Abstract
Although high tidal volume ventilation exacerbates lung injury, the mechanisms underlying the inflammatory response are not clear. Here, we exposed isolated lungs to high or low tidal volume ventilation, while perfusing lungs with whole blood, or blood depleted of leukocytes and platelets. Then, we determined signaling responses in freshly isolated lung endothelial cells by means of immunoblotting and immunofluorescence approaches. In depleted blood perfusion, high tidal volume induced modest increases in both P-selectin expression on the endothelial surface, and in endothelial protein tyrosine phosphorylation. Both high tidal volume–induced responses were markedly enhanced in the presence of whole blood perfusion. However, a P-selectin–blocking antibody given together with whole blood perfusion inhibited the responses down to levels corresponding to those for depleted blood perfusion. These findings indicate that the full proinflammatory response occurs in two stages. First, lung distension causes modest endothelial activation. Second, subsequent endothelial–inflammatory cell interactions augment P-selectin expression and tyrosine phosphorylation. We conclude that interactions of circulating inflammatory cells with P-selectin critically determine proinflammatory endothelial activation during high tidal volume ventilation.
Keywords: leukocytes, lung, mechanical, P-selectin, phosphorylation
Although mechanical ventilators provide essential respiratory support in lung injury, a potential difficulty is that the high tidal volumes delivered by mechanical ventilation may independently exacerbate lung injury (1). Since high tidal volume (HV) causes excessive expansion of pulmonary alveoli, and since stretching cultured endothelial cells activates kinases (2), it is proposed that alveolar overexpansion stretches endothelial cells, causing inflammatory responses. However, no direct data in situ support this view.
Because endothelial cells are critical for initiating lung inflammation, it is important to understand the endothelial signaling sequence activated by HV ventilation. An important but unanswered question is whether lung expansion is the predominant factor that induces proinflammatory endothelial signaling, or whether secondary inputs are required. The stretch hypothesis is derived largely from findings in cultured cells (2). Hence, the possibility exists that even under HV conditions, lung endothelial cells in situ may not stretch sufficiently to account entirely for the induced inflammatory consequences.
To test this hypothesis, we exposed lungs to HV ventilation. Our aim was to determine the extent to which alveolar stretch, as opposed to inflammatory cells secondarily recruited by the stretch, cause HV-induced endothelial activation. In primary isolates of endothelial cells, we detected tyrosine kinase activation and expression of the leukocyte adhesion receptor, P-selectin, as proinflammatory endpoints (3). We report here our new finding that the proinflammatory endothelial signaling induced by HV resulted less from the stretch effects of lung expansion than from the effects of leukocytes and platelets that were recruited consequent to stretch.
MATERIALS AND METHODS
Reagents and Antibodies
The following were purchased: PBS (GIBCO Laboratories, Grand Island, NY); sulfosuccinimidobiotin (sulfo-NHS-biotin; Pierce Chemical, Rockford, IL); gadolinium chloride (Sigma Chemical Co., St. Louis, MO); mouse monoclonal anti-phosphotyrosine antibody (PY99), anti-mouse IgG-HRP, protein A/Protein G-agarose, mouse monoclonal anti-P-Selectin antibody (CTB201), mouse monoclonal IgG1 (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit anti-factor VIIIR:Ag/von Willebrand factor (vWf; Zymed, South San Francisco, CA); Alexa 568–tagged goat anti-mouse IgG and Rhodamine 6G (Molecular Probes, Eugene, OR); Dynabeads M-270 tosylactivated (Dynal, Oslo, Norway); and Strept Avidin HRP conjugate (Jackson ImmunoResearch, Inc., West Grove, PA). Anti-rat P-selectin nonblocking and blocking mAbs RP-2 and RMP-1, respectively, were gifts of Dr A.C. Issekutz (Department of Pediatrics, Dalhousie University, Halifax, NS, Canada) (3, 4).
Lung Preparation
We prepared the isolated, blood-perfused (14 ml/min) rat lung as before (3). Briefly, we anesthetized rats (Sprague-Dawley, 600 g), heparinized them (1,200 IU heparin/kg), collected their blood, and excised their heart and lungs. To blood-perfuse the lungs (37°C, 14 ml/min), we held pulmonary artery and left atrial pressures at 12 and 6 cm H2O, respectively. By means of a mechanical ventilator (Harvard Apparatus, Holliston, MA) attached to the tracheal cannula, we ventilated the lungs at low (LV) or high (HV) tidal volumes of 6 or 12 ml/kg, respectively. The corresponding inspiratory pressures were 11 and 22 cm H2O, and the corresponding integrated mean airway pressures were 6 and 10 cm H2O. Throughout, ventilatory rate and end-expiratory pressure were 30/min and 5 cm H2O, respectively.
Preparation of Blood Perfusates
To determine the effects of leukocytes and platelets, we established the following blood preparations.
Whole blood.
We heparinized anesthetized rats (100 U/kg), then we withdrew whole blood (WB).
Leukocyte- and platelet-depleted blood.
We centrifuged heparinized blood (2,000 rpm, 10 min, 4°C), removing and saving the leukocyte- and platelet-containing buffy coat that was layered immediately above the RBCs following each spin. To ensure complete removal of leukocytes and platelets from red cells, we washed the red cells three times by resuspension in buffer (4% albumin/PBS, 4°C) followed by centrifugation (×3). Again, each time we saved the supernatants. Finally, we suspended the washed red cells in 4% albumin.
Reconstituted whole blood.
To control for procedural effects in the preparation of blood perfusates, we first removed, then added back leukocytes and platelets to RBCs to prepare reconstituted WB (Rec-WB).
To determine leukocyte and platelet counts, we obtained blood perfusate aliquots at the beginning of the experiment for fluorescently labeling cells with the marker, rhodamine 6G (5 μg/ml, 10 min).
FLEC Isolation
We isolated FLEC as we previously described (3). Briefly, we terminated the experiment after 2 h of perfusion, using ice-cold buffer perfusion (200 ml, 0.1% albumin/PBS, 4°C) to clear blood from the lung. Then, with the lung immersed in cold PBS (4°C) we sequentially perfused collagenase, trypsin, and buffer (4°C) to dislodge luminal cells from the lung vasculature. We washed the dislodged cells in buffer (×3) and then exposed them to magnetic beads (Dynabeads M-270, 2.8 μm) labeled with endothelial-specific, anti-vWf antibody (5). We held the mixed cells in a magnetic chamber (magnetic particle concentrator; Dynal), collected the cells retained and confirmed their endothelial phenotype (3).
Immunofluorescent Labeling of FLEC for P-Selectin
We labeled cells using anti–P-selectin mAb (10 μg/ml, 60 min, 4°C) and fluorescent, Alexa 568–tagged goat anti-mouse IgG (20 μg/ml, 30 min, 4°C). Labeling was done at 4°C to avoid antibody internalization and enable surface labeling. We fixed cells (2% paraformaldehyde, 10 min, room temperature) before viewing them by confocal microscopy (LSM 510; Zeiss, Germany) (MCID-M4; Imaging Research, St. Catherine's, Ontario, Canada). We digitally imaged single cells to quantify fluorescence intensity (MCID-M4; Imaging Research).
Immunoblotting and Immunoprecipitation
FLEC were lysed as reported previously (3) (4°C, 30 min), lysates cleared (14,000 RPM, 15 min), protein concentrations determined (BCA Protein Assay; Pierce), and anti-tyrosine phosphorylation blotting performed as previously described (6). Briefly, equal amounts of protein run in duplicate on 10% SDS polyacrylamide gels (SDS-PAGE) under reducing conditions were transfered to nitrocellulose. Then phosphotyrosyl-containing proteins were detected using affinity-purified anti-tyrosine phosphorylation antibody, followed by streptavidin–horseradish peroxidase. We developed blots using enhanced chemiluminescence. Duplicate gels were stained with Coomassie Blue.
To determine P-selectin surface expression, we surface biotinylated FLEC (sulfo-NHS-biotin, 0.5 mg/ml, pH 8.0, 4°C, 30 min) and, using monoclonal anti–P-selectin antibody, immunoprecipitated P-selectin from the lysates (3). For each immunoprecipitation (IP), to ensure adequacy of FLEC protein (150–200 μg), we pooled cells from the lungs of two rats that we subjected to identical ventilation and perfusion conditions. Thus we used the lungs of four rats for one paired experiment. We used equal quantities of lysate protein for IP.
Statistics
All data are mean ± SE. Differences between groups were tested by the paired t test for two groups and by the Newman-Keuls test for > 2 groups. Statistical significance was accepted at P < 0.05.
RESULTS
Cell Depletion
We perfused lungs either with WB or blood depleted of leukocytes and platelets (DB). Counts for leukocytes and platelets in DB were, respectively, 9.3 ± 2 and 14.8 ± 2% of those in WB (Table 1).
TABLE 1.
CELL COUNTS
Definition of abbreviations: DB, leukocyte- and platelet-depleted blood perfusate; WB, reconstituted whole blood perfusate.
Data are shown as mean ± SE.
P < 0.05 compared to WB.
P-Selectin Expression
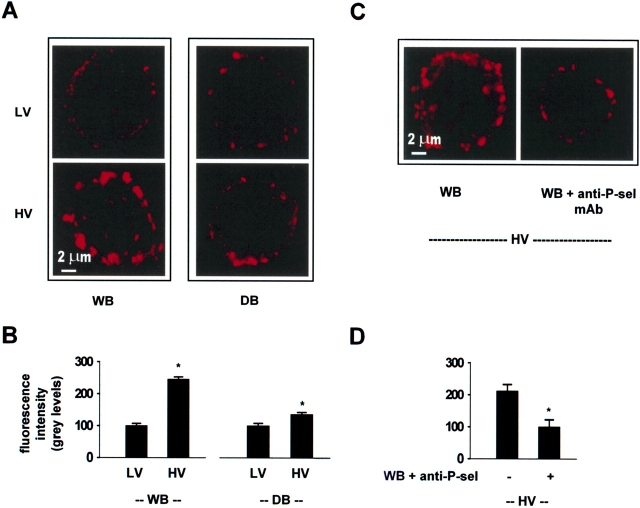
In non-permeabilized FLEC, immunofluorescence of P-selectin reveals cell surface expression of the receptor, since the fluorescent probe is excluded from cells and causes no cytosolic labeling (3). Under WB perfusion, surface expression of P-selectin was markedly greater for HV than LV ventilation (Figure 1A, left panels). In LV ventilation, P-selectin expression was similar for both WB and DB perfusion (Figure 1A, top panels). However, the HV-induced P-selectin expression was markedly lower in DB than WB perfusion (Figure 1A, bottom panels). In control FLEC obtained immediately (5 min) after lung excision, P-selectin immunofluorescence was 21 ± 7% of the expression in LV lungs under WB perfusion (P < 0.05, n = 3).
Figure 1.
Confocal images of single lung endothelial cells showing P-selectin expression on the cell surface. anti-P-sel mAb, anti–P-selectin monoclonal antibody, RMP-1 (20 μg/ml). HV, high tidal volume; LV, low tidal volume. WB, whole blood perfusion; DB, perfusion with leukocyte- and platelet-depleted blood. The single endothelial cells shown were obtained from lungs treated either under different combinations of ventilation and perfusion conditions as indicated (A, B), or from lungs exposed only to HV ventilation under different perfusion conditions (C, D). In paired experiments, we quantified the mean fluorescence in 30–40 cells from each lung. We processed cells obtained from each lung in parallel but in separate aliquots (n = 3 paired experiments). Mean ± SE. *P < 0.05, compared with bar on left by paired t test.
A comparison of the fluorescence distributions induced by HV ventilation indicated that P-selectin expression occurred in large clumps at the cell periphery for WB perfusion, but was sparse for DB perfusion (Figure 1A, lower panels). Fluorescence quantification indicated that under WB perfusion, HV increased P-selectin fluorescence more than two times above LV levels (Figure 1B). By contrast, under DB perfusion HV increased the fluorescence by only ∼ 25%. A nonspecific primary mAb resulted in dark images (not shown), ruling out the presence of nonspecific fluorescence.
The inclusion of the P-selectin–blocking mAb, RMP-1 (4) in WB perfusion markedly inhibited the HV-induced P-selectin expression (Figure 1C). Fluorescence quantification indicated that this inhibition (Figure 1D) was not different from the extent to which the HV-induced expression was inhibited in the presence of DB perfusion.
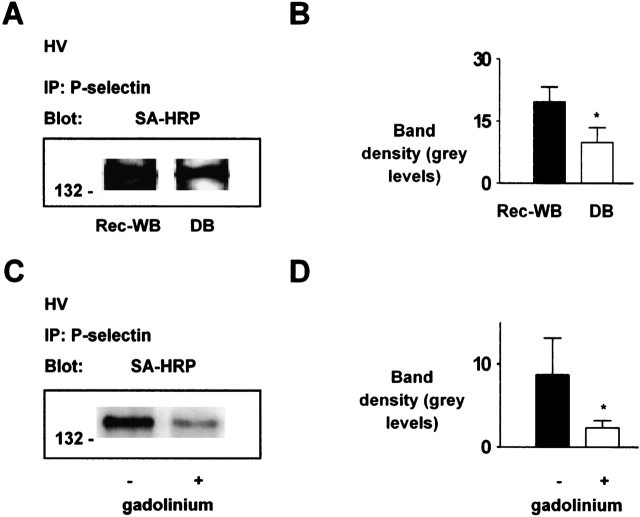
To determine bulk expression of P-selectin, we exposed FLEC to biotin, which specifically binds to the external cell surface (4). Then, after immunoprecipitating P-selectin from FLEC lysates, we ran samples on SDS-PAGE and blotted membranes with streptavidin. These procedures revealed the presence of a band at ∼ 140 kD (Figure 2A) that we confirmed to be P-selectin by reprobing the blot with specific mAb (not shown). In paired lungs ventilated under HV conditions, the band was 48 ± 13% lower for DB than Rec-WB perfusion (Figure 2B). In another set of paired experiments in which we held both lungs under WB perfusion and HV ventilation conditions, addition of the Ca2+ mobilization inhibitor, gadolinium, decreased band density for P-selectin (Figures 2C and 2D), affirming the known Ca2+ dependence of P-selectin expression (7).
Figure 2.
Immunoprecipitation of P-selectin from lung endothelial cells. IP, immunoprecipitate. SA-HRP, streptavidin–horseradish peroxidase. HV, high tidal volume. WB, whole blood perfusion; Rec-WB, reconstituted whole blood perfusion; DB, perfusion with leukocyte- and platelet- depleted blood. P-selectin was immunoprecipitated from lysates of primary endothelial isolates obtained from HV ventilated lungs. (A–D) SA-HRP blots show endothelial surface expression of P-selectin, under perfusion conditions of WB or DB in A and WB in C. Molecular weights are indicated on the left. Bars show densitometry. Gadolinium, 10 μM. Each bar: n = 3. Mean ± SE. *P < 0.05, compared with bar on left by paired t test.
Taken together, these immunoprecipitation and immunofluorescence data indicate that HV ventilation induced P-selectin expression, and that bloodborne leukocytes and platelets played an important role in achieving the full extent of the expression.
Tyrosine Kinase Activation
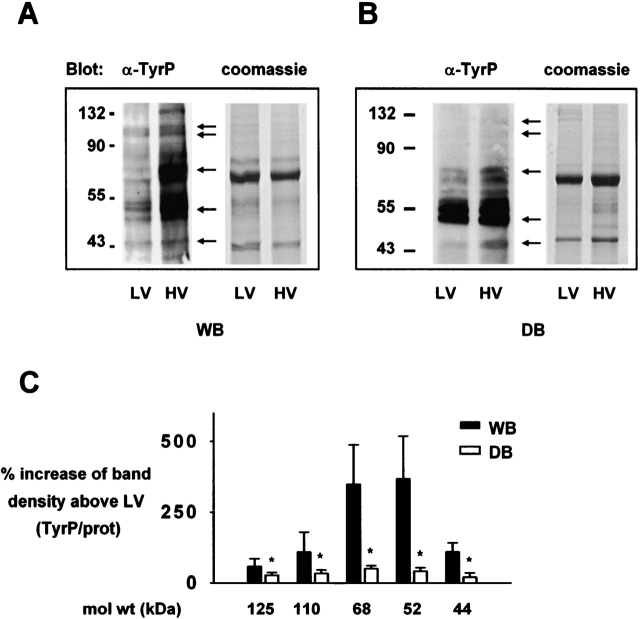
To determine cell-signaling responses, we determined protein tyrosine phosphorylation in FLEC lysates by Western blotting. Lanes run in parallel and stained with Coomassie Blue served as indicators of equal protein loading. Under WB perfusion, protein tyrosine phosphorylation was markedly greater for HV than LV ventilated lungs (Figure 3A). However, under DB perfusion the HV-induced response was markedly attenuated (Figure 3B). Thus, although HV always increased tyrosine phosphorylation in endothelial cells (P < 0.05), the increase under DB perfusion was 70–80% less on several proteins than under WB perfusion (Figure 3C).
Figure 3.
Lung endothelial protein tyrosine phosphorylation. α-tyrP, anti-phosphotyrosine; HV, high tidal volume; LV, low tidal volume; WB, whole blood perfusion; DB, perfusion with leukocyte- and platelet- depleted blood. (A and B) anti-phosphotyrosine blots are of lysates of endothelial cells from lungs ventilated and perfused for 2 h as indicated. Arrows indicate prominent bands. Coomassie staining is on duplicate gels. Molecular weights are indicated on the left. (C) Bars represent HV-induced increases in band density (tyrosine phosphorylation/protein ratios). n = 3 for each bar. Mean ± SE, *P < 0.05 versus left bar by paired t test.
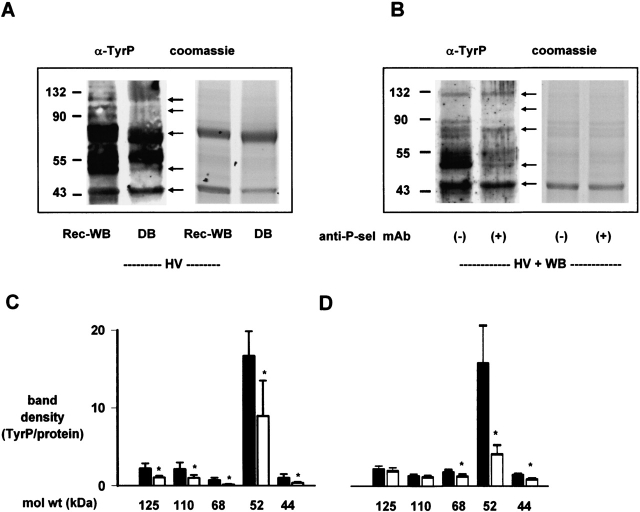
To rule out nonspecific effects that might have contributed to the differences in band density between separately run gels, in a group of paired experiments, holding both lungs under HV conditions, we ran lysates from Rec-WB and DB together in the same gel. These experiments also indicated that protein tyrosine phosphorylation was markedly reduced under DB than Rec-WB perfusion conditions (Figures 4A and 4C). Hence, we interpret that under DB conditions, namely in the absence of inflammatory cells in blood, HV ventilation had a considerably diminished effect on endothelial tyrosine kinase activation.
Figure 4.
4P-selectin blockade inhibits endothelial protein tyrosine phosphorylation in ventilation stress. anti-P-sel mAb, anti–P-selectin monoclonal antibody, RMP-1 (20 μg/ml). α-tyrP, anti-phosphotyrosine; HV, high tidal volume; WB, whole blood perfusion; Rec-WB, reconstituted whole blood perfusion; DB, perfusion with leukocyte- and platelet-depleted blood. (A and B) Representative anti-phosphotyrosine blot of lysates of endothelial cells derived from lungs ventilated and perfused for 2 h as indicated. Arrows indicate prominent bands. Coomassie staining is on duplicate gels. Molecular weights are indicated on the left. (C and D) Bars represent band densities (tyrosine phosphorylation/protein ratios). C: solid bars indicate Rec-WB; open bars indicate DB. D: solid bars indicate WB; open bars indicate WB + anti–P-sel mAb. n = 6 (A) and 3 (B). Mean ± SE. *P < 0.05 by paired t test against left bar.
To determine the role of the surface-expressed P-selectin in the FLEC phosphorylation response, we added the anti–P-selectin mAb, RMP-1 to the perfusate under WB conditions. RMP-1, which blocks leukocyte binding to P-selectin, markedly attenuated HV-induced tyrosine phosphorylation (Figures 4B and 4D). These findings indicated that P-selectin mediated the HV-induced activation of endothelial tyrosine kinases.
To determine the extent to which Rec-WB contained activated leukocytes and platelets that may have in turn, activated endothelial cells, we perfused paired lungs with either WB, or Rec-WB under HV conditions. Not shown are results from these experiments in which endothelial surface P-selectin expression and protein tyrosine phosphorylation were similar for WB and Rec-WB perfusion (n = 3). We cannot rule out the possibility that some leukocyte activation might have taken place during Rec-WB preparation. However, evidently this activation did not detectably contribute to the P-selectin and tyrosine phosphorylation responses under HV.
Extravascular Lung Water
We have shown previously (3) that the present protocol for HV ventilation does not increase the extravascular lung water (EVLW). Here, in lungs exposed to HV during WB or DB perfusion, EVLW averaged 4 ± 0.1 and 4.1 ± 0.1 g/g dry weight, respectively. These values, which are within the range of normal for our laboratory, indicate that neither group developed pulmonary edema.
DISCUSSION
We show here for the first time, that in HV ventilation, endothelial P-selectin expression critically enhanced proinflammatory activation of endothelial cells. To determine the roles played by circulating inflammatory cells, we depleted leukocytes and platelets from the blood. Our HV protocol did not increase extravascular lung water, thereby ruling out any contribution of pulmonary edema to these signaling responses. Lung perfusion with depleted blood led to the important finding that in the absence of bloodborne inflammatory cells, HV ventilation caused a markedly attenuated P-selectin expression. Notably, in the presence of inflammatory cells the same distension caused a 4-fold greater enhancement of P-selectin expression. Further, the P-selectin blocking mAb RMP-1 inhibited increases in both HV-induced tyrosine phosphorylation and P-selectin expression.
We interpret that the primary effect of lung overdistension was to activate endothelial P-selectin expression modestly, but sufficiently to initiate leukocyte recruitment. Thus HV in the absence of leukocytes and platelets increased tyrosine phosphorylation, indicating that overdistension alone was sufficient to activate endothelial signaling. The increased tyrosine phosphorylation may have triggered P-selectin expression (3), initiating the first wave of leukocyte recruitment. The recruited leukocytes and platelets then interacted with P-selectin to further spike tyrosine phosphorylation and, thereby, P-selectin expression. Evidently, the endothelial signaling initiated by HV ventilation occurred in a sequence in which lung distension evoked modest inflammatory activation, but subsequently, recruited leukocytes were required to induce the full extent of the response (8, 9). We propose that ventilation stress incurs a feedback loop in which recruited leukocytes act on endothelial cells to enhance the gain of the inflammatory process.
To our knowledge, this is the first evidence that interplay between mechanical strain and leukocyte recruitment sensitizes the lung's proinflammatory response. In the first step of this process, lung distension is likely to induce stretch of blood vessels (10), causing displacement of the endothelial cell membrane against the interstitial matrix. In cultured cells, mechanically induced cell-matrix displacement induces integrin aggregation (11), which induces further downstream signaling (12). Our previous studies indicate that HV aggregates the αvβ3 integrin, causing it to associate with the focal adhesion kinase (3). The extent to which these mechanisms contributed to the present findings requires further consideration.
In endothelial cells, mechanically induced cell-matrix displacement induces integrin-mediated increase in cell Ca2+ (13), causing exocytosis and cell-surface expression of P-selectin (14). We speculate that endothelial integrin aggregation and focal adhesion formation resulting from lung distension may induce several proinflammatory pathways in lung. In cultured lung endothelial cells, integrin aggregation causes tyrosine phosphorylation– mediated increases in cytosolic Ca2+ (Ca2+cyt) (15). Integrin aggregation also induces release of arachidonate, which activates Ca2+ entry (16). Cytosolic Ca2+ increases activate exocytosis of P-selectin containing Weibel-Palade bodies (WPB) (7), resulting in P-selectin expression on the endothelial surface. The present responses support these considerations. The nonspecific Ca2+ inhibitor gadolinium blocked the present P-selectin expression, indicating that the response was Ca2+ mediated.
Although our findings point to P-selectin as being critical to the present responses, the role of P-selectin in lung inflammation continues to be controversial (reviewed in Ref. 14). According to Kotani and colleagues (17), ventilation stress does not induce P-selectin expression in lung vessels. The reason for the difference from our findings is not clear. In lung, P-selectin expression occurs only in the arterial and venous segments (18). Our evidence for P-selectin expression in FLEC from freshly isolated lungs confirms previous reports that unstressed P-selectin expression occurs in lung vessels (19). It is possible that FLEC include cells from these segments, while Kotani and coworkers' immunohistochemical data largely address the alveolar capillary segment that does not express P-selectin (17).
The potential concern that platelets adherent to FLEC contribute d to P-selectin fluorescence is unlikely on several counts. First, the 10-min perfusion of a large volume (200 ml) of ice-cold buffer probably removed all vascular leukocytes and platelets. Second, the collagenase and trypsin infusions and the triple washing of the isolated cells were also likely to have removed any cells or other material adherent on FLEC. Third, no adherent platelets or leukocytes were evident through direct microscopy of > 250 FLEC. We conclude that the P-selectin fluorescence was of endothelial origin.
The extent to which the present findings in the isolated lung reflect responses in the intact lung requires consideration. The high vascular resistance (20) and ex vivo perfusion conditions are potential sources of nonspecific responses. However, since systemic effects are excluded, isolated lungs are widely used in the investigation of ventilation-induced lung injury (21). To reduce nonspecific influences such as those attributable to vinblastine (22), an agent sometimes used to deplete leukocytes (17), we avoided drug-induced leukocyte depletion procedures. We opted instead for blood reconstitution methods that were evidently not in themselves cell activating, since P-selectin expression and tyrosine phosphorylation were not affected. We point out that our procedures were identical for all groups. Hence, the HV-induced responses were not procedure-driven, but were specific consequences of mechanical stress.
In selecting the HV tidal volume, our aim was to establish a modest ventilation challenge that could cause stretch-mediated endothelial signaling, while avoiding lung injury. Although lung overexpansion exacerbates lung injury in mechanically ventilated patients (1), our aim was to avoid excessive hyperinflation that might elicit independent signaling effects secondary to events such as pulmonary edema (23) and stress fracture (24). Previous reports indicate that stretch-mediated increase of vascular diameter by ∼ 8% induces Ca2+ and NO signaling in lung endothelial cells in situ (25, 26). To achieve comparable levels of stretch, we held tidal volume at physiologic levels (27) for LV, but at two times this volume for HV. The increase of tidal volume increased mean airway pressure from 6 in LV to 10 cm H2O in HV. This increase in airway pressure stretches lung vascular diameters by ∼ 8% (10). Thus, although the present HV conditions did not cause lung injury, at least to the extent that lung water did not increase, the vascular stretch was sufficient to induce endothelial signaling, which may reflect the initial phases of the proinflammatory response to ventilation stress. Further studies are required to determine whether secondary events enhance this signaling to enhance inflammation and induce overt lung injury (28, 29).
In conclusion, our findings indicate a novel role for leukocytes and platelets in the proinflammatory responses to ventilation stress. In VILI and other forms of lung injury, leukocytes and platelets are traditionally viewed as direct agents of tissue injury. For example, damage to the endothelial barrier by activated leukocytes causes lung microvascular hyperpermeability and pulmonary edema (30, 31). However, here the HV-recruited leukocytes and platelets played a novel role. Thus, once recruited through P-selectin expression, these cells facilitated the endothelial proinflammatory response. Although shown here for ventilation stress, the extent to which leukocytes and platelets similarly drive inflammation in other forms of tissue injury requires consideration.
This study was supported by grant HL54157 to S.B. from the National Institutes of Health.
Originally Published in Press as DOI: 10.1165/rcmb.2005-0133OC on August 25, 2005
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Eisner MD, Thompson T, Hudson LD, Luce JM, Hayden D, Schoenfeld D, Matthay MA. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med 2001;164:231–236. [DOI] [PubMed] [Google Scholar]
- 2.Naruse K, Yamada T, Sai XR, Hamaguchi M, Sokabe M. Pp 125FAK is required for stretch dependent morphological response of endothelial cells. Oncogene 1998;17:455–463. [DOI] [PubMed] [Google Scholar]
- 3.Bhattacharya S, Sen N, Yiming MT, Patel R, Parthasarathi K, Quadri S, Issekutz AC, Bhattacharya J. High tidal volume ventilation induces proinflammatory signaling in rat lung endothelium. Am J Respir Cell Mol Biol 2003;28:218–224. [DOI] [PubMed] [Google Scholar]
- 4.Walter UM, Ayer LM, Wolitzky BA, Wagner DD, Hynes RO, Manning AM, Issekutz AC. Characterization of a novel adhesion function blocking monoclonal antibody to rat/mouse P-selectin generated in the P-selectin-deficient mouse. Hybridoma 1997;16:249–257. [DOI] [PubMed] [Google Scholar]
- 5.Richard L, Velasco P, Detmar M. A simple immunomagnetic protocol for the selective isolation and long-term culture of human dermal microvascular endothelial cells. Exp Cell Res 1998;240:1–6. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya S, Fu C, Bhattacharya J, Greenberg S. Soluble ligands of the αvβ3 integrin mediate enhanced tyrosine phosphorylation of multiple proteins in adherent bovine pulmonary artery endothelial cells. J Biol Chem 1995;270:16781–16787. [DOI] [PubMed] [Google Scholar]
- 7.Datta YH, Romano M, Jacobson BC, Golan DE, Serhan CN, Ewenstein BM. Peptido-leukotrienes are potent agonists of von Willebrand factor secretion and P-selectin surface expression in human umbilical vein endothelial cells. Circulation 1995;92:3304–3311. [DOI] [PubMed] [Google Scholar]
- 8.Reyes LI, Escobar P, Bono MR, Rosemblatt M. Adhesion of B cell lines to endothelial cells from human lymphoid tissue modulates tyrosine phosphorylation and endothelial cell activation. J Immunol 2002;169:5881–5888. [DOI] [PubMed] [Google Scholar]
- 9.Ohmori T, Yatomi Y, Okamoto H, Miura Y, Rile G, Satoh K, Ozaki YG. (i)-mediated Cas tyrosine phosphorylation in vascular endothelial cells stimulated with sphingosine 1-phosphate: possible involvement in cell motility enhancement in cooperation with Rho-mediated pathways. J Biol Chem 2001;276:5274–5280. [DOI] [PubMed] [Google Scholar]
- 10.Albert RK, Lamm WJ, Rickaby DA, Al-Tinawi A, Dawson CA. Lung inflation distends small arteries (< 1 mm) in excised dog lungs. J Appl Physiol 1993;75:2595–2601. [DOI] [PubMed] [Google Scholar]
- 11.Chen KD, Li YS, Kim M, Li S, Yuan S, Chien S, Shyy JY. Mechanotransduction in response to shear stress: roles of receptor tyrosine kinases, integrins, and Shc. J Biol Chem 1999;274:18393–18400. [DOI] [PubMed] [Google Scholar]
- 12.Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res 2000;261:25–36. [DOI] [PubMed] [Google Scholar]
- 13.Sasamoto A, Nagino M, Kobayashi S, Naruse K, Nimura Y, Sokabe M. Mechanotransduction by integrin is essential for IL-6 secretion from endothelial cells in response to uniaxial continuous stretch. Am J Physiol Cell Physiol 2005;288:C1012–C1022. [DOI] [PubMed] [Google Scholar]
- 14.Ichimura H, Parthasarathi K, Quadri S, Issekutz AC, Bhattacharya J. Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J Clin Invest 2003;111:691–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharya S, Ying X, Fu C, Patel R, Kuebler W, Greenberg S, Bhattacharya J. αvβ3 integrin induces tyrosine phosphorylation- dependent Ca2+ influx in pulmonary endothelial cells. Circ Res 2000;86:456–462. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharya S, Patel R, Sen N, Quadri S, Parthasarathi K, Bhattacharya J. Dual signaling by the αvβ3 -integrin activates cytosolic phospholipase A2 in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol 2001;280:L1049–L1056. [DOI] [PubMed] [Google Scholar]
- 17.Kotani M, Kotani T, Ishizaka A, Fujishima S, Koh H, Tasaka S, Sawafuji M, Ikeda E, Moriyama K, Kotake Y, et al. Neutrophil depletion attenuates interleukin-8 production in mild-overstretch ventilated normal rabbit lung. Crit Care Med 2004;32:514–519. [DOI] [PubMed] [Google Scholar]
- 18.Burns AR, Smith CW, Walker DC. Unique structural features that influence neutrophil emigration into the lung. Physiol Rev 2003;83:309–336. [DOI] [PubMed] [Google Scholar]
- 19.Clark JG, Mandac-Dy JB, Dixon AE, Madtes DK, Burkhart KM, Harlan JM, Bullard DC. Trafficking of Th1 cells to lung: a role for selectins and a P-selectin glycoprotein-1-independent ligand. Am J Respir Cell Mol Biol 2004;30:220–227. [DOI] [PubMed] [Google Scholar]
- 20.Raj JU, Kaapa P, Hillyard R, Anderson J. Pulmonary vascular pressure profile in adult ferrets: measurements in vivo and in isolated lungs. Acta Physiol Scand 1991;142:41–48. [DOI] [PubMed] [Google Scholar]
- 21.Frank JA, Gutierrez JA, Jones KD, Allen L, Dobbs L, Matthay MA. Low tidal volume reduces epithelial and endothelial injury in acid-injured rat lungs. Am J Respir Crit Care Med 2002;165:242–249. [DOI] [PubMed] [Google Scholar]
- 22.Birukova AA, Birukov KG, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol 2005;289:L75–L84. [DOI] [PubMed] [Google Scholar]
- 23.Daffara R, Botto L, Beretta E, Conforti E, Faini A, Palestini P, Miserocchi G. Endothelial cells as early sensors of pulmonary interstitial edema. J Appl Physiol 2004;97:1575–1583. [DOI] [PubMed] [Google Scholar]
- 24.West JB. Invited review: pulmonary capillary stress failure. J Appl Physiol 2000;89:2483–2489. (discussion 2497). [DOI] [PubMed] [Google Scholar]
- 25.Kuebler WM, Ying X, Bhattacharya J. Pressure-induced endothelial Ca(2+) oscillations in lung capillaries. Am J Physiol Lung Cell Mol Physiol 2002;282:L917–L923. [DOI] [PubMed] [Google Scholar]
- 26.Kuebler WM, Uhlig U, Goldmann T, Schael G, Kerem A, Exner K, Martin C, Vollmer E, Uhlig S. Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am J Respir Crit Care Med 2003;168:1391–1398. [DOI] [PubMed] [Google Scholar]
- 27.Walker JK, Lawson BL, Jennings DB. Breath timing, volume and drive to breathe in conscious rats: comparative aspects. Respir Physiol 1997;107:241–250. [DOI] [PubMed] [Google Scholar]
- 28.Kiely JM, Hu Y, Garcia-Cardena G, Gimbrone MA Jr. Lipid raft localization of cell surface E-selectin is required for ligation-induced activation of phospholipase C gamma. J Immunol 2003;171:3216–3224. [DOI] [PubMed] [Google Scholar]
- 29.van IJzendoorn SC, van Gool RG, Reutelingsperger CP, Heemskerk JW. Unstimulated platelets evoke calcium responses in human umbilical vein endothelial cells. Biochim Biophys Acta 1996;1311:64–70. [DOI] [PubMed] [Google Scholar]
- 30.Mulligan MS, Polley MJ, Bayer RJ, Nunn MF, Paulson JC, Ward PA. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest 1992;90:1600–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyriakides C, Austen W Jr, Wang Y, Favuzza J, Moore FD Jr, Hechtman HB. Endothelial selectin blockade attenuates lung permeability of experimental acid aspiration. Surgery 2000;128:327–331. [DOI] [PubMed] [Google Scholar]