Abstract
Like many tumors, malignant mesothelioma exhibits significant chemoresistance and resistance to apoptosis in vivo that is not seen in current in vitro models. To study the mechanisms of this multicellular resistance, biologically relevant in vitro models are necessary. Therefore, we characterized and tested human mesothelioma tissue grown in vitro as tumor fragment spheroids. After 5–10 d in culture, fragments from each of 15 human mesothelioma tumors rounded into spheroids. The tumor fragment spheroids maintained multiple characteristics of the original tumors for up to 3 mo including the presence of viable mesothelioma cells, macrophages, and a collagen- rich stroma. In 14-d-old spheroids, mesothelioma cells showed the same proliferation rate and expression of a death receptor, DR5, as in the original tumor. To determine responses to treatment, we treated tumor fragment spheroids grown from three separate tumors with agents, TNF-related apoptosis-inducing ligand (TRAIL) plus cycloheximide, that induced near total apoptosis in three human mesothelioma cell lines (M28, REN, MS-1) grown as monolayers (94 ± 6% apoptosis; mean ± SEM). Compared with mesothelioma cells in monolayers, mesothelioma cells in the spheroids were resistant to TRAIL plus cycloheximide (32 ± 4% apoptosis; mean ± SEM). Apoptotic resistance of mesothelioma cells was significantly reduced by inhibiting either the PI3K/Akt pathway with LY294002 (47 ± 6% apoptosis) or the mTOR pathway with rapamycin (50 ± 17% apoptosis). We conclude that human mesothelioma can be maintained in vitro in a biologically relevant model that exhibits apoptotic resistance, thereby permitting study of its tumor biology and of novel approaches to therapy.
Keywords: collagen, death receptor DR5, mTOR, multicellular resistance, PI3K/Akt survival pathway, TNF-related apoptosis-inducing ligand (TRAIL), tumor-associated macrophage, tumor fragment spheroid
Resistance to apoptosis, or programmed cell death, is now considered to be a critical step in the generation and maintenance of cancer (1). Resistance to apoptosis may underlie the resistance of tumors to chemotherapy and radiotherapy (2). Mechanisms of resistance have been identified on a cellular level, for example via P-glycoprotein efflux pumps, DNA repair mechanisms, or from expression of anti-apoptotic proteins such as Bcl-2 (3). Additional mechanisms of resistance are now recognized to involve stimuli from the cell's external environment, termed multicellular resistance (3). These multicellular resistance mechanisms have been attributed to cell–cell contacts, cell–matrix contacts, and the three-dimensional shape found in tissues but not in monolayer cultures. Other mechanisms of multicellular resistance may derive from resident nonmalignant cells, such as tumor-associated macrophages (4). To study multicellular resistance, systems other than monolayer cultures of highly selected homogeneous immortal cell lines are needed.
Two types of in vitro models used to study the complex resistance found in tumors are multicellular spheroids and tumor fragment spheroids (5, 6). In the first, cells are allowed to grow into three-dimensional structures called multicellular spheroids (5). In these structures, certain features contributing to multicellular resistance can be studied including cell–cell contact and a three-dimensional architecture (7). These multicellular spheroids, though still artificial, can recreate a resistance not found in monolayers and have been used particularly in studies of resistance to radiotherapy (8). However, these structures still lack much of the complexity of the original tumor because they are generated from cell lines, themselves highly selected clonal subpopulations derived from the original tumor cells, and they contain none of the original stroma or other cell types found in tumors. In the second major in vitro model, small fragments of the original tumor tissue are allowed to grow into three-dimensional structures called tumor fragment spheroids. In the tumor fragment spheroid model, there is the potential for preserving the original heterogeneity of the tumor, including the actual, not selected, tumor cells, resident nonmalignant cells, and the tumor extracellular matrix. In recent years, the interactions between tumor cells, nonmalignant cells, and extracellular matrix have been recognized as important in cell growth, migration, and differentiation, as well as in survival and resistance to apoptosis (9, 10).
Tumor fragment spheroids have been generated successfully mainly from glioblastoma (11, 12), meningioma (13, 14), head and neck squamous tumors (15), and lung cancer (16). In several studies, tumor fragment spheroids were shown to maintain tumor markers and viability better than either monolayers or multicellular spheroids (12–14, 17). In a few cases, the effect of different treatments has been studied—for example, on the proliferation or histology of the tumor (13, 18–20). We are not aware of any study in which tumor fragment spheroids were specifically examined for apoptosis, although this in vitro model has much promise for such studies. Studies of in vitro tumor responses to treatments may be particularly relevant in infrequent and recalcitrant tumors in which novel treatments can be tested.
One such tumor, malignant mesothelioma, is an aggressive cancer that has not responded to standard therapies (21) and is considered highly resistant to apoptosis (22). Despite its increasing frequency, mesothelioma remains an unusual tumor and thus difficult to study in large clinical trials. Cell lines and animal models have greatly advanced the understanding of this tumor; however, an in vitro human tumor model could be particularly useful for screening and testing treatment strategies. An in vitro mesothelioma model derived from a patient's tumor could possibly be used to test targeted therapy designed for the individual patient (23).
In studies using mesothelioma cell lines grown as monolayers, we have successfully overcome apoptotic resistance by activating both the death receptor and DNA damage apoptotic pathways (24, 25). Whereas activating either pathway alone failed to induce apoptosis, activating the two pathways together produced a synergistic apoptosis (24). The damaging agent sensitized the mesothelioma cell lines to the death receptor ligand, TNF- related apoptosis-inducing ligand (TRAIL), and induced synergistic apoptosis via interactions at the level of the mitochondria (25, 26). Because TRAIL, unlike other death receptor ligands such as fas ligand and TNF, has been largely safe and effective in in vivo studies (27), TRAIL is considered a potentially valuable agent for therapy (28). We thus wished to test this combinatorial treatment approach using TRAIL in a more biologically relevant model of mesothelioma.
In this study, we asked whether tumor fragment spheroids could be generated from human mesothelioma tissue and whether these spheroids could be used to study responses of mesothelioma to treatment. Using tumors from 15 patients, we developed approaches to allow spheroid formation in vitro and examined the spheroids serially over a period of up to three months. The tumor fragment spheroids formed successfully from each tumor and maintained multiple characteristics of the original tumor, including mesothelioma cell viability, proliferation, and expression of the TRAIL receptor, DR5, as well as the presence of tumor-associated macrophages and a collagen-rich stroma. When compared with mesothelioma cell lines grown in monolayer culture, the mesothelioma cells in tumor fragment spheroids were resistant to apoptosis induced by the potent combinatorial stimulus, TRAIL plus cycloheximide. Efforts to block various anti-apoptotic pathways showed a contribution of the PI3K and mTOR pathways to the resistance. We conclude that human mesothelioma can be maintained as a complex, viable, biologically relevant system that will expand opportunities to study tumor biology and responses to therapy.
MATERIALS AND METHODS
Antibodies and Reagents
Antibodies were used at the indicated dilutions to detect cytokeratin (1:200, AE1/AE3; DakoCytomation, Carpinteria, CA), calretinin (1:200, M7245; DakoCytomation), Ki67 (1:200, MIB1; DakoCytomation), TRAIL death receptor-2 or DR5 (1:200; Calbiochem, San Diego, CA), CD68, a macrophage marker, (1:200, PG-M1 clone; DakoCytomation), cleaved caspase 3 (1:100, AB3623; Chemicon, Temecula, CA), phospho-Akt (1:50, Ser473, 736E11; Cell Signaling, Beverly, MA), and phospho-S6 kinase (1:500, Thr389 [1A5]; Cell Signaling). TRAIL (375-TEC) was purchased from R&D Systems (Rosen, MN). Inhibitors, LY294002 and rapamycin, were purchased from Sigma (St. Louis, MO). MG132, a proteasome inhibitor, was purchased from EMD Biosciences (San Diego, CA).
Single immunohistochemical staining was performed with the EnVision plus kit with peroxidase detection (DakoCytomation), and double immunohistochemical staining with the EnVision Doublestain System with peroxidase/alkaline phosphatase detection (DakoCytomation). Control staining was performed with omission of the primary antibody to confirm specificity for each antibody.
Formation of Tumor Fragment Spheroids
Fifteen freshly resected tumor samples were obtained under approval from the Committee on Human Research at the University of California, San Francisco. Tumor pathology was later determined to be epithelial (n = 9), sarcomatous (n = 4), or biphasic (n = 2) by examination of stained sections by one of our authors (S.L.N.). The tissue was aseptically transferred to a container containing Dulbecco's modification of Eagle's medium (DMEM; Difco Laboratories, Sparks, MD) supplemented with 10% heat inactivated FCS, penicillin (100 IU/ml), and streptomycin (100 μg/ml). A part of the tumor was fixed in 10% formalin (Fisher Scientific, Fair Lawn, NJ) and embedded in paraffin for histologic examination.
For spheroid culture, tumor tissue was diced finely with scalpels to pieces smaller than 1 mm in diameter that were suspended in medium in 10-cm plates coated with 0.8% agar (Agar Noble; Sigma). Some tumor fragments were maintained in DMEM and some in a mesothelial cell–specific media, LHC-MM (Lechner and LaVeck medium; Biosource International, Camarillo, CA). The volume of overlay media was 15 ml, and half the volume of the overlay media was changed twice a week. Individual cells or clumps of cells obtained at the time of tumor harvest were also plated onto uncoated 10-cm plates in an attempt to grow cells in monolayer culture. The cultures were maintained at 37°C in 5% CO2 with 100% relative humidity. The agar-coated plates were regularly observed using an inverted phase microscope during the incubation period, up to a maximum of 3 mo. Spheroids were collected at different time points, fixed in 10% formalin, and embedded in paraffin.
Tumor fragments grown in LHC-MM failed to form spheroids consistently; therefore, we have limited our report to the spheroids grown in DMEM.
Identification and Characterization of Mesothelioma Cells in Spheroids
To confirm the presence of mesothelioma cells, the tumor fragment spheroids were stained for the mesothelioma markers, cytokeratin or calretinin. In brief, after deparaffinization and rehydration, antigens were retrieved by boiling in sodium citrate solution (pH 6.0) with 0.1% Tween in a pressure cooker for 15 min. The slides were then cooled at room temperature for 20 min. After antigen retrieval, sections were blocked in hydrogen peroxide for 20 min to remove endogenous peroxidase. The primary antibody, anti-cytokeratin or anti-calretinin, was applied for 1 h and the secondary antibody conjugated to a horseradish peroxidase–labeled polymer was applied for 30 min and detected by the 3′, 3′-diaminobenzidine tetrahydrochloride (DAB) method.
To assess expression of Ki67 or DR5 specifically in mesothelioma cells, we used a double immunohistochemical staining approach (DakoCytomation; Envision Doublestain System). After initial immunohistochemical staining for Ki67 or DR5 with the peroxidase method as described above, blocking was performed by protocol and the next primary antibody, anti-cytokeratin, was applied, followed by a secondary antibody conjugated to an alkaline phosphatase–labeled polymer and substrate chromagen (fast red).
Determination of Collagen and Macrophages in Spheroids
Tumor fragment spheroids of various ages were stained with hematoxylin and eosin (H&E). Some were also stained using Gomori's one-step trichrome stain for collagen, by standard techniques.
Macrophages were detected using an antibody to CD68, a macrophage marker (29). To confirm that macrophages and mesothelioma cells were separate populations, double staining for CD68 and cytokeratin was also performed using the protocol described above.
Treatment of Spheroids with TRAIL plus Cycloheximide
At 14 d of culture, we selected rounded spheroids from three separate tumor samples, and plated 15–20 in each well of a 6-well agar-coated plate in new media for 24 h. Three human mesothelioma cell lines, M28 (obtained from Dr. Brenda Gerwin, NCI), REN (obtained from Dr. Roy Smythe, Texas A&M University), and MS-1 (obtained from Dr. Steven Idell, University of Texas at Tyler) grown as monolayers, were also studied in parallel. Mesothelioma cell lines were plated in 6-well plates the day before study to achieve either 60–70% confluence or, in an attempt to maximize apoptotic resistance, to achieve complete confluence. Tumor fragment spheroids and the mesothelioma cell monolayers were then treated either with nothing (control) or with agents that are known to induce synergistic and potent apoptosis, TRAIL (5 ng/ml) plus cycloheximide (10 μg/ml). Doses of TRAIL are in the high range of clinically used concentrations (27, 30), while cycloheximide, a protein synthesis inhibitor, is known to enhance TRAIL-induced apoptosis (24). Because each agent alone failed to induce significant apoptosis in mesothelioma cell monolayers in our prior studies, TRAIL and cycloheximide were not used individually. Initially, both the tumor fragment spheroids and the cells were treated for 24 h. However, because the spheroids had no obvious apoptotic response, tumor fragment spheroids and cell monolayers were treated for 48 h with two treatment doses, one given at 0 h and another at 24 h. Data are reported for both monolayers and tumor fragment spheroids treated identically for 48 h. In separate studies, additional spheroids were treated with inhibitors of putative resistance pathways including LY294002 (a PI3K inhibitor, 100 μM), rapamycin (an mTOR inhibitor, 5 nM), or MG132 (a proteasome inhibitor, 100 μM). These doses were selected from separate experiments in cell lines or spheroids for the ability to enhance the apoptosis of TRAIL or TRAIL plus cycloheximide without toxicity (data not shown). These agents were added to spheroids alone or together with TRAIL and cycloheximide at the start of the experiment and again at 24 h, for a total exposure of 48 h.
Tumor fragment spheroids were collected immediately after treatment, fixed in 10% formalin, and embedded in paraffin. After rehydration and antigen retrieval as above, specimens were doubly stained for immunofluorescent detection of both cleaved caspase 3 and cytokeratin. The slides were first incubated with a rabbit polyclonal antibody to cleaved caspase 3 (Chemicon) followed by the primary murine monoclonal antibodies to cytokeratin. After washing, the cytokeratin was detected with a secondary biotinylated sheep anti-mouse antibody (Amersham Biosciences Corp., Piscataway, NJ), followed by washing, and then a streptavidin-conjugated Oregon Green 488 (Molecular Probes, Eugene, OR). The cleaved caspase 3 was separately detected using a secondary goat anti-rabbit IgG conjugated with Alex Fluor 594 (Molecular Probes). Positive immunofluorescent staining for cytokeratin was green and cleaved caspase 3 was red. Cells treated as monolayers were stained with a nuclear stain, 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA), and examined by microscopy for the dense, shrunken nuclear morphology characteristic of apoptosis, as previously described (24).
To confirm the activity of the survival pathways and their inhibition by the kinase inhibitors, mesothelioma spheroids exposed to TRAIL plus cycloheximide with and without inhibitors were stained for expression of p-Akt or p-S6 kinase, downstream targets of PI3K and mTOR, respectively. Intensity of staining of individual cells was assessed by an observer blinded to the experimental condition on a scale of 0–3 for cytoplasmic staining. An average staining intensity for each spheroid was calculated for each of five different spheroids from two tumors.
Fluorescent images were captured using a fluorescent microscope (Zeiss, Gottingen, Germany) and image acquisition software (Spot Advanced, Chantilly, VA), with standardized acquisition times for the different colors. The separate images were then merged. The gain and contrast of each image was adjusted until the background was black.
Methods for Quantifying Proliferation, DR5 Expression, and Apoptosis
For quantification purposes, images of stained spheroids were captured as above, overlaid with a grid, and examined by independent observers blinded to the experimental conditions.
To determine the proliferation rate of mesothelioma cells, three observers analyzed spheroids from three different tumors. An average of 300 cytokeratin-positive cells from between 5 and 10 spheroids were counted from each tumor for each time point. Positivity for cytokeratin was identified as pink (fast red; DakoCytomation) cytoplasmic staining above background. Cytokeratin-positive cells with visible nuclei were counted as Ki67-positive (brown) or Ki67-negative (not brown). The mesothelioma cell proliferation rate was determined as the percentage of all cytokeratin-positive cells with Ki67-positive nuclei.
To determine the frequency of DR5 staining, two observers examined 14-d-old spheroids grown from four different tumors compared with the original tumor. An average of 300 cells from between 5 and 10 spheroids were counted for each tumor at each time point. Positivity for DR5 was determined by brown cytoplasmic or membrane staining above background. The total cell number was counted as the number of DAPI-positive nuclei. The frequency of DR5 expression was determined as the percentage of all DAPI-stained cells with DR5 expression.
To determine the percentage of mesothelioma cell apoptosis, two observers analyzed spheroids from three different tumors treated with apoptotic agents in separate experiments. An average of 200 and at least 100 cytokeratin-positive cells from between 10 and 20 spheroids were counted for each experimental condition in each tumor. Cytokeratin- positive cells were identified as having cell-specific green staining above background. All cytokeratin-positive cells were counted as either nonapoptotic (green only) or as apoptotic if the red staining for cleaved caspase 3 exceeded background levels. Mesothelioma cell apoptosis was calculated as the percentage of all cytokeratin-positive cells with staining for cleaved caspase 3.
Statistics
Statistical analysis was performed using ANOVA with post hoc analysis using Tukey's test for parametric data (e.g., apoptotic responses to treatment) or using the Mann-Whitney rank sum test for nonparametric data (e.g., intensity of staining) using GraphPad Prism version 4.0 for Windows (GraphPad Software, San Diego, CA). Data are represented as mean ± SEM or SD as appropriate for at least three separate experiments. A P value < 0.05 was considered significant. Images shown are representative of at least three experiments.
RESULTS
Mesothelioma Can Be Cultured as Tumor Fragment Spheroids
Spheroids could be grown from all mesothelioma cases. Most tumor fragments became rounded into spheroids by 5–10 d. From each tumor, hundreds of spheroids formed. Large numbers of individual cells and clumps of cells obtained from minced tumor tissue were also plated on plastic dishes. However, despite adherence and spreading, tumor cells grown as monolayers failed to proliferate. No tumor cells were successfully propagated in monolayer culture.
By hematoxylin and eosin staining, tumor fragments were found to change from an irregular shape to a rounded and smooth shape as the cells appeared at the periphery (Figures 1A–1G). By inverted phase microscopy, cells could be seen as a bright border at the periphery of the spheroids (Figure 1H). At least for 3 mo, spheroids maintained their shape and size. Fragments that failed to form spheroids were found to be acellular (data not shown).
Figure 1.
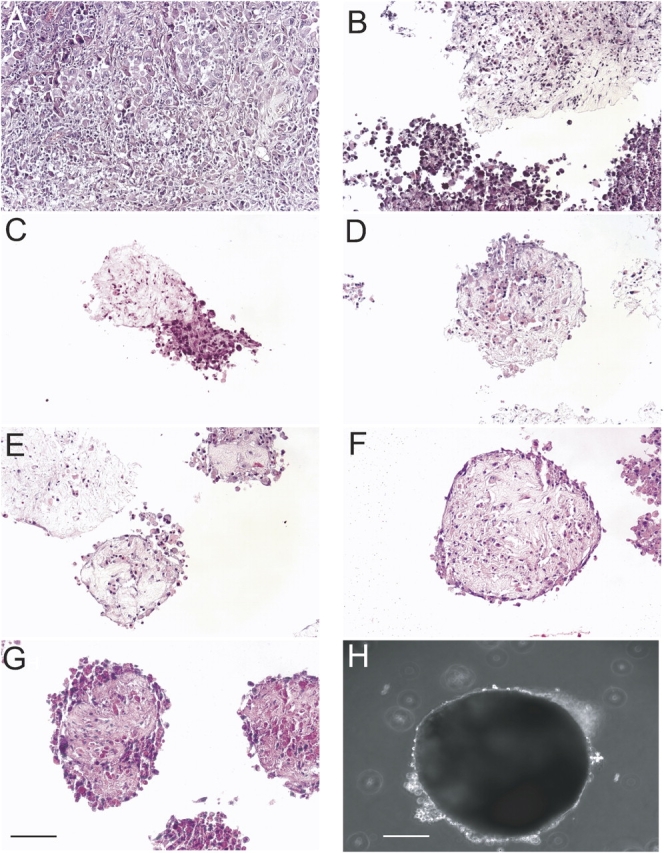
Tumor fragment spheroids form from human mesothelioma tissue. By hematoxylin and eosin staining, the parental tumor is shown (A), followed by tumor fragment spheroids at Days 1 (B), 3 (C), 5 (D), 7 (E), 10 (F), and 14 (G). Tumor fragment spheroids had consistently formed by Day 10 and showed little change in size during growth in culture. At 2 wk of age, a tumor fragment spheroid is shown with cells around the margin seen as a bright halo (H). Bar, 50 μm.
Mesothelioma Cells Are Identified within Tumor Fragment Spheroids
The majority of cells in spheroids were found to be cytokeratin- and calretinin-positive, strongly supporting their identity as mesothelioma cells (Figures 2A–2D). Staining for cytokeratin and calretinin was sustained up to at least 3 mo in culture (data not shown).
Figure 2.
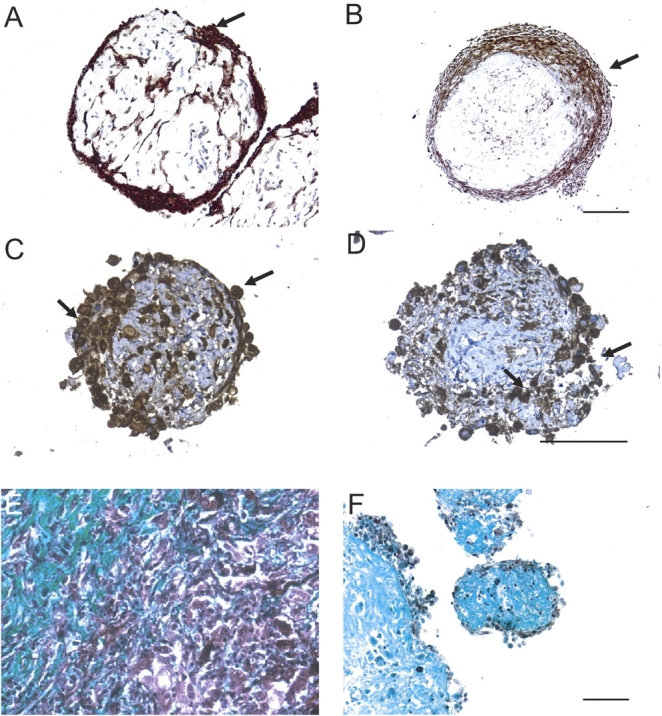
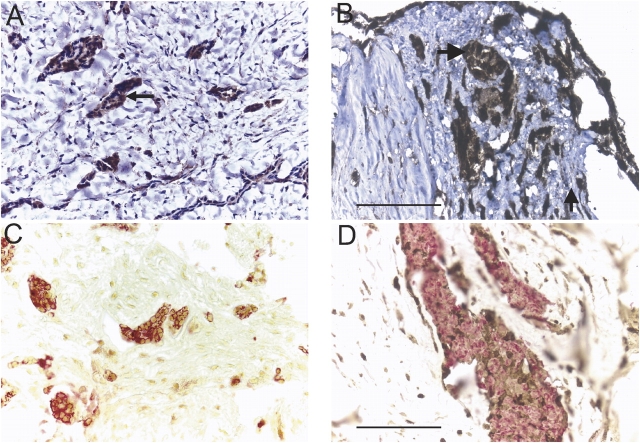
Mesothelioma cells and collagen are identified in tumor fragment spheroids. Mesothelioma cells were identified by immunohistochemical stains for cytokeratin or calretinin. Shown is expression of cytokeratin (arrows) in a 2-wk-old spheroid (A) and a 6-wk-old spheroid (B) from two different tumors. In 2-wk-old tumor fragment spheroids grown from the same tumor, there is expression of cytokeratin (C) and of calretinin (D) (arrows). By Gomori Trichrome staining, blue-green staining identifies the collagen-rich stroma present in both the original mesothelioma tumor (E) and in the spheroids grown from this tumor at Day 14 (F). Bar, 50 μm.
By Gomori trichrome staining, there was strong staining for collagen in the original tumor and in spheroids (Figures 2E and 2F). In spheroids, collagen was most apparent in the central, less cellular region (Figure 2F).
Mesothelioma Cell Proliferation Is Maintained in Tumor Fragment Spheroids
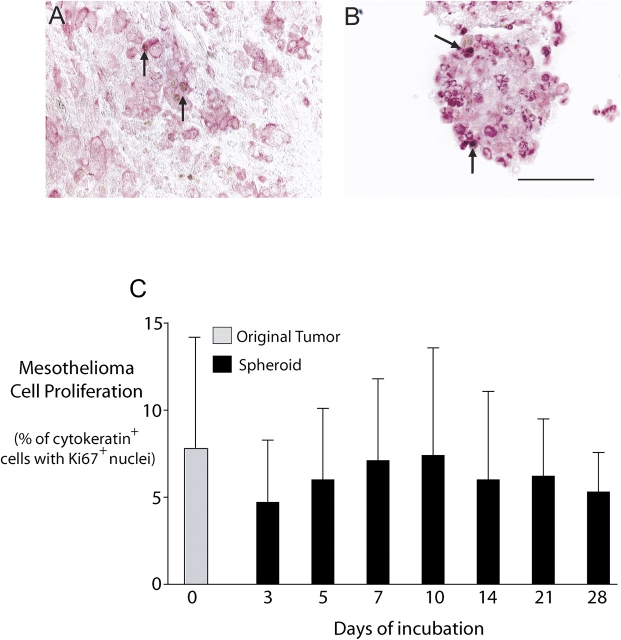
Cells in both the original tumor samples and in the tumor fragment spheroids were shown to be proliferating by virtue of positive staining for Ki67, a nuclear proliferation marker. The proliferation was determined specifically for the mesothelioma cells by double staining for Ki67 and cytokeratin (Figures 3A and 3B). Mesothelioma cell proliferation was demonstrated for at least 4 wk, in an experiment using mesothelioma tumor fragment spheroids from three different tumors (Figure 3C). The rate of proliferation of mesothelioma cells in the spheroids was not significantly different from that in the original tumor at any time (Figure 3C). Mesothelioma cell proliferation could still be demonstrated at 3 mo (data not shown).
Figure 3.
Mesothelioma cells proliferate within tumor fragment spheroids. By double immunochemical staining, cytokeratin-positive cells (pink) could be examined for Ki67 staining (brown) so that proliferation could be determined specifically for mesothelioma cells. Both the original tumor (A) and a 10-d-old tumor fragment spheroid grown from it (B) show Ki67-positive cells (arrows). Bar, 50 μm. (C) In spheroids grown from three tumors, proliferation of mesothelioma cells in the tumor fragment spheroids over 4 wk was compared with that of the original tumor. The proliferation rate was not significantly different from that of the original tumor during the 4 wk. (Mean ± SD; n = 3). Mesothelioma cells continued to proliferate for at least 3 mo (data not shown).
Macrophages Are Detected in Tumor Fragment Spheroids
Both the original tumor and spheroids grown from those tumors contained numerous CD68-positive cells (Figure 4). In double staining experiments, these CD68-positive cells and cytokeratin-positive cells were shown to be two separate populations, strongly supporting their identity as macrophages and mesothelioma cells, respectively. Both cell populations could be found in the original tumor (Figures 4A and 4C) and in the tumor fragment spheroids (Figures 4B and 4D).
Figure 4.
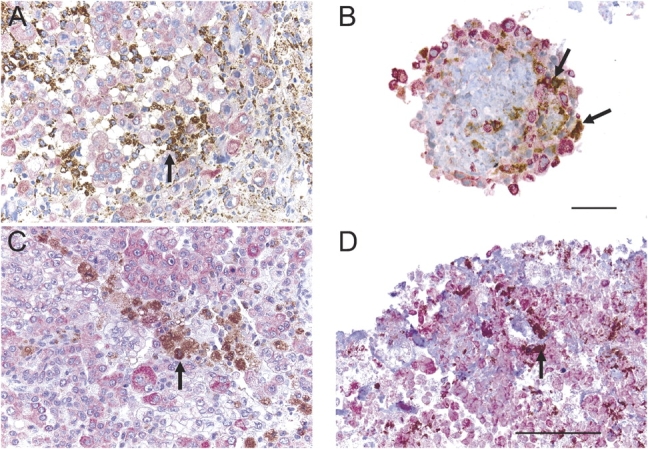
Macrophages are identified in tumor fragment spheroids. Macrophages, staining with anti-CD68 (brown), were found in the original tumors (A and C) and in tumor fragment spheroids (B and D) grown from those tumors, shown at 2 wk (B) or at 3 mo of age (D) (see arrows). The macrophages could be identified in close proximity to the cytokeratin-positive mesothelioma cells (pink). Bar, 50 μm.
Expression of DR5 Is Maintained over 14 d
A major death receptor for TRAIL, DR5, was present in both the original tumor and in the tumor fragment spheroids, in a similar distribution (Figures 5A and 5B). With double immunohistochemical staining for DR5 and cytokeratin, DR5 co-localized with mesothelioma cells (Figures 5C and 5D). Compared with the original tumors, DR5 expression was not altered in the spheroids. In four of the original tumors, DR5 was expressed by 41 ± 12% (mean ± SEM) of all cells and, in 14-d-old spheroids grown from these four different mesotheliomas, DR5 was expressed by 49 ± 13% of cells (P > 0.05, n = 4).
Figure 5.
Mesothelioma cells in tumor fragment spheroids express DR5. By staining for DR5 alone followed by a hematoxylin counterstain, DR5 was shown to be present in clusters of cells (arrow) in both the original tumor (A) and in tumor fragment spheroids at Day 14 (B). By double immunohistochemical staining for DR5 (brown) and cytokeratin (red) without hematoxylin counterstaining, DR5 staining could be localized to the cytokeratin-positive mesothelioma cells in both the original tumor (C) and the tumor fragment spheroid grown from it at Day 14 (D). Bar, 50 μm.
Mesothelioma Cell Monolayers Undergo Complete Apoptosis with TRAIL plus Cycloheximide
Three mesothelioma cell lines (M28, REN, MS-1), grown to 60–70% confluence or to 100% confluence, were exposed to nothing or to two doses of TRAIL plus cycloheximide over 48 h. All cell lines showed complete apoptosis (94 ± 6%) as measured by characteristic nuclear morphology, compared with the untreated cells (6 ± 4%, mean ± SEM; n = 3).
Mesothelioma Cells within Tumor Fragment Spheroids Demonstrate Apoptotic Resistance
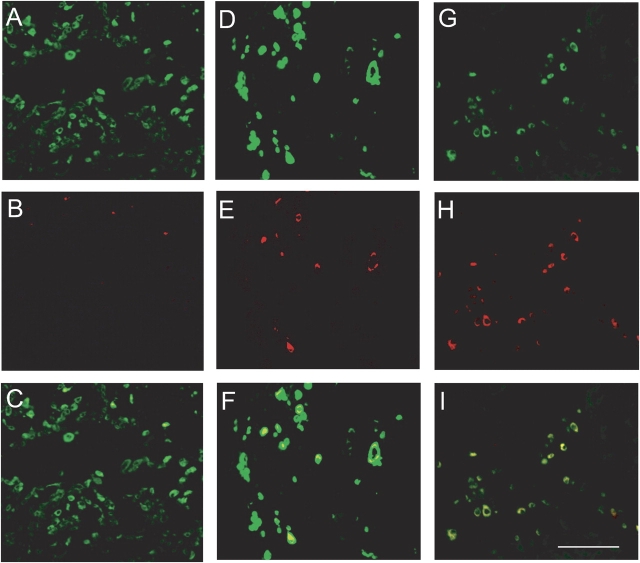
Tumor fragment spheroids were exposed to nothing or to two doses of TRAIL plus cycloheximide over 48 h. By the use of double staining for cytokeratin and cleaved caspase 3, apoptosis could be identified in some, but not all, of the mesothelioma cells in the treated spheroids (Figures 6A–6F). Apoptotic cells were evenly distributed throughout the spheroid (data not shown). Rapamycin appeared to increase the apoptotic response to TRAIL plus cycloheximide (Figures 6G–6I).
Figure 6.
Mesothelioma cell apoptosis can be identified within spheroids. Spheroids were either not treated (A–C), treated with TRAIL plus cycloheximide for 48 h (D–F), or treated with TRAIL plus cycloheximide with rapamycin for 48 h (G–I) and then stained for the presence of cytokeratin (green) or cleaved caspase 3 (red). In the top panels (A, D, G), the green represents cytokeratin, identifying the mesothelioma cells; in the middle panels (B, E, H), red represents cleaved caspase 3, identifying ongoing apoptosis; in the lower panels (C, F, I) the images have been merged, showing the colocalization of red with the green. Apoptotic cells can be identified within the treated spheroids (E and H) and can be demonstrated to be cytokeratin-positive in the merged images (F and I). Bar, 50 μm.
PI3K/Akt and mTOR Contribute to Apoptotic Resistance
Quantification of apoptosis showed that, in contrast to the nearly complete apoptosis of the mesothelioma cell monolayers, TRAIL plus cycloheximide induced apoptosis in 32 ± 4% (mean ± SEM) of the mesothelioma cells within the spheroids versus 7 ± 1% apoptosis in untreated spheroids. Addition of a proteasome inhibitor (MG132) had no effect on apoptosis (Figure 7A).
Figure 7.
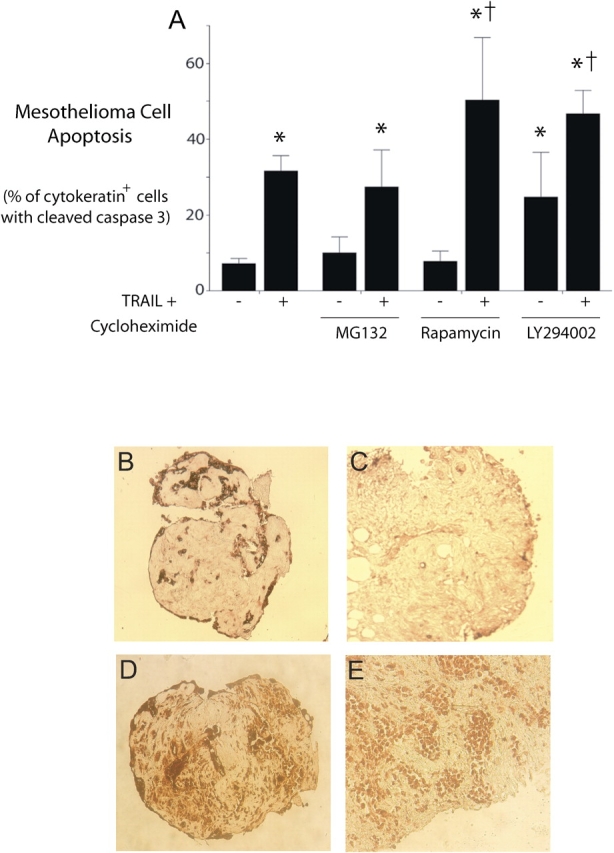
Mesothelioma cell apoptosis within spheroids is enhanced by blocking PI3K/Akt and mTOR pathways. (A) Apoptosis in treated spheroids quantified as the percentage of cytokeratin-positive cells with co-expression of cleaved, active caspase 3. Spheroids from three separate tumors were either not treated or treated with the strong apoptotic stimulus, TRAIL plus cycloheximide, for 48 h. To determine a possible contribution of known survival pathways, spheroids were also exposed to inhibitors of the proteasome (MG132), the mTOR pathway (rapamycin), or the PI3K/Akt pathway (LY294002). Apoptosis induced by TRAIL plus cycloheximide was enhanced by co-treatment with rapamycin or with LY294002, while LY294002 also had an effect on baseline apoptosis. (mean ± SEM; *different from untreated or inhibitor alone, †different from TRAIL plus cycloheximide without inhibitor; P < 0.05, n = 3). (B–E) Immunohistochemical confirmation of activity of PI3K/Akt and mTOR pathways and of effectiveness of inhibitors. In spheroids stained for p-Akt (B and C), spheroids treated with TRAIL plus cycloheximide alone (B) demonstrated staining for p-Akt that was reduced in spheroids co-treated with LY294002 (C). Similarly, in spheroids stained for p-S6 kinase (D and E), spheroids treated with TRAIL plus cycloheximide alone (D) demonstrated staining for p-S6 kinase, a target of mTOR, which was reduced in spheroids co-treated with rapamycin (E).
Rapamycin, an inhibitor of the mTOR pathway, had no effect on baseline apoptosis, but significantly increased apoptosis due to TRAIL plus cycloheximide to 50 ± 17% (mean ± SEM) (Figure 7A). LY294002, an inhibitor of the PI3K/Akt pathway, increased the apoptosis in spheroids by itself and also enhanced the response to TRAIL plus cycloheximide to 47 ± 6% (mean ± SEM) (Figure 7A).
For confirmation of the activity and the inhibition of these survival pathways, spheroids exposed for 48 h to TRAIL plus cycloheximide with or without inhibitors were stained for p-Akt or p-S6 kinase, targets of the PI3K and mTOR pathways, respectively. On a scale of 0–3 for intensity of cytoplasmic staining, the intensity of staining for p-Akt decreased significantly with use of LY294002 from 2.5 ± 0.1 to 0.4 ± 0.2 (mean ± SD, P < 0.05) and the intensity of cell staining for p-S6 kinase decreased with use of rapamycin from 2.9 ± 0.1 to 1.9 ± 0.1 (mean ± SD, P < 0.05) (Figures 7B–7E).
DISCUSSION
Mesothelioma, a tumor with a high degree of resistance to therapy, remains incurable despite aggressive therapy. Much of this resistance to available chemotherapy or radiotherapy is thought to be due to resistance to apoptosis (22). The mechanisms of apoptosis are difficult to study in immortal cell lines, where apoptotic mechanisms can be expected to be altered significantly compared with the original tissue. The complexity of the original tumor is also lost in the selection of tumor cells for growth as monolayers. Here, we have grown mesothelioma as fragments in which the three-dimensional form of the tumor and its complexity of cell types and extracellular matrix are maintained. We have further shown that this in vitro model demonstrates apoptotic resistance when compared with mesothelioma cell lines in monolayer culture and thereby represents a more realistic model of the tumor in vivo.
Tumor fragment spheroids retained many characteristics of the original tumor, despite weeks to months in in vitro culture. In particular, the mesothelioma cells showed retention of several key features. First of all, mesothelioma cells in the spheroids remained viable. Individual tumor cells or clumps of cells that were plated on plastic on the first day of harvest never propagated successfully, whereas tumor cells in spheroids remained viable for weeks. Second, mesothelioma cells continued to proliferate, at the same rate as in the original tumor, for at least 4 wk. Finally, the mesothelioma cells continued to express a major TRAIL receptor, DR5, thus showing that the resistance to exogenous TRAIL was not due to loss of TRAIL receptors. In addition, the tumor fragment spheroids appeared to retain key cell types and constituents. For example, it was interesting that both the tumor fragment spheroids and the original mesothelioma tumors contained a large number of macrophages. Macrophages have not been described, to our knowledge, as a feature of mesothelioma. In other tumors, tumor-associated macrophages have been recognized as important contributors to tumor biology, generally because of cytokine production that promotes angiogenesis and supports the growth and survival of tumor cells (4). The specificity of the CD68 marker for macrophages in our study is supported by the use of an antibody to CD68 (clone PG-M1) thought to be highly specific for macrophages (29); our use of tissue, not cell culture (29); and the lack of staining for cytokeratin in these cells. There still remained cell types not staining for either marker that may represent fibroblasts or endothelial cells, cell types that have been identified in spheroids from other tumors (31). Tumor fragment spheroids also contained a large amount of collagen as in the original tumor. A collagen-rich stroma has been shown to contribute to cell survival and resistance to apoptosis in other models (32). The mesothelioma tumor fragment spheroids retain a complexity representative of the original tumor, a complexity that constitutes both a challenge and an opportunity for study.
Mesothelioma may form similar structures in vivo, as has been described in cytologic studies (33). Fragments of tumor found in cytologic preparations of malignant pleural fluid are strongly associated with malignancy, and are much more frequently found in mesothelioma than in adenocarcinoma. These fragments often have an internal structure composed of collagen called a collagen core that, in one study, was found in 64% of mesothelioma cases versus 4% of adenocarcinoma cases (34). The source of these fragmentary tumor structures is unknown, but is speculated to be due to shedding of tumor cells or breakage of papillary structures. This natural tendency of mesothelioma to form tumor fragments may favor the formation of similar structures in our in vitro culture system and explain the high success rate of growing spheroids from this tumor.
To measure apoptosis in tissue, we addressed two major issues not found in cell culture. First, because tissue presents a complex mixture of cell types, identifying the cell of interest required use of a specific biomarker. For apoptosis studies, this biomarker should persist despite apoptosis. For our purposes, cytokeratin was used as an identifying biomarker of mesothelioma cells, as we have used successfully in an in vivo model of apoptosis in the pleural space (35). Second, assays for apoptosis in tissue are currently being reappraised. For example, TUNEL and the in situ ligation assay may be nonspecific due to detection of DNA strand breaks not solely due to apoptosis (36). Thus, we used the detection of the caspase 3 cleavage fragment as a measure of activated “executioner” caspases and presumably an irreversible and specific step in apoptosis (37).
Using this approach, we found that treatment induced apoptosis in the mesothelioma cells within spheroids but that the degree of apoptosis was significantly less than in cell lines grown as monolayers. This resistance to apoptosis did not appear to be due to reduced diffusion of the reagents into the spheroids because resistant and apoptotic cells were equally distributed in all regions of the spheroid and because the effect of the kinase inhibitors on their targets was also homogeneous. Instead, we found that resistance could be attributed in part to known survival pathways.
Various survival or anti-apoptotic mechanisms have been identified in tumors and shown to contribute to their survival and resistance to therapy. We found that inhibitors of the PI3K pathway and of the mTOR pathway were successful in enhancing apoptosis in the human mesothelioma spheroids. Inhibition of the proteasome did not enhance apoptosis in this system. The PI3K/Akt pathway is a major survival pathway in several tumors and has been shown to be active in mesothelioma cell lines (38–40). mTOR, a kinase downstream of Akt, is best known as a sensor of nutrients and a key initiator of cell cycle progression and protein translation (41). A role in survival signaling has been identified in overexpression studies in mice, where the mTOR pathway was found to account for the major survival effect of Akt (42). We found that inhibition of PI3K/Akt with LY294002 increased apoptosis both as a single agent and together with TRAIL and cycloheximide. Inhibition of mTOR did not increase apoptosis by itself but significantly increased apoptosis due to TRAIL and cycloheximide in the human tumor spheroids. This study is the first to our knowledge to show a role for mTOR in mesothelioma. Because several inhibitors of mTOR now exist and are entering clinical trials in oncology, mTOR represents a potentially interesting target.
Despite efforts to maximize apoptosis in this system, a significant degree of apoptotic resistance remained, which may indicate additional mechanisms or combination of mechanisms of resistance. Resistance may derive from interaction of mesothelioma cells with the three-dimensional structure, the extracellular matrix, or the products of nonmalignant cells within the tumor. This in vitro model opens new avenues for the study of tumor biology and resistance mechanisms active in human mesothelioma.
Acknowledgments
The authors thank Dr. Kirk Jones for his assistance.
The research was supported by an NIH RO1 NCI-095671 grant (V.C.B.) and a grant from the Norwegian Cancer Society (L.F.).
Originally Published in Press as DOI: 10.1165/rcmb.2004-0355OC on August 25, 2005
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Hanahan D, Weinberg R. The hallmarks of cancer. Cell 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 2.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell 2002;108:153–164. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Man S, Graham CH, Kapitain SJ, Teicher BA, Kerbel RS. Acquired multicellular-mediated resistance to alkylating agents in cancer. Proc Natl Acad Sci USA 1993;90:3294–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004;4:71–78. [DOI] [PubMed] [Google Scholar]
- 5.Mueller-Klieser W. Three dimensional cell cultures: from molecular mechanisms to clinical applications. Am J Physiol 1997;273:C1109–C1123. [DOI] [PubMed] [Google Scholar]
- 6.Kunz-Schughart LA. Multicellular tumor spheroids: intermediates between monolayer culture and in vivo tumor. Cell Biol Int 1999;23:157–161. [DOI] [PubMed] [Google Scholar]
- 7.Boudreau N, Werb Z, Bissell MJ. Suppression of apoptosis by basement membrane requires three-dimensional tissue organization and withdrawal from the cell cycle. Proc Natl Acad Sci USA 1996;93:3509–3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santini MT, Rainaldi G, Indovina PL. Multicellular tumour spheroids in radiation biology. Int J Radiat Biol 1999;75:787–799. [DOI] [PubMed] [Google Scholar]
- 9.Fracasso G, Colombatti M. Effect of therapeutic macromolecules in spheroids. Crit Rev Oncol Hematol 2000;36:159–178. [DOI] [PubMed] [Google Scholar]
- 10.Chrenek MA, Wong P, Weaver VM. Tumour-stromal interactions: integrins and cell adhesions as modulators of mammary cell survival and transformation. Breast Cancer Res 2001;3:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bjerkvig R, Tonnesen A, Laerum OD, Backlund EO. Multicellular tumor spheroids from human gliomas maintained in organ culture. J Neurosurg 1990;72:463–475. [DOI] [PubMed] [Google Scholar]
- 12.Paulus W, Huettner C, Tonn JC. Collagens, integrins and the mesenchymal drift in glioblastomas: a comparison of biopsy specimens, spheroid and early monolayer cultures. Int J Cancer 1994;58:841–846. [DOI] [PubMed] [Google Scholar]
- 13.Tonn JC, Ott MM, Bouterfa H, Kerkau S, Kapp M, Muller-Hermelink HK, Roosen K. Inverse correlation of cell proliferation and expression of progesterone receptors in tumor spheroids and monolayer cultures of human meningiomas. Neurosurgery 1997;41:1992–1998. [DOI] [PubMed] [Google Scholar]
- 14.Tonn JC, Ott MM, Meixensberger J, Paulus W, Roosen K. Progesterone receptors are detectable in tumor fragment spheroids of meningiomas in vitro. Anticancer Res 1994;14:2453–2456. [PubMed] [Google Scholar]
- 15.Heimdal JH, Aarstad HJ, Olofsson J. Monocytes secrete interleukin-6 when co-cultured in vitro with benign or malignant autologous fragment spheroids from squamous cell carcinoma patients. Scand J Immunol 2000;51:271–278. [DOI] [PubMed] [Google Scholar]
- 16.Fjellbirkeland L, Bjerkvig R, Laerum OD. Tumor fragment spheroids from human non-small cell lung cancer maintained in organ culture. Virchows Arch 1995;426:169–178. [DOI] [PubMed] [Google Scholar]
- 17.Janka M, Fischer U, Tonn JC, Kerkau S, Roosen K, Meese E. Comparative amplification analysis of human glioma tissue and glioma derived fragment spheroids using reverse chromosome painting. Anticancer Res 1996;16:2601–2606. [PubMed] [Google Scholar]
- 18.Fehlauer F, Stalpers LJA, Panayiotides J, Kaaijk P, Gonzalez Gonzalez D, Leenstra S, van der Valk P, Sminia P. Effect of single dose irradiation on human glioblastoma spheroids in vitro. Oncol Rep 2004;11:477–485. [PubMed] [Google Scholar]
- 19.Kaaijk P, Troost D, de Boer O, Van Amstel P, Bakker P, Leenstra S, Bosch D. Daunorubicin and doxorubicin but not BCNU have deleterious effects on organotypic multicellular spheroids of gliomas. Br J Cancer 1996;74:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaaijk P, Troost D, Sminia P, Hulshof MC, van der Kracht AH, Leenstra S, Bosch DA. Hypofractionated radiation induces a decrease in cell proliferation but no histological damage to organotypic multicellular spheroids of human glioblastomas. Eur J Cancer 1997;33:645–651. [DOI] [PubMed] [Google Scholar]
- 21.Treasure T, Sedrakyan A. Pleural mesothelioma: little evidence, still time to do trials. Lancet 2004;364:1183–1185. [DOI] [PubMed] [Google Scholar]
- 22.Fennell DA, Rudd RM. Defective core-apoptosis signalling in diffuse malignant pleural mesothelioma: opportunities for effective drug development. Lancet Oncol 2004;5:354–362. [DOI] [PubMed] [Google Scholar]
- 23.Berglund A, Glimelius B, Bergh J, Brodin O, Fjallskog ML, Hagberg H, von Heideman A, Larsson R, Tholander B, de la Torre M, et al. Selection of chemotherapy by ex vivo assessment of tumor sensitivity to cytotoxic drugs: results of a clinical trial. Med Oncol 2002;19:151–159. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Bodle E, Chen JY, Gao M, Rosen GD, Broaddus VC. Tumor necrosis factor-related apoptosis-inducing ligand and chemotherapy cooperate to induce apoptosis in mesothelioma cell lines. Am J Respir Cell Mol Biol 2001;25:111–118. [DOI] [PubMed] [Google Scholar]
- 25.Vivo C, Liu W, Broaddus VC. c-Jun N-terminal kinase contributes to apoptotic synergy induced by tumor necrosis factor-related apoptosis-inducing ligand plus DNA damage in chemoresistant, p53 inactive mesothelioma cells. J Biol Chem 2003;278:25461–25467. [DOI] [PubMed] [Google Scholar]
- 26.Broaddus VC, Dansen TB, Abayasiriwardana KS, Wilson SM, Finch AJ, Swigart LB, Hunt AE, Evan GI. Bid mediates apoptotic synergy between TNF-related apoptosis-inducing ligand (TRAIL) and DNA damage. J Biol Chem 2005;280:12486–12493. [DOI] [PubMed] [Google Scholar]
- 27.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest 1999;104:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhojani MS, Rossu BD, Rehemtulla A. TRAIL and anti-tumor responses. Cancer Biol Ther 2003;2:S71–S78. [PubMed] [Google Scholar]
- 29.Woodward J, Sisley K, Reeves G, Nichols C, Parsons MA, Mudhar H, Rennie I. Evidence of macrophage and lymphocyte, but not dendritic cell, infiltration in posterior uveal melanomas, whilst cultured uveal melanomas demonstrate pluripotency by expressing CD68 and CD163. Int J Exp Pathol 2004;85:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Braybrooke JP, Levitt NC, Joel S, Davis T, Madhusudan S, Turley H, Wilner S, Harris AL, Talbot DC. Pharmacokinetic study of cisplatin and infusional etoposide phosphate in advanced breast cancer with correlation of response to topoisomerase IIalpha expression. Clin Cancer Res 2003;9:4682–4688. [PubMed] [Google Scholar]
- 31.Kaaijk P, Troost D, Das PK, Leenstra S, Bosch DA. Long-term culture of organotypic multicellular glioma spheroids: a good culture model for studying gliomas. Neuropathol Appl Neurobiol 1995;21:386–391. [DOI] [PubMed] [Google Scholar]
- 32.Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a β1 integrin viability signaling pathway. J Biol Chem 2004;279:33024–33034. [DOI] [PubMed] [Google Scholar]
- 33.Whitaker D. The cytology of malignant mesothelioma. Cytopathology 2000;11:139–151. [DOI] [PubMed] [Google Scholar]
- 34.Delahaye M, de John AA, Versnel MA, Hoogsteden HC, Teeling P, van der Kwast TH. Cytopathology of malignant mesothelioma. Reappraisal of the diagnostic value of collagen cores. Cytopathology 1990;1:137–145. [DOI] [PubMed] [Google Scholar]
- 35.Marchi E, Liu W, Broaddus VC. Mesothelial cell apoptosis is confirmed in vivo by morphologic change in cytokeratin distribution. Am J Physiol Lung Cell Mol Physiol 2000;278:L528–L535. [DOI] [PubMed] [Google Scholar]
- 36.Hughes SE. Detection of apoptosis using in situ markers for DNA strand breaks in the failing human heart. Fact or epiphenomenon? J Pathol 2003;201:181–186. [DOI] [PubMed] [Google Scholar]
- 37.Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA. Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol 2003;199:221–228. [DOI] [PubMed] [Google Scholar]
- 38.Mohiuddin I, Cao X, Ozvaran MK, Zumstein L, Chada S, Smythe WR. Phosphatase and tensin analog gene overexpression engenders cellular death in human malignant mesothelioma cells via inhibition of Akt phosphorylation. Ann Surg Oncol 2002;9:310–316. [DOI] [PubMed] [Google Scholar]
- 39.Cacciotti P, Barbone D, Porta C, Altomare DA, Testa JR, Mutti L, Gaudino G. SV40-dependent Akt activity drives mesothelial cell transformation after asbestos exposure. Cancer Res 2005;65:5256–5262. [DOI] [PubMed] [Google Scholar]
- 40.Ramos-Nino ME, Vianale G, Sabo-Atwood T, Mutti L, Porta C, Heintz N, Mossman BT. Human mesothelioma cells exhibit tumor cell-specific differences in phophatidylinositol 3-kinase/AKT activity that predict the efficacy of Onconase. Mol Cancer Ther 2005;4:835–842. [DOI] [PubMed] [Google Scholar]
- 41.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004;4:335–348. [DOI] [PubMed] [Google Scholar]
- 42.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, Cordon-Cardo C, Pelletier J, Lowe SW. Survival signalling by Akt and elF4E in oncogenesis and cancer therapy. Nature 2004;428:332–337. [DOI] [PubMed] [Google Scholar]