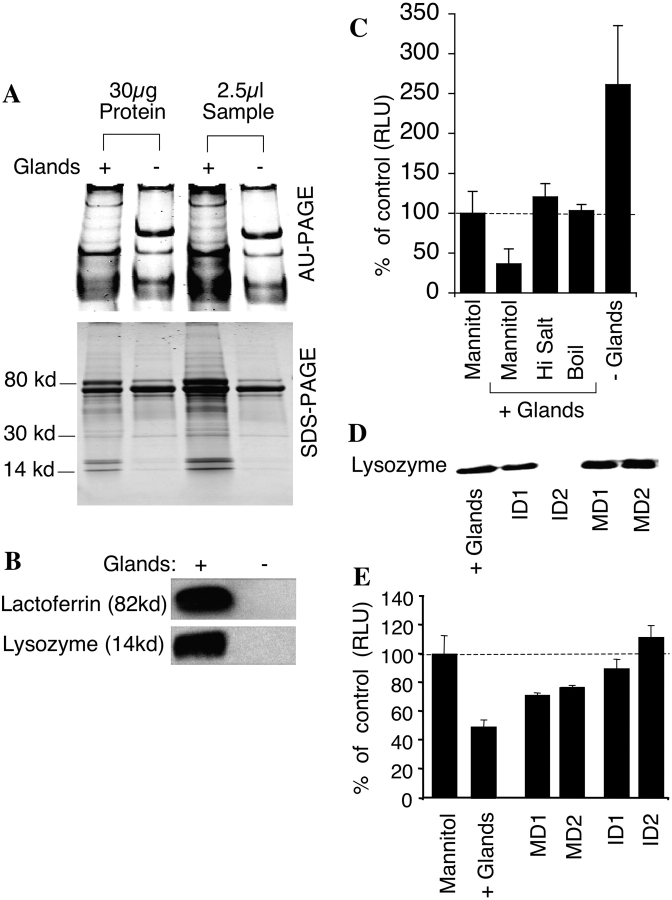
To begin to identify the factors that participate in airway innate immunity, equal volumes and equal protein concentrations of secretions from xenografts with and without SMGs were analyzed by AU-PAGE (22) and SDS-PAGE (Figure 3A) and stained with Coomassie brilliant blue. Glandular secretions were more complex as compared with secretions from xenografts without SMGs. However, unique bands existed in both types of secretions. Glandular secretions contained unique protein bands on AU-PAGE that migrated in a similar, but not identical, manner to human lysozyme and human lactoferrin standards (data not shown). These differences in migration were likely due to amino acid sequence differences between human and ferret proteins. Hence, to conclusively determine if lysozyme and lactoferrin were enriched in glandular secretions, we performed Western blots for these proteins. Results from this analysis confirmed that both lysozyme and lactoferrin were significantly enriched in glandular secretions (Figure 3B). The presence of a highly abundant unique AU-PAGE protein band in secretions from xenografts lacking glands was an unexpected finding. This may be a result of a proteolytic factor secreted by SMGs that partially degrades a protein secreted by the surface airway epithelium. Alternatively, SMGs may secrete a factor that inhibits secretion of a protein from surface airway epithelium. Because lysozyme is both salt-sensitive and heat-labile (11), we examined these two features of SMG-derived antibacterial activity. Both high salt- and heat-treated secretions had reduced antibacterial activity (Figure 3C). Cumulatively, these findings suggested that lysozyme could be a major component of antibacterial activity of glandular airway secretions.
Figure 3.
Analysis of ferret xenograft airway secretions. (A) AU-PAGE and SDS-PAGE of xenograft airway secretions with and without glands. Each lane was loaded with 2.5 μl of secretions or 30 μg of protein. (B) Western blot of lysozyme and lactoferrin from xenograft airway secretions (2.5 μl/lane). (C) The effect of NaCl or boiling on antibacterial activity of xenograft airway secretions. E. coli were incubated with 50 μg of secretions harvested from xenografts with glands in the absence or presence of 125 mM NaCl added to the standard assay buffer. E. coli were also incubated with 50 μg of secretions harvested from xenograft with glands after boiling for 30 min. Values are given as percent of control luminescence (5% mannitol), Mean ± SEM, n = 4. (D) Western blot showing immunodepletion of lysozyme from secretions of xenografts with glands. + Glands, secretions from xenografts with glands; ID1, supernatant following the first immuno-depletion; ID2, supernatant following the second immunodepletion; MD1, supernatant following the first mock immunodepletion; MD2, supernatant following the second mock immunodepletion. All lanes were loaded with 5 μl and are representative of four independent experiments. (E) The effect of immunodepletion of lysozyme from secretions of xenograft with glands on antibacterial activity was measured using the bioluminescent assay on 50 μg of sample. Values represent the percent of control luminescence (5% mannitol), n = 4. Results depict the mean (± SEM) luminescence as compared with control samples.
Because lysozyme levels were significantly higher in secretions from xenograft airways containing SMGs, we tested the hypothesis that this enzyme is at least partially responsible for the antibacterial activity observed in glandular airways. Lysozyme was removed from secretions of xenograft airways with SMGs by two rounds of immunodepletion with an antilysozyme antibody (Figure 3D). The mock-depleted secretions lost ∼ 50% of the antibacterial activity due to nonspecific absorption of antibacterial factors other than lysozyme by the Dyna beads. The remaining antibacterial activity seen in the mock-depleted secretions was lost after lysozyme depletion. Lysozyme-depleted secretions were much less effective at inhibiting bacterial growth than mock-depleted samples, suggesting that lysozyme is partially responsible for the antibacterial activity of the glandular airway secretions (Figure 3E). Consistent with our findings using glandular ferret tracheal xenograft secretions, immunodepletion of lysozyme from human nasal secretions also reduced antibacterial activity by 50% as compared with mock-depleted samples (data not shown).
These studies provide in vivo evidence that lysozyme secreted by SMGs imparts important antibacterial activity to the airway. Although glandular lysozyme appears to be an important component of airway innate immunity, it is likely that other factors also contribute to the antibacterial activity found in SMG secretions. For example, lactoferrin has been recently shown to prevent bacterial biofilm development (23), and our present study demonstrates that lactoferrin found in airway secretions is predominantly produced by SMGs. These findings, and the fact that CFTR is expressed in SMGs, support the notion that SMGs play an important role in host defense and the pathophysiology of CF lung disease.
This study was supported by research funding from NIDDK (DK47967 to J.F.E.), NHLBI (HL061234 to M.J.W.), and the Animal Models Core of the Center for Gene Therapy NIDOK P30 DK54759.
Conflict of Interest Statement: R.D. has no declared conflicts of interest; Y.Z. has no declared conflicts of interest; P.J.T. has no declared conflicts of interest; S.M.T. has no declared conflicts of interest; T.D.S. has no declared conflicts of interest; A.O. has no declared conflicts of interest; J.Z. has no declared conflicts of interest; M.J.W. has no declared conflicts of interest; and J.F.E. has no declared conflicts of interest.
References
- 1.Verkman AS, Song Y, Thiagarajah JR. Role of airway surface liquid and submucosal glands in cystic fibrosis lung disease. Am J Physiol Cell Physiol 2003;284:C2–C15. [DOI] [PubMed] [Google Scholar]
- 2.Welsh MM, Tsui L-C, Boat TF, Beaudet AL, Sly WS, Valle D. The metabolic and molecular bases of inherited disease, 7th ed. New York: McGraw-Hill; 1995.
- 3.Tizzano EF, O'Brodovich H, Chitayat D, Benichou JC, Buchwald M. Regional expression of CFTR in developing human respiratory tissues. Am J Respir Cell Mol Biol 1994;10:355–362. [DOI] [PubMed] [Google Scholar]
- 4.Jacquot J, Puchelle E, Hinnrasky J, Fuchey C, Bettinger C, Spilmont C, Bonnet N, Dieterle A, Dreyer D, Pavirani A, et al. Localization of the cystic fibrosis transmembrane conductance regulator in airway secretory glands. Eur Respir J 1993;6:169–176. (see comments). [PubMed] [Google Scholar]
- 5.Engelhardt JF, Yankaskas JR, Ernst SA, Yang Y, Marino CR, Boucher RC, Cohn JA, Wilson JM. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat Genet 1992;2:240–248. [DOI] [PubMed] [Google Scholar]
- 6.Ballard ST, Inglis SK. Liquid secretion properties of airway submucosal glands. J Physiol 2004;556:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joo NS, Irokawa T, Wu JV, Robbins RC, Whyte RI, Wine JJ. Absent secretion to vasoactive intestinal peptide in cystic fibrosis airway glands. J Biol Chem 2002;277:50710–50715. [DOI] [PubMed] [Google Scholar]
- 8.Jayaraman S, Joo NS, Reitz B, Wine JJ, Verkman AS. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na(+)] and pH but elevated viscosity. Proc Natl Acad Sci USA 2001;98:8119–8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyrick B, Sturgess JM, Reid L. A reconstruction of the duct system and secretory tubules of the human bronchial submucosal gland. Thorax 1969;24:729–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flemming A. On a remarkable bacteriolytic element in tissues and secretions. Proc R. Soc Lond B Biol Sci 1922;93:306–317. [Google Scholar]
- 11.Travis SM, Conway BA, Zabner J, Smith JJ, Anderson NN, Singh PK, Greenberg EP, Welsh MJ. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol 1999;20:872–879. [DOI] [PubMed] [Google Scholar]
- 12.Basbaum CB, Jany B, Finkbeiner WE. The serous cell. Annu Rev Physiol 1990;52:97–113. [DOI] [PubMed] [Google Scholar]
- 13.Tom-Moy M, Basbaum CB, Nadel JA. Localization and release of lysozyme from ferret trachea: effects of adrenergic and cholinergic drugs. Cell Tissue Res 1983;228:549–562. [DOI] [PubMed] [Google Scholar]
- 14.Cole AM, Liao HI, Stuchlik O, Tilan J, Pohl J, Ganz T. Cationic polypeptides are required for antibacterial activity of human airway fluid. J Immunol 2002;169:6985–6991. [DOI] [PubMed] [Google Scholar]
- 15.Dohrman A, Tsuda T, Escudier E, Cardone M, Jany B, Gum J, Kim Y, Basbaum C. Distribution of lysozyme and mucin (MUC2 and MUC3) mRNA in human bronchus. Exp Lung Res 1994;20:367–380. [DOI] [PubMed] [Google Scholar]
- 16.Dubin RF, Robinson SK, Widdicombe JH. Secretion of lactoferrin and lysozyme by cultures of human airway epithelium. Am J Physiol Lung Cell Mol Physiol 2004;286:L750–L755. [DOI] [PubMed] [Google Scholar]
- 17.Choi HK, Finkbeiner WE, Widdicombe JH. A comparative study of mammalian tracheal mucous glands. J Anat 2000;197:361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akinbi HT, Epaud R, Bhatt H, Weaver TE. Bacterial killing is enhanced by expression of lysozyme in the lungs of transgenic mice. J Immunol 2000;165:5760–5766. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Zhang Y, Amberson A, Engelhardt JF. New models of the tracheal airway define the glandular contribution to airway surface fluid and electrolyte composition. Am J Respir Cell Mol Biol 2001;24:195–202. [DOI] [PubMed] [Google Scholar]
- 20.Leigh MW, Gambling TM, Carson JL, Collier AM, Wood RE, Boat TF. Postnatal development of tracheal surface epithelium and submucosal glands in the ferret. Exp Lung Res 1986;10:153–169. [DOI] [PubMed] [Google Scholar]
- 21.Steinberg DA, Lehrer RI. Designer assays for antimicrobial peptides: disputing the “one-size-fits-all” theory. Methods Mol Biol 1997;78:169–186. [DOI] [PubMed] [Google Scholar]
- 22.Cole AM, Ganz T. Human antimicrobial peptides: analysis and application. Biotechniques 2000;29:822–826, 828, 830–831. [DOI] [PubMed] [Google Scholar]
- 23.Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature 2002;417:552–555. [DOI] [PubMed] [Google Scholar]