Abstract
Human sporadic Creutzfeldt–Jakob disease (sCJD), endemic sheep scrapie, and epidemic bovine spongiform encephalopathy (BSE) are caused by a related group of infectious agents. The new U.K. BSE agent spread to many species, including humans, and clarifying the origin, specificity, virulence, and diversity of these agents is critical, particularly because infected humans do not develop disease for many years. As with viruses, transmissible spongiform encephalopathy (TSE) agents can adapt to new species and become more virulent yet maintain fundamentally unique and stable identities. To make agent differences manifest, one must keep the host genotype constant. Many TSE agents have revealed their independent identities in normal mice. We transmitted primate kuru, a TSE once epidemic in New Guinea, to mice expressing normal and ≈8-fold higher levels of murine prion protein (PrP). High levels of murine PrP did not prevent infection but instead shortened incubation time, as would be expected for a viral receptor. Sporadic CJD and BSE agents and representative scrapie agents were clearly different from kuru in incubation time, brain neuropathology, and lymphoreticular involvement. Many TSE agents can infect monotypic cultured GT1 cells, and unlike sporadic CJD isolates, kuru rapidly and stably infected these cells. The geographic independence of the kuru agent provides additional reasons to explore causal environmental pathogens in these infectious neurodegenerative diseases.
Keywords: sporadic CJD, bovine spongiform encephalopathy, viral adaptation, prion protein receptor, tissue culture infection
Kuru, a progressive and fatal neurodegenerative disease in New Guinea, was the first human transmissible spongiform encephalopathy (TSE) shown to be caused by a virus-like agent (1). Kuru reached local epidemic levels in the 1960s and revealed remarkable similarities to scrapie, an endemic infection of sheep (2, 3). Infection with the kuru agent was linked to ritual cannibalism and probably spread into the bloodstream from superficial wounds smeared with infected brain and from direct consumption of infected tissues (1). Kuru died out with the cessation of these rituals and hence is not created spontaneously by the human host. As with endemic scrapie (4), the infectious disease clearly requires an environmental source or external pathogen for its reproduction.
Originally, human TSE agents such as kuru and sporadic Creutzfeldt–Jakob disease (sCJD) were thought to be distinguished from sheep agents by transmission to primates but not to rodents (1). Transmission of sCJD to several rodent species in the 1970s, including normal mice, undermined this difference and opened the way for direct comparison of various TSE agents in normal mice expressing WT murine prion protein (PrP) (5, 6). Serial propagation of different TSE isolates in a foreign species demonstrated that these agents, as classical viruses, could adapt and evolve, as shown by their increased virulence and shorter incubation times on serial passage (7). Despite this adaptation, each agent maintains its distinctive identity, and reinoculation of the initial host recreates the original disease (8–11). In normal mice with WT PrP, one can reliably distinguish many different TSE agents by (i) the incubation time to clinical disease, (ii) the distribution of pathological brain lesions, (iii) the relative involvement of the lymphoreticular system, and (iv) tissue PrP pathology; some agents provoke major PrP amyloid deposits, whereas others do not. Major differences in agent-determined incubation times (80 vs. 350 days) and extremely restricted versus widespread brain lesions are obvious in comparisons of western sCJD and geographically limited Japanese CJD isolates (12, 13). Similarly, many different sheep scrapie agents also appear to possess an independent genome with only exceptional sudden mutation (14, 15). The propagation of a variety of TSE agents in monotypic murine cultures further extends the reality of individual agent-encoded identities not determined by host PrP (16).
Standard WT mouse models are very useful for studying human TSEs because they faithfully recapitulate key features of human disease in a relatively short time frame of ≤1 year. In humans, CJD agents can exist as persistent latent infections that are clinically silent for >30 years, probably hiding in the lymphoreticular system, and brought out by later stresses, including aging itself (17, 18). Whereas normal mice with WT murine PrP have been used to reproducibly discriminate many distinct TSE agents, transgenic (Tg) mice expressing PrP sequences from other species such as cow and human have been used to evaluate only a few agents. Foreign PrPs in Tg mice are typically expressed at higher than normal levels, sometimes inducing their own pathological consequences (19). Thus, Tg PrP models are not ideal for extensive agent comparisons. Furthermore, agents such as bovine encephalopathy (BSE) spread unpredictably with respect to PrP sequences and can maintain an unanticipated species preference. This U.K. BSE agent clearly causes a variant form of CJD (vCJD) in younger people; it induces the same unique pathology in WT mice when transmitted from cow brain and from human brain (11). Whereas this unfortunate cross-species transmission was considered a reasonable possibility from the virus-like biology and experimental transmissions of TSEs, it was dismissed by proponents of the prion hypothesis (20).
According to the prion hypothesis, normal host PrP misfolds to become an infectious agent by rare spontaneous events, by inherited germ-line mutations, or by inoculation or ingestion of misfolded PrP in a tissue homogenate (21, 22). The conversion of PrP into an infectious form requires interaction with a homologous PrP. The “revolutionary” aspect of this paradigm is that the infectious agent contains no nucleic acid. Hence, host PrP must encode individual agent properties through its misfolding (assayed as digested PrP-res bands on Western blots). PrP-res is absent in uninfected samples. However, (i) samples without PrP-res, such as blood (23, 24), microglia (25), and digestive material (26) can transmit infection, (ii) very different agents show indistinguishable PrP-res band profiles, and (iii) PrP-res band patterns vary with tissue type while the agent remains invariant (10). Dramatically altering the PrP-res pattern by cell culture also does not alter an agent's distinctive identity because it reproduces the original disease in mice (9). Although host PrP is required for infection and probably acts as a receptor or scaffold for the infectious agent (27), it is unlikely to be the agent itself. The amount of PrP-res does not correlate with infectivity levels in many in vivo TSE models, and infectious particles can be isolated from most PrP and PrP-res without loss of titer (17, 28, 29). Most problematic for prion proponents is the lack of any significant or reproducible infectivity in purified, recombinant, or misfolded amyloid forms of PrP (10).
The key prion assumption that only a highly similar PrP sequence between the donor and the recipient will provide fertile ground for infection, or PrP conversion, has fueled many studies of Tg mice with PrP inserts that correspond to the donor. This concept has not been borne out experimentally [e.g., the vCJD agent that replicated in a human brain for >5 years transmitted readily to normal mice but poorly to Tg mice expressing only human PrPs (11)]. Kuru has not been transmitted to any standard small rodents, including mice. However, transmission of kuru to several Tg mouse lines expressing human PrPs but not to normal mice has been reported (22). From incubation times, the authors concluded they had “established that kuru prions have prion strain properties equivalent to those of classical (sporadic and iatrogenic) CJD prions” and further stated that kuru probably originated from chance consumption of an individual with sCJD.
Our kuru transmission and neuropathology data are not in accord with this conclusion and are based on agent-induced incubation times, PrP-res profiles, brain and spleen pathology, and species susceptibility and culture transmissions. We compare kuru to a variety of other TSE agents in normal CD-1 and in Tga20 mice that express 8-fold higher than normal levels of murine PrP. These studies show the kuru agent is a unique geographic isolate unrelated to the sCJD agent. It is also different from the BSE vCJD agent, the geographic Japanese CJD agent, and representative scrapie agents derived from sheep and goats. Furthermore, kuru infected monotypic GT1 murine cells stably support infection by a variety of different TSE agents (11). These in vitro studies again showed that the kuru agent is different from 3 independent sCJD isolates, including one from a patient with a very rare 102L PrP mutation proposed to infect via the germ line (30). The fundamental realization that kuru represents yet another unique and geographically restricted TSE agent provides additional reasons to explore nonhost environmental pathogens as causal agents in these infectious neurodegenerative diseases.
Results
Transmissible spongiform encephalopathy agents can differ dramatically in their virulence for species, by incubation time and by neuropathological sequelae. Because PrP sequence variants and other host factors can modulate susceptibility to infection and disease phenotype, we here compare kuru to other agents using mice and GT1 neuronal cultures expressing standard WT PrP. For simplicity, we designate all human agents according to the natural host species from which they were isolated (Table 1). This table summarizes some of the major features that distinguish various CJD and scrapie agents, including kuru (kCJD), in both CD-1 and Tga20 mice. First, there are obvious differences in incubation time that persist after serial passages. Notably, the same prolonged incubation time with limited brain lesions is generated by 3 different sCJD isolates with diverse species passage histories regardless of passage number (passages 2–8). In contrast, kCJD produced severe clinical signs and neuropathological lesions at short times (154 vs. >300 days). Mice with sCJD also develop a unique stereotypic scratching syndrome, whereas kCJD and other agents elicited very different behaviors (Movies S1–S4). Kuru and sCJD agents also provoke markedly different patterns of neurodegeneration in CD-1 mice, as summarized in Table 1. These incubation and neuropathological differences were not apparent from the PrP-res band sizes (both type 1). In addition, human vCJD and BSE from cow brain showed no clear relation to any other CJD or scrapie agents by either incubation time or neuropathology. Unlike any of the other agents, BSE and vCJD isolates both provoke a diagnostic 19-kDa PrP-res band. This diagnostic band was remarkable because it is present in many different infected species with nonhomologous PrPs (cow, human, other primates, and mouse). Thus, the vCJD agent induces a PrP response that is not host-encoded but probably results from agent-specific binding to the host PrP receptor as diagrammed in ref. 27.
Table 1.
Comparison of different TSE agents in mice with WT PrP
| Country–origin (no. of species before mouse) | Diagnosis | Agent name | Mouse incubations CD-1 (Tga20) ≥passage 2 | Mouse brain pathology | Mouse brain PrP-res | |
|---|---|---|---|---|---|---|
| 1 | U.S.–human (++) | sCJD | SY-CJD | 370 (307) days | Restricted | Type 1 |
| 2 | Italy–human (+) | sCJD | LU-CJD | 380 (324) days | Restricted | Type 1 |
| 3 | U.K.–human | GSS | MA-CJD | 380 (350) days | Restricted | Type 1 |
| 4 | Japan–human (+) | GSS | FU-CJD | 120 (70) days | Widespread + amyloid | Type 1 |
| 5 | Japan–human | GSS | YAM-CJD | 130 days | Widespread + amyloid | Type 1 |
| 6 | U.K.–human | vCJD | vCJD | 185 (126) days | BSE pattern + amyloid | Type 2 |
| 7 | U.K.–cow | BSE | BSE | 160 days | BSE pattern + amyloid | Type 2 |
| 8 | kuru–human (+) | kuru | kCJD | 440 (154) days | kCJD specific | Type 1 |
| 9 | U.K.–sheep | Scrapie | 22L-sc | 140 (87) days | Scrapie variant | Type 1 |
| 10 | U.K.–sheep | Scrapie | Ch (RML)-sc | 120 days | Scrapie variant | Type 1 |
| 11 | U.K.–sheep (++++) | Scrapie | 263K-Sc | 330 (310) days | 263K specific | Type 1 |
Key features of transmissible spongiform encephalopathy (TSE) agents propagated in mice with country of origin, original species, and disease diagnosis. The agent name indicates the host from which it was isolated, e.g., CJD for human. Sporadic Creutzfeldt–Jakob disease (sCJD) causes a rapidly progressive dementia globally in older people. In Gerstmann–Sträussler–Sheinker disease (GSS), patients with a 102L prion protein (PrP) mutation have symptoms for ≈5 years with cerebellar PrP amyloid plaques. A plus sign (+) indicates prior nonmurine species passages. SY-CJD was first passaged in guinea pigs and hamsters (++) (6). LU-CJD was passaged in hamsters (+), whereas MA-CJD brain homogenate was inoculated directly in mice. The Japanese GSS-102L PrP samples yield short mouse incubations and widespread brain lesions with the same PrP-res band pattern as sCJD (11,12), and FU-CJD was first passaged in rats. The human variant CJD (vCJD) and cow bovine spongiform encephalopathy (BSE) isolates produce type 2 PrP-res (Fig. 2) and a unique BSE lesion profile (11). Mutant 263K-sc was cloned at limiting dilution in 5 different species and then again selected for low mouse pathogenicity [>600 versus 65 days in hamsters (15)]. When retransmitted to mice, its biological properties were changed profoundly, but PrP-res remained type 1 (11). All mice were inoculated with 1% brain homogenates as detailed in refs. 5 and 6 and SI Methods.
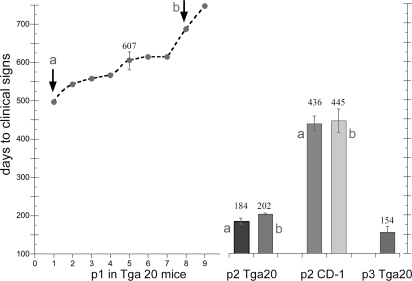
Sequential Incubation Time Changes with kCJD.
Fig. 1 details serial passages of kCJD from a macaque brain homogenate (31) to Tga20 mice overexpressing WT murine PrP. All inoculated mice developed clinical symptoms and brain disease. As expected, the primary passage across species is very prolonged but completely effective. These data contrast with the lack of transmission to normal FVB mice with clinical signs scored for a shorter time (22). Two representative kCJD mouse brains at passage 1, including one with the shortest and another with a prolonged incubation (Fig. 1, a and b, respectively), were used for passage 2 infection of Tga20 and CD-1 mice. Both samples yielded significantly reduced incubation times at passage 2 (t < 0.0001). The Tga20 incubation times were cut by two-thirds.
Fig. 1.
Transmission of kuru Creutzfeldt–Jakob disease (kCJD) to mice. Graph shows days of incubation in the 9 intracerebrally inoculated Tga20 mice at passage 1 (p1) [607 ± 26 (SEM)]. Brain homogenates from passage 1 mice a and b with an ≈200-day difference in incubation time then were inoculated into both Tga20 and CD-1 mice. Brains a and b yield the same incubation time. Reductions in passage 2 (p2) incubations indicate agent adaptation in both mouse genotypes, with Tga20 mice showing a greater reduction. By passage 3 (p3), Tga20 mice provide a rapid kCJD model.
The much longer incubation times in CD-1 versus Tga20 mice at passage 2 are likely due to the relatively lower expression of murine PrP in CD-1 mice, as would be expected for a viral receptor. Nevertheless, both groups of recipient CD-1 mice at passage 2 (Fig. 1) showed a significantly shorter incubation time than passage 1 Tga20 mice (440 vs. 607 days), indicating adaptation or selection of the foreign agent. At passage 2, there was a >100-day difference between kCJD and sCJD in Tga20 mice (195 vs. 335 days, Table 1). Because Tga20 mice are highly inbred, the kCJD agent rather than the invariant murine genes must encode this dramatic and progressive reduction. A third passage of kCJD in Tga20 mice decreased the incubation time to 154 days, far below that seen even after 8 murine passages of the sporadic SY-CJD isolate (>300 days) and, notably, 50 days shorter than that of kuru in humanized PrP mice (22).
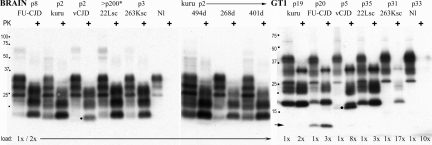
PrP-res Profiles in Brain.
The PrP and PrP-res profiles in kCJD remained the same in serial passages despite the agent's adaptation to its new murine host. There was no evidence that host PrP-res folding encoded this progressively enhanced agent virulence. Fig. 2 shows that Japanese FU-CJD, 22L-sc, and the cloned mutant 263K-sc agents all provoke the same basic type 1 PrP-res profiles as kCJD in brain. The only obvious difference was a greater proteinase K sensitivity of the most glycosylated PrP band (at 27 kDa) in kCJD. This profile was unchanged by propagation in various normal inbred, outbred, and Tga20 mice. The SY-CJD agent also induces the same basic PrP-res brain profile (32). Only the vCJD agent reveals a clearly different diagnostic PrP-res band of 19 kDa (at dot). Fig. 2 also shows representative samples encompassing the entire span of kCJD incubation times and clinical durations in CD-1 mice. Only minor differences in PrP-res amounts, as would be expected for animals killed at varying stages of clinical disease, are seen. In contrast, pathological PrP-res amounts in brain can differ 10-fold by agent type, as shown for SY-CJD and FU-CJD (32).
Fig. 2.
The prion protein (PrP) and PrP-res bands [proteinase K (PK)+ lanes] in CD-1 mouse brain homogenates and in GT1 cultures assayed on Western blots (SI Methods). Only the variant Creutzfeldt–Jakob disease (vCJD) agent shows a unique ≈19-kDa band (at dot). Kuru samples show one mouse with a long (55-day) clinical course (494-day lane) with more PrP-res than mice with typical 15–20 day signs; all kuru (kCJD) mice (268- and 401-day lanes), including Tga20 mice, show the same PrP-res profile. The 22L-sc CD-1 mouse (asterisk) was inoculated with 22L-sc-infected N2a cells displaying an N2a-specific but not agent-specific PrP band pattern (9–11), yet these cells reproduced their original 22L-sc incubation time and neuropathology (9–11) and the type I brain PrP pattern in mice (22L-sc lane). Protein loads and mouse passages are noted. The GT1 lanes show GT1-cell-specific PrP and PrP-res patterns (e.g., the heavily glycosylated top band in undigested brain homogenates is at 34 kDa, whereas it is at 39 kDa in GT1 homogenates). Kuru brain homogenates rapidly, reproducibly, and stably infected GT1 cells, as shown by the high levels of PrP-res (kCJD lane, >4 experiments), whereas sCJD gave only subliminal infection with the same homogenate exposure (SI Methods). The 13-kDa PrP-res marker in GT1 cells (arrow) is diagnostic for Japanese isolates in GT1 cells but not brain (FU-CJD lanes), but the 19-kDa doublet, found only in vCJD brain infections (at dot), is again seen in GT1 cultures. The band profile in kCJD is comparable with those of 22L-sc and 263K-sc in GT1 cells but required much higher amounts of protein to be loaded to each lane, as noted. Uninfected lanes (Nl) show no PrP-res.
Infection of Monotypic GT1 Cultures.
Because we found that GT1 tissue cultures support stable infection by a wide variety of CJD and scrapie agents (11, 16), evaluating the relative transmission capability of kCJD in GT1 cells was advantageous. Sporadic SY-CJD barely elicits detectable PrP-res and displays this pathological marker only transiently (9). Sporadic LU-CJD and MA-CJD also failed to elicit PrP-res. In marked contrast, kCJD readily infected GT1 cells when tested with 3 different kCJD brain homogenates, including CD-1 and Tga20 samples from different passages. Furthermore, cells infected with kCJD stably display large amounts of PrP-res from passages 2 to >50 in culture. Hence, the virulence of kCJD for these cells is much greater than that shown by all 3 sCJD isolates.
Passages of various TSE agents in GT1 cells also show the predominance of a cell-type- rather than an agent-specific PrP-res band pattern (Fig. 2). Notably, unlike complex brain tissue, the relative proteinase K sensitivity of the most glycosylated PrP-res band (now at 39 kDa) no longer discriminated kCJD form the other agents, such as 22L-sc and 263K-sc. Hence, this PrP sensitivity in kCJD is not agent-intrinsic. However, the kCJD agent is far more virulent for GT1 cells than the 263K-sc agent. A 17× load was necessary to clearly reveal the PrP-res bands in 263K-sc infections. The GT1 culture also brought out other agent-specific interactions with PrP that enhanced their discrimination. In GT1 cells, kCJD was different from Japanese isolates. Both FU-CJD (arrow) and YAM-CJD provoke a unique 13-kDa PrP-res band (22). This confirms their common geographic origin. Moreover, both Japanese “familial” 102L PrP patient isolates propagate a markedly different agent than the U.K. 102L PrP patient (MA-CJD). Thus, this 102L PrP mutation does not define these different geographic isolates. Rather, it influences host susceptibility and PrP amyloid responses to infection. Whereas PrP band patterns did not breed true in brain and GT1 cells infected with typical CJD and scrapie agents, the BSE-linked vCJD agent distinguished itself by inducing the same 19-kDa PrP-res band in both brain and GT1 cells (at dot).
Clinical Signs and Neuropathology.
The clinical signs of kCJD are markedly different from those of sCJD, vCJD, and 263K-sc. These signs were not dependent on the mouse genotype. Each agent provoked its own characteristic behavior in both Tga20 and CD-1 mice. Movies S1–S4 show the unique stereotypic scratching in sCJD-infected mice (Movie S1), the head shaking, unsteadiness, poor grooming, and extreme kyphosis (hunching) in kCJD-infected mice (Movie S2), hyperactivity with a diagnostic twisting response in vCJD-infected mice (Movie S3), and extreme obesity in 263K-sc-infected mice (Movie S4). These distinct behaviors were in accord with major neuropathological differences.
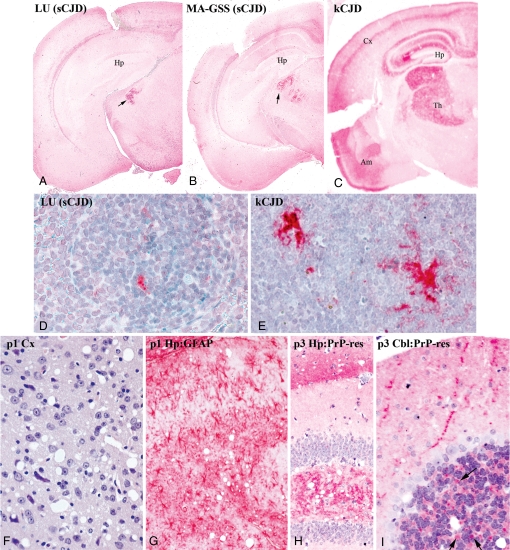
Fig. 3 shows representative neuropathological changes induced by the sCJD and kCJD agents. These changes further substantiate profound differences between these two CJD agents. Brain cross-sections from CD-1 mice at passage 2 are shown at low-power magnification (Fig. 3 A–C). Essential findings depicted also were apparent in Tga20 mice at both low- and high-power magnification and in different serial passages (Fig. 3 F–I). Sporadic SY-CJD induces brain changes in WT mice only in a very limited region of the medial thalamus (6, 13). Fig. 3A shows that the LU-CJD agent and the MA-CJD agent from the U.K. 102L PrP patient (Fig. 3B) both induce the same anatomically restricted sCJD lesions. Strong staining of the medial thalamus for abnormal PrP (dark red) highlights the focal change. Vast regions of cerebrum do not show PrP pathology, and cerebellar PrP amyloid plaques of 102L PrP patients were not reproduced, even though WT mice carry this same mutation. In contrast to sCJD agents, kCJD produced a far more extensive and unique neurodegenerative pattern (Fig. 3C). Not only is the thalamus broadly involved, but other regions of brain also are affected. The more extensive pathology in kCJD was not due to a longer incubation, as shown. The distribution of brain lesions also differed from that of vCJD, where severe hypothalamic and thalamic involvement, relative sparing of the cortex, and large PrP plaques are found (11). The widespread brain lesions in Japanese CJD, including marked involvement of the cerebral cortex and hippocampus, define yet another agent-specific distribution (13).
Fig. 3.
Sporadic Creutzfeldt–Jakob disease (sCJD) and kuru (kCJD) tissue differences. The first row (A–C) shows low-magnification images of brain from passage 2 CD-1 mice with sCJD versus those with kCJD. All mice became sick at similar times (377, 384, and 403 days) yet still show that specific neuronal nuclei are affected. Both sCJD isolates (LU and MA) induce pathological prion protein (PrP) in the medial thalamus (dark red at arrow) like SY-CJD (12). Kuru infection (C) provokes extensive PrP deposits in a much larger thalamic region (Th) and in hippocampus (Hp), a band of cortical gray (Cx), and temporal lobe/Ammon's horn (Am). Profound differences in spleen PrP are seen with sCJD (D) versus kCJD (E) in passage 2 CD-1 mice. The third row (F–I) shows kCJD brains in Tga20 mice with spongiform lesions in temporal cortex at passage 1 (F), intense gliosis with red-stained astrocytes (G), and abnormal PrP deposits in hippocampus at passage 3 (H). Cerebellum (I) shows pathological PrP deposits consistent with degenerating synaptic boutons (arrows) that become more prominent with further digestion (SI Methods) (i.e., they contain PrP amyloid).
The presence of large deposits of PrP-res in follicular dendritic cells (FDCs) of spleens infected with kCJD further distinguished this agent from all 3 sCJD isolates. Only rare small PrP-res refractile bodies are found in sCJD (Fig. 3D, LU-CJD). In contrast, kCJD induces extensive deposits over FDC processes (Fig. 3E). This extensive labeling of pathological PrP in kCJD is comparable with that seen with Japanese CJD, where confocal studies demonstrate abnormal PrP in FDC plasma membranes (33). Similarly, vCJD-infected mice also display extensive deposits of abnormal PrP in lymphoid FDCs (11). Abundant abnormal PrP deposits in spleen typically indicate a propensity for bloodstream infection and transfusion-related transmissions.
Higher-power magnification images of Tga20 brains further demonstrate the consistent changes in kCJD not found in any sCJD-infected mice. The regional distribution of lesions in kCJD remained constant from passages 1 to 3. Severe spongiform change in the temporal cortex (Fig. 3F) and marked gliosis in the hippocampus at passage 1 are shown. Fig. 3G depicts hypertrophic astrocytes in red. Spongiform change and PrP-res are again present in hippocampus at passage 3 (Fig. 3H); Fig. 3I shows synapse-sized pathological PrP-res deposits in the molecular layer and internal granule layer of cerebellum (arrows). None of these features were seen in sCJD.
Discussion
The kuru agent stands out from the other CJD and scrapie agents here on the basis of incubation times, behavior, and neuropathology in 2 mouse genotypes. There was no evidence that it resembles or derives from sCJD. The clearly unique behavioral changes documented with each different agent in mice are compatible with the vastly different regional neuropathological changes that they induce. Additionally, whereas >15 independent sCJD isolates readily transmitted to hamsters (8), the kCJD sample did not. Remarkably, even after several prolonged 350-day serial passages in mice, the sCJD agent retained its capacity to reinfect hamsters with a short 150-day incubation and provoked the original widespread hamster lesions (8). The stability of sCJD agents, regardless of whether first passaged in multiple or in no other species, is also evident in the invariant scratching behavior and the highly restricted thalamic lesions produced in mice. The GT1 culture experiments recapitulated major kCJD and sCJD differences. Whereas sCJD produced negligible PrP-res, kCJD brain homogenates reproducibly infected and stably induced large amounts of PrP-res in GT1 cells. As in brain, kCJD did not provoke any unique agent-specific PrP-res band pattern that bred true. Rather, it showed only the standard GT1 pattern. On the basis of previous results, the GT1 PrP-res pattern confers no demonstrable change in the fundamental identity and behavior of TSE infectious agents (9, 16, 34).
Spleen studies further separated kuru and sCJD agents. The kCJD agent induced major accumulations of abnormal PrP in FDCs of the lymphoreticular system, whereas sCJD isolates provoked very few. Although different species infected with a single agent can show different levels of infectivity and pathological PrP deposits in the spleen, abnormal PrP in the spleen has important biological consequences, given the person-to-person transmission of vCJD by transfusion (11). The observation of abundant deposits in kCJD-infected mouse spleen, in addition to the infectivity of kCJD in primate spleens (31), supports transmission of kuru not only through ingestion but also via the bloodstream from wounds incurred during ritual cannibalism (1). The bloodstream is an excellent conduit for rapid and effective infection of many tissues, including the gastrointestinal tract (12, 35).
We have shown 100% transmission of the infectious kuru agent to both normal and Tg mice with WT murine PrP. Recently, kuru was transmitted to Tg mice overexpressing human PrP sequences (22) but not to normal mice. High numbers of PrP copies can enhance the development of rapid murine models, and the kCJD model here, like the vCJD model previously (11), yielded shorter incubation times than humanized PrP mice. Thus, the TSE infectious particle rather than the host's PrP sequence defines cross-species virulence. The sCJD agent is also relatively incapable of adaptive evolution in mice. Even after 8 passages, it has a very prolonged incubation time of >300 days, in contrast to kCJD and vCJD. Current and previous data do not support a conversion of homologous PrP to an infectious form, but are in accord with the proposal that host PrP is a required agent receptor that can modulate disease expression. Host receptor differences often modulate viral infection and disease progression. The kCJD and other TSE agents here maintain their fundamental identity, despite their adaptation to a new species and to monotypic cells with disparate cell-type-specific PrP bands.
The PrP-res band patterns often are presumed to encode agent-specific information, yet they are a relatively poor indicator of the biological diversity and stability of TSE agents. In the present studies, only the vCJD agent elicited a consistent PrP-res marker in different species and cell types that bred true. Eleven other independent isolates did not (11, 32). In contrast, incubation times are clearly distinctive for each agent group and nonoverlapping when both CD-1 and Tga20 values are considered. The extreme precision of incubation time with each type of agent is really quite remarkable and remains unexplained by prion theory. It also begs the question of what type of viral (nucleic acid) sequence could lead to this diversity, and this further underscores both complex and subversive interactions between these TSE agents and their hosts. The differential susceptibility of neuronal subtypes with the same PrP to the various TSE agents presents yet another conundrum for prion theory.
There are many aspects of TSE agent biology and structure that remain unresolved, but these agents clearly encode individual virus-like properties. None of these diverse biological properties can be explained parsimoniously by the prion hypothesis or by actual PrP observations. The many distinct geographic TSE agents, the local outbreak of epidemic kuru and BSE, the rare but sudden mutation or progressive selection of new virulent strains (14, 15), and the endemic perpetuation of infection only in exposed hosts (as in sheep scrapie) strongly implicate a viral structure in the environment (10). On a structural level, the presence of nucleic acid in all infectious preparations and the consistent observation of virus-like ultrastructural particles support this hypothesis. Although host PrP as well as other factors may modulate susceptibility to infection and disease phenotype, there is also no simple genetic inheritance pattern, nor evidence for a spontaneous conversion of PrP into an infectious form.
The clear distinction of kuru from western sCJD, its occurrence only in New Guinea, and the observation that Japanese CJD is limited to Asia make one suspect that additional geographic TSE isolates in the environment may be uncovered. The U.S. stopped its scrapie eradication program years ago, before comparative rodent and culture models were introduced. Since then, scrapie agents with new properties may have evolved, possibly facilitating their spread to deer in the U.S. Geographic French scrapie agents are different (31), and Europe appears to be free of U.S. cervid TSE. An understanding of the origin, relative virulence, individual properties, and molecular structure of TSE agents remains a fundamental problem and a public health issue. The distinctive biology of a wide variety of TSE agents revealed in mice and in monotypic cultures already has defined intrinsic agent properties, and simplified cultures may allow one to follow and develop ways to limit the spread of different environmental TSE agents.
Methods
Standard methods for intracerebral inoculation, Western blot analysis and immunocytochemistry were as described in refs. 6, 7, and 9. See SI Methods for more details.
Supplementary Material
Acknowledgments.
We thank Brian Mullins for help with the mice. This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 012674 and National Institutes of Health National Institute of Allergy and Infectious Diseases Grant R21 A1076645. K.M. was supported by a Japanese JSPS fellowship.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905825106/DCSupplemental.
References
- 1.Gajdusek DC. Unconventional viruses and the origin and disappearance of kuru. Science. 1977;197:943–960. doi: 10.1126/science.142303. [DOI] [PubMed] [Google Scholar]
- 2.Hadlow WJ. Scrapie and kuru. Lancet. 1959;274:289–290. [Google Scholar]
- 3.Sigurdsson B. Rida: A chronic encephalitis of sheep. With general remarks on infections which develop slowly and some of their characteristics. Br Vet J. 1954;110:341–354. [Google Scholar]
- 4.Hunter N, Cairns D. Scrapie-free Merino and Poll Dorset sheep from Australia and New Zealand have normal frequencies of scrapie-susceptible PrP genotypes. J Gen Virol. 1998;79:2079–2082. doi: 10.1099/0022-1317-79-8-2079. [DOI] [PubMed] [Google Scholar]
- 5.Manuelidis E, Kim J, Angelo J, Manuelidis L. Serial propagation of Creutzfeldt–Jakob disease in guinea pigs. Proc Natl Acad Sci USA. 1976;73:223–227. doi: 10.1073/pnas.73.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manuelidis EE, Gorgacz EJ, Manuelidis L. Transmission of Creutzfeldt–Jakob disease to mice with scrapie-like syndromes. Nature. 1978;271:778–779. doi: 10.1038/271778a0. [DOI] [PubMed] [Google Scholar]
- 7.Manuelidis L, Fritch W, Xi YG. Evolution of a strain of CJD that induces BSE-like plaques. Science. 1997;277:94–98. doi: 10.1126/science.277.5322.94. [DOI] [PubMed] [Google Scholar]
- 8.Manuelidis L, Murdoch G, Manuelidis E. Potential involvement of retroviral elements in human dementias. Ciba Found Symp. 1988;135:117–134. doi: 10.1002/9780470513613.ch8. [DOI] [PubMed] [Google Scholar]
- 9.Arjona A, Simarro L, Islinger F, Nishida N, Manuelidis L. Two Creutzfeldt–Jakob disease agents reproduce prion protein-independent identities in cell cultures. Proc Natl Acad Sci USA. 2004;101:8768–8773. doi: 10.1073/pnas.0400158101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manuelidis L. A 25 nm virion is the likely cause of transmissible spongiform encephalopathies. J Cell Biochem. 2007;100:897–915. doi: 10.1002/jcb.21090. [DOI] [PubMed] [Google Scholar]
- 11.Manuelidis L, Liu Y, Mullins B. Strain-specific viral properties of variant Creutzfeldt–Jakob disease (vCJD) are encoded by the agent and not by host prion protein. J Cell Biochem. 2009;106:220–231. doi: 10.1002/jcb.21988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manuelidis L, Lu ZY. Virus-like interference in the latency and prevention of Creutzfeldt–Jakob disease. Proc Natl Acad Sci USA. 2003;100:5360–5365. doi: 10.1073/pnas.0931192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manuelidis L, Lu ZY. Attenuated Creutzfeldt–Jakob disease agents can hide more virulent infections. Neurosci Lett. 2000;293:163–166. doi: 10.1016/s0304-3940(00)01514-7. [DOI] [PubMed] [Google Scholar]
- 14.Bruce ME, Dickinson AG. Biological evidence that scrapie has an independent genome. J Gen Virol. 1987;68:79–89. doi: 10.1099/0022-1317-68-1-79. [DOI] [PubMed] [Google Scholar]
- 15.Kimberlin RH, Walker CA, Fraser H. The genomic identity of different strains of mouse scrapie is expressed in hamsters and preserved on reisolation in mice. J Gen Virol. 1989;70:2017–2025. doi: 10.1099/0022-1317-70-8-2017. [DOI] [PubMed] [Google Scholar]
- 16.Nishida N, Katamine S, Manuelidis L. Reciprocal interference between specific CJD and scrapie agents in neural cell cultures. Science. 2005;310:493–496. doi: 10.1126/science.1118155. [DOI] [PubMed] [Google Scholar]
- 17.Manuelidis L. Transmissible encephalopathies: Speculations and realities. Viral Immunol. 2003;16:123–139. doi: 10.1089/088282403322017875. [DOI] [PubMed] [Google Scholar]
- 18.Manuelidis L. Dementias, neurodegeneration, and viral mechanisms of disease from the perspective of human transmissible encephalopathies. Ann NY Acad Sci. 1994;724:259–281. doi: 10.1111/j.1749-6632.1994.tb38916.x. [DOI] [PubMed] [Google Scholar]
- 19.Barron R, Manson J. A gene-targeted mouse model of P102L Gerstmann–Sträussler–Scheinker syndrome. Clin Lab Med. 2003;1:161–173. doi: 10.1016/s0272-2712(02)00067-7. [DOI] [PubMed] [Google Scholar]
- 20.Manuelidis L. Penny wise, pound foolish—A retrospective. Science. 2000;290:2257. doi: 10.1126/science.290.5500.2257b. [DOI] [PubMed] [Google Scholar]
- 21.Prusiner S. Prions. Proc Natl Acad Sci USA. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wadsworth J, et al. Kuru prions and sporadic Creutzfeldt–Jakob disease prions have equivalent transmission properties in transgenic and wild-type mice. Proc Natl Acad Sci USA. 2008;105:3885–3890. doi: 10.1073/pnas.0800190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manuelidis EE, Gorgacz EJ, Manuelidis L. Viremia in experimental Creutzfeldt-Jakob disease. Science. 1978;200:1069–1071. doi: 10.1126/science.349691. [DOI] [PubMed] [Google Scholar]
- 24.Pincock S. Government confirms second case of vCJD transmitted by blood transfusion. Brit Med J. 2004;329:351. doi: 10.1136/bmj.329.7460.251-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker CA, Martin D, Manuelidis L. Microglia from CJD brain are infectious and show specific mRNA activation profiles. J Virol. 2002;76:10905–10913. doi: 10.1128/JVI.76.21.10905-10913.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeffrey M, et al. Transportation of prion protein across the intestinal mucosa of scrapie-susceptible and scrapie-resistant sheep. J Pathol. 2006;209:4–14. doi: 10.1002/path.1962. [DOI] [PubMed] [Google Scholar]
- 27.Manuelidis L. Beneath the emperor's clothes: The body of data in scrapie and CJD. Ann Inst Pasteur. 1997;8:311–326. [Google Scholar]
- 28.Manuelidis L, Sklaviadis T, Akowitz A, Fritch W. Viral particles are required for infection in neurodegenerative Creutzfeldt–Jakob disease. Proc Natl Acad Sci USA. 1995;92:5124–5128. doi: 10.1073/pnas.92.11.5124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun R, Liu Y, Zhang H, Manuelidis L. Quantitative recovery of scrapie agent with minimal protein from highly infectious cultures. Viral Immunol. 2008;21:293–302. doi: 10.1089/vim.2008.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prusiner SB. Prion disease and the BSE crisis. Science. 1997;278:245–251. doi: 10.1126/science.278.5336.245. [DOI] [PubMed] [Google Scholar]
- 31.Lasmézas C, et al. Adaptation of the bovine spongiform encephalopathy agent to primates and comparison with Creutzfeldt–Jakob disease: Implications for human health. Proc Natl Acad Sci USA. 2001;98:4142–4147. doi: 10.1073/pnas.041490898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manuelidis L. Vaccination with an attenuated CJD strain prevents expression of a virulent agent. Proc Natl Acad Sci USA. 1998;95:2520–2525. doi: 10.1073/pnas.95.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manuelidis L, et al. Follicular dendritic cells and the dissemination of Creutzfeldt-Jakob disease. J Virol. 2000;74:8614–8622. doi: 10.1128/jvi.74.18.8614-8622.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Sun R, Chakrabarty T, Manuelidis L. A rapid accurate culture assay for infectivity in transmissible encephalopathies. J Neurovirol. 2008;14:352–361. doi: 10.1080/13550280802105283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radebold K, Chernyak M, Martin D, Manuelidis L. Blood borne transit of CJD from brain to gut at early stages of infection. BMC Infect Dis. 2001;1:20–25. doi: 10.1186/1471-2334-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.