Abstract
Bronchopulmonary dysplasia in premature infants is characterized by inhibited alveolarization and vasculogenesis. Our goal was to generate a mouse model of inhibited alveolarization by the administration of an inhibitor of angiogenesis. We then examined the effects of retinoic acid (RA) and erythropoietin (EPO) on alveolar development in this model. Three-day-old mice were injected with a single dose of SU1498 (30 mg/kg, subcutaneously) and either concomitant RA (2 mg/kg, intraperitoneally) or EPO (2,000 IU/kg, subcutaneously) for 10 consecutive days, then harvested on Day 21. Morphometric and electron microscopic analysis, and platelet endothelial cell adhesion molecule (PECAM) immunostaining of endothelial cells, were performed on the lung tissue. In vitro assays were also performed to characterize the effects of RA on endothelial cell growth. Alveolar development was attenuated in the SU1498-treated mice, and electron microscopy demonstrated dilated and dysmorphic capillaries in alveolar walls comparable to previous findings in lungs of infants with bronchopulmonary dysplasia. RA or EPO maintained mean alveolar volume, alveolar surface area, and endothelial cell volume density in the SU1498-treated animals. RA also increased the proliferation of human fetal lung capillary endothelial precursor cells in vitro. These results suggest that the maintenance or growth of the endothelial cell population of the distal lung plays a major role in postnatal alveolar development.
Keywords: alveolar development, erythropoietin, retinoic acid, SU1498, angiogenesis inhibition
Although advances in newborn care have improved the survival of very low birth weight (VLBW) infants, ∼ 40% of VLBW survivors suffer from bronchopulmonary dysplasia (BPD) as a consequence of pulmonary immaturity, ventilator-related lung injury, and oxygen toxicity (1). The “new BPD” lung of the post–surfactant replacement era is hallmarked by an attenuation of pulmonary blood vessels and limited alveolar development (2). A role for an abnormality of vascular development in the pathogenesis of impaired alveolar development in BPD supports the “vascular hypothesis” of BPD (3).
Vascular endothelial growth factor (VEGF) is an important endothelial cell mitogen and survival factor (4). VEGF actions are primarily mediated by two tyrosine kinase receptors, VEGF receptor 1 (VEGFR1, also known as Flt-1) and VEGFR2 (also known as Flk-1/KDR). VEGFR1 may be involved in capillary network formation, whereas VEGFR2 transmits a mitogenic signal in endothelial cells (5). Null mutants of VEGFR are embryonic lethal (6). Administration of the antiangiogenic compound, SU5416, a VEGFR inhibitor, results in airspace enlargement and pulmonary hypertension in newborn rats 3 wk after a single injection on Day 1 of life (7).
Vitamin A supplementation to extremely low birth weight infants requiring respiratory support at 24 h of age reduces the rate of death or BPD by 11% (8). Retinoic acid (RA), a product of vitamin A oxidation, is important in lung function and development (9). As the lung matures, increased demand for RA results in a decrease in the retinyl-ester storage form of RA in the lung as it is used during the process of cellular differentiation and surfactant production (9). RA is required for normal alveolar development in the mouse, and exogenous administration of RA restores alveolar architecture to the lungs of dexamethasone-treated mice (10). Mice with a deletion of RA receptor γ have decreased lung elastin, decreased alveolar number, and increased alveolar size (11). Exogenous RA increases alveolar septal formation in adult tight skin mice, a genetic model associated with a failure in alveolar septation resulting in emphysema (12).
In addition to its erythropoietic functions, erythropoietin (EPO) is an endothelial cell mitogen that also has antiapoptotic effects on certain cells in the brain (13). EPO receptors have been identified in the developing human lung (14), and we have recently demonstrated effects of EPO on the epithelium of developing human lung in vitro and a mitogenic effect of EPO on endothelial precursor cells derived from human fetal lung (15). These data are supportive of a role for EPO in lung development, and suggest that EPO may be able to preserve lung development in the lung exposed to an antiangiogenic agent.
To further examine the role of angiogenesis in lung development, we sought to generate a murine model of inhibited alveolar development by employing SU1498, a commercially available selective inhibitor of VEGFR2 (16). After successfully generating the model, we investigated whether RA or EPO could support normal lung development in this model of impaired alveolarization.
MATERIALS AND METHODS
Study Animals and Treatments
The animal care committee at the University of Iowa Carver College of Medicine approved all procedures and protocols used in this study. Timed-pregnant mice (Institute for Cancer Research [ICR] strain, Harlan, Indianapolis, IN) were housed in the animal care facility, fed ad libitum, and exposed to a 12:12 h light:dark cycle in room air at room temperature. A single dose of SU1498 [(E)-3–93,5-Diisopropyl-4-hydroxyphenyl]-2-[(3-phenyl-n-propyl)amino-carbonyl] acrylonitrile] (30 mg/kg; Calbiochem, Oakland, CA), dissolved in DMSO and mixed with 2% methylcellulose as vehicle, or DMSO and vehicle alone was injected to the pups subcutaneously on Day 3 of life (day of birth = Day 1 of life). Concomitant intraperitoneal injections with all trans retinoic acid (RA, 2 mg/kg; Sigma, St. Louis, MO) dissolved in DMSO and mixed in corn oil as a diluent or DMSO and corn oil as a sham injection were performed after SU1498 injection in 3-d-old mice for 10 consecutive days. This protocol resulted in the following study groups: (1) control: DMSO and 2% methylcellulose sc plus DMSO and corn oil intraperitoneally; (2) RA: DMSO and 2% methylcellulose sc plus RA in DMSO and corn oil intraperitoneally; (3) SU1498: SU1498 in DMSO and 2% methylcellulose sc plus DMSO and corn oil intraperitoneally; and (4) SU1498 and RA: SU1498 in DMSO and 2% methylcellulose sc plus RA in DMSO and corn oil intraperitoneally. In a separate series of experiments, newborn mice were treated as above with subcutaneous erythropoietin (EPO, 2,000 IU/kg in PBS; Amgen, Thousand Oaks, CA) for 10 d instead of RA. Therefore, for the EPO experiment, all animals received a single subcutaneous injection of SU1498 with DMSO or DMSO alone on Day 3 of life, and 10 d of subcutaneous EPO in PBS or PBS alone for 10 d starting on Day 3 of life. In total, two litters of mice were used in a pilot experiment that demonstrated an effect of SU1498 to inhibit alveolar development (data not shown) and seven litters of mice were used for the experiments involving RA and EPO. The pups were randomized within each litter for all experiments. The dose of SU1498 was a molar equivalent to the dose of SU5416 administered to newborn rats by Jakkula and coworkers (7). The dose of RA and duration of treatment was the same as previously demonstrated to cause regeneration of alveoli in mice treated with dexamethasone (10). The daily dose of EPO was chosen to be approximately ten times the dose used to stimulate erythropoiesis in premature infants to ensure that a possible effect on alveolar development would be detected (17). Because alveolar development in the mouse occurs during Days 4–14 of life (18), we reasoned that the administration of the SU1498 on the day before the onset of alveolarization would inhibit the endothelium just as it is being recruited to the nascent alveoli. Both RA and EPO were given for 10 consecutive days in an effort to support the lung throughout the majority of alveolarization. Importantly, the animals in the RA experiments all received daily intraperitoneal doses of DMSO and corn oil with or without RA. In contrast, the animals in the EPO experiments never received intraperitoneal injections, but only subcutaneous injections of PBS with or without EPO. Therefore, the conditions of the two distinct experiments were much different, making it impossible to directly compare the results of the RA experiment with the EPO experiment. Only the lungs of animals that survived to 21 d of life and were optimally inflated were used for analysis.
Tissue Preparation
On Day 21 of life, all mice were anesthetized by intraperitoneal injection of pentobarbital sodium (200 mg/kg). Lungs were exposed by thoracotomy, the right ventricle was punctured, and the lungs were perfused with ice-cold PBS. The trachea was cannulated and zinc acetate fixative (0.1 Tris buffer pH 7.4, 0.5 g calcium acetate, 5.0 g zinc acetate, and 5.0 g zinc chloride) was instilled at 25 cm H2O (19). After 5 min, the trachea was closed with a suture and the lungs were removed. Lung volume was measured by volume displacement. After overnight immersion in zinc acetate fixative, the lungs were dehydrated with 50% and 70% ethanol, placed in cassettes, then embedded in paraffin. A separate litter was harvested and fixed in 4% paraformaldehyde for tissue processing for transmission electron microscopy.
Histologic Staining and Morphometry
After fixation, 5-μm sections were cut and mounted on Super Frost Plus slides (VWR Scientific, West Chester, PA) and heated overnight at 55°C. After staining with hematoxylin and eosin, four random nonoverlapping fields were chosen in the distal lung. Three to eight animals were analyzed per condition. Slides were viewed under brightfield using a Nikon microscope (Melville, NY). For volume density (VD) analysis, slides were viewed with a ×20 lens. VD of airspace, alveolar tissue, conducting airways, and large blood vessels were counted using a 121-point grid by two independent observers blinded to the treatment conditions. The number of points falling on each compartment were counted and divided by the total number of points falling over the lung tissue to calculate the VD. Images for further morphometric analysis were acquired with a SPOT digital camera (Sterling Heights, MI). The photomicrographs were enlarged uniformly, overlaid with transparent grids and used for morphometric analysis as previously described (11). Mean cord lengths were determined by counting intersections with an array of 70 lines, each 33 mm long. The alveolar surface area was calculated as previously described (20, 21). For the calculation of the mean alveolar volume, a grid that contained equally spaced crosses was placed on the photomicrograph of the distal lung and the diameters of the alveoli that contained a cross were measured along the horizontal axis of the cross. The cube of the alveolar diameter times π and divided by 3 was used to estimate the mean alveolar volume (20, 21).
Platelet Endothelial Cell Adhesion Molecule Immunostaining
Paraffin-embedded lung tissue sections were deparaffinized with xylene, then rehydrated through an ethanol-water series. Sections were blocked with 5% normal horse serum at room temperature, and endogenous biotin was blocked with an avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA). The paraffin sections were then incubated with primary antibody (goat polyclonal anti CD-31 [platelet endothelial cell adhesion moelcule {PECAM}] IgG, 1:500; Vector Laboratories) for 1 h at room temperature. The tissue sections were washed two times in PBS, 5 min per rinse, incubated for 30 min in biotinylated horse anti-goat secondary antibody (1:50 dilution in PBS), then rinsed two times in PBS, 5 min per rinse. The sections were then incubated for 45 min in avidin-peroxidase reagent using a Vectastain Elite kit (Vector Laboratories). After being rinsed two times in PBS, 5 min per rinse, the sections were incubated in diaminobenzidine (700 g/ml) for 1 min. The sections were counterstained with hematoxylin for 30 s, rinsed in PBS for 5 min, rinsed quickly in distilled water, dehydrated, and mounted with glass coverslips. Sections were viewed under a Nikon FX photomicroscope. A negative control using 5% nonimmune goat serum in the place of primary antibody demonstrated no immunoreactivity. The distal part of the lung was viewed with a ×40 lens and a point-counting method in which the number of points landing on the lung tissue served as the denominator to determine the PECAM immunoreactive tissue VD on four nonoverlapping fields by two independent investigators blinded to the treatment conditions. The VD of immunoreactive PECAM-positive tissue (positive stained points / points on parenchyma) was then calculated.
Isolation of CD34+ Cells from Human Fetal Lung and Proliferation Assay with [3H]-Thymidine Uptake into Cellular DNA
Human fetal lung capillary endothelial precursor CD34+ cells (HFLCEPC) were isolated from human fetal lung tissue as previously described (22). The selected cells were plated on plastic dishes coated with rat-tail collagen (5 g/cm2; Becton Dickinson, Franklin Lakes, NJ) and maintained in a commercially available endothelial cell growth media (E-Stim; Becton Dickinson). Before plating, the cells were counted on a hemocytometer, and cell viability was evaluated by trypan blue exclusion. HFLCEPC were seeded into 12-well tissue culture plates at a density of 50,000 cells/well and incubated in E-Stim cell culture media for 24 h. The medium was changed to serum-free EMEM for 24 h, followed by incubation for 24 h in EMEM, with or without added all trans RA (Sigma) at a concentration of 10−6 M, 10−7 M, or 10−8 M. [3H]-thymidine (0.5 μCi/ml; Amersham, Piscataway, NJ) was added to the cell cultures for the final 4 h of incubation. The cells were washed twice with cold PBS, incubated with 10% trichloroacetic acid a 4°C for 20 min to precipitate the DNA, then washed twice with ice-cold H2O. The precipitate was solubilized by the addition of 0.75 ml 0.2 N NaOH and then neutralized with 0.75 ml 0.2 N HCl. A 0.5-ml aliquot was removed from each well and added to 10 ml of scintillation cocktail. Radioactivity was measured by scintillation spectroscopy. Triplicate determinations were made for each condition and each experiment was repeated at least five times with cells derived from different fetal lungs.
Statistical Analysis
Data are expressed as mean ± SEM. Statistical analysis was performed by ANOVA followed by the Mann-Whitney test or Kruskal-Wallis test using a Sigma Stat, Version 2.0 software package (Jandel Corporation, San Rafael, CA). Statistical significance was set at P < 0.05.
RESULTS
Treatment Effect on Subject Weight, Mortality, and Lung Volume
We treated newborn mice with vehicle, the VEGFR inhibitor, SU1498, and/or RA or EPO. The body weights of the treated animals were not different among the four treatment conditions on Postnatal Day 21. In addition, no significant difference was found in the lung volumes, measured by volume displacement, with each treatment. However, injection of the mice on Day 3 of life with SU1498 resulted in a mortality rate of nearly 36% at 21 d compared with a 11% loss of control animals, which was similar to the loss in RA-treated mice (Table 1A). In contrast, there were no losses in the animals in the EPO experiment (Table 1B), perhaps as a result of the different diluent used in this experiment (no daily intraperitoneal injections of DMSO and corn oil).
TABLE 1.
EFFECTS OF TREATMENT WITH SU1498 AND RETINOIC ACID ON BODY WEIGHT, LUNG VOLUMES, AND NEWBORN MORTALITY IN TREATED MICE
| Groups | Body Weight (g) | Lung Volume (cm3) | Mortality (%) |
|---|---|---|---|
| A. RA Experiment | |||
| Control (n = 5) | 15.3 ± 0.7 | 0.95 ± 0.2 | 10.8 |
| RA (n = 5) | 13.6 ± 1.2 | 0.70 ± 0.2 | 12.9 |
| SU1498 (n = 8) | 12.7 ± 1.1 | 0.91 ± 0.2 | 35.7* |
| RA + SU1498 (n = 6) | 12.8 ± 1.4 | 0.78 ± 0.2 | 20.0 |
| B. EPO Experiment | |||
| Control (n = 3) | 13.4 ± 0.6 | 1.00 ± 0.0 | 0.0 |
| EPO (n = 3) | 12.5 ± 0.7 | 0.75 ± 0.7 | 0.0 |
| SU1498 (n = 3) | 13.1 ± 0.9 | 1.43 ± 0.4 | 0.0 |
| EPO + SU1498 (n = 3) | 12.2 ± 0.9 | 0.93 ± 0.1 | 0.0 |
Definition of abbreviations: EPO, erythropoietin; RA, retinoic acid.
Values represent mean ± SEM (n = 3–8).
P < 0.05 versus control group.
Light Microscopic Morphology
Although the 21-d-old lungs were not different in gross appearance or in volume, a striking difference in lung structure was observed under the light microscope. As demonstrated in Figure 1, lungs of mice exposed to SU1498 had larger airspace with less alveolar tissue. The lungs of mice treated concurrently with SU1498 and RA (Figure 1A) or EPO (Figure 1B) were grossly similar in light microscopic morphology to the lungs of the control animals. These observations were then quantified by detailed morphometric analysis. The VD of airspace was significantly larger in mice treated with SU1498 alone or in combination with RA when compared with the control mice. A reciprocal decrease in the alveolar tissue VD was also observed in SU1498-treated lungs compared with the lungs of the control animals (Table 2A). RA had no effect on airspace or tissue VD. Similarly, airspace volume densities were increased in SU1498-treated mice compared with the controls in the EPO experiments. No other significant differences were found in the VDs in the EPO experiment (Table 2B). Volume densities of blood vessels or conducting airways were not different between the various conditions in either experiment.
Figure 1.
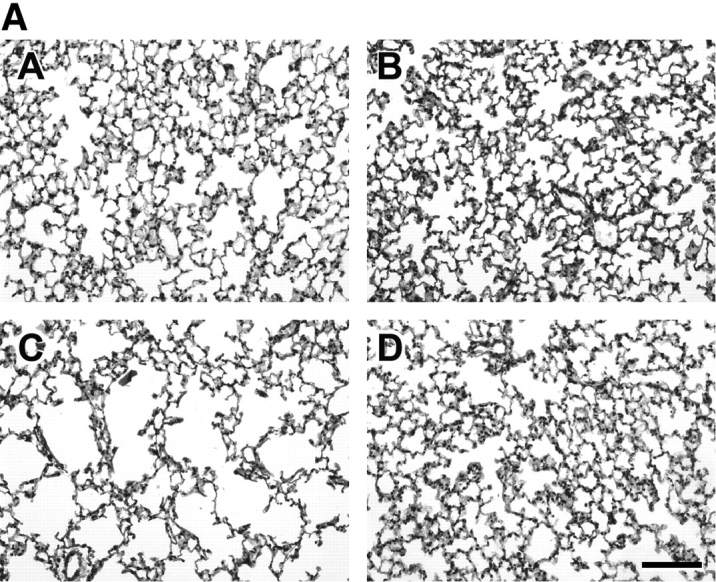
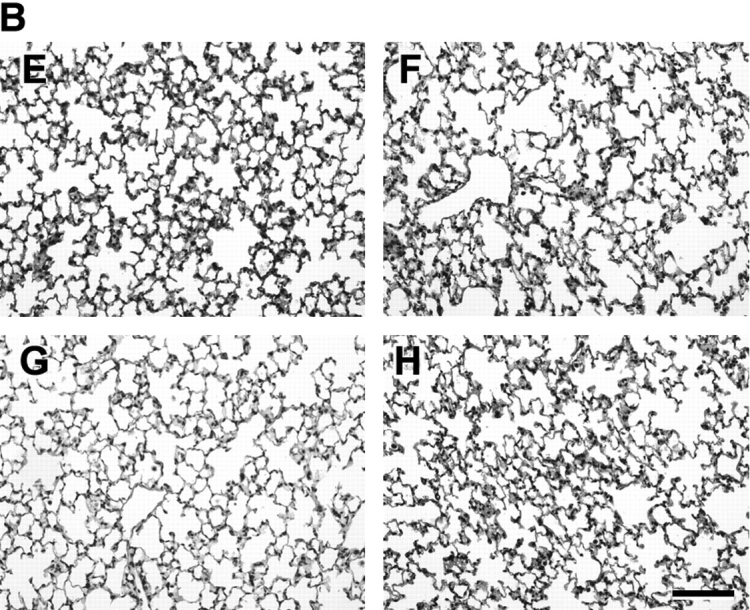
Representative photomicrographs of Day 21 mouse lungs stained with hematoxylin and eosin and observed at ×20 magnification. The lungs of mice in the RA experiment are demonstrated in A. Control (panel A), animals treated with RA (panel B), animals treated with SU1498 (panel C), animals treated with SU1498 + retinoic acid (panel D). The lungs of mice in the EPO experiment are demonstrated in B. Control (panel E), animals treated with EPO (panel F), animals treated with SU1498 (panel G), animals treated with SU1498 + EPO (panel H). Larger airspaces can be appreciated in the SU1498-treated mice (panels C and G) when compared with untreated controls (panels A and E). Concomitant RA or EPO treatment maintains alveolar size similar to that of control animals (panels D and H). Bars denote 100 μm.
TABLE 2.
EFFECTS OF SU1498, RETINOIC ACID, AND ERYTHROPOIETIN ON VOLUME DENSITY OF AIRSPACE AND ALVEOLAR TISSUE IN TREATED MICE
| Groups | Airspace VD (%) | Alveolar Tissue VD (%) | Large Blood Vessel VD (%) | Conducting Airway VD (%) |
|---|---|---|---|---|
| A. RA Experiment | ||||
| Control | 40.1 ± 2.5 | 49.0 ± 3.1 | 5.4 ± 0.5 | 5.6 ± 0.8 |
| RA | 42.1 ± 1.6 | 48.9 ± 1.9 | 4.8 ± 0.8 | 5.2 ± 0.7 |
| SU1498 | 50.8 ± 1.7* | 41.0 ± 1.2* | 4.3 ± 0.9 | 4.2 ± 0.7 |
| RA + SU1498 | 49.2 ± 1.9* | 42.4 ± 2.2 | 4.2 ± 0.8 | 4.8 ± 0.6 |
| B. EPO Experiment | ||||
| Control | 44.1 ± 5.3 | 48.2 ± 4.3 | 3.8 ± 0.7 | 4.7 ± 1.2 |
| EPO | 49.9 ± 4.1 | 42.3 ± 2.6 | 2.4 ± 0.6 | 5.1 ± 0.4 |
| SU1498 | 55.2 ± 1.7* | 41.7 ± 2.9* | 2.0 ± 1.0 | 2.2 ± 0.6 |
| EPO + SU1498 | 50.1 ± 1.7 | 43.9 ± 1.7 | 3.5 ± 2.7 | 2.7 ± 0.9 |
Definition of abbreviations: EPO, eryhtropoietin; RA, retinoic acid; VD, volume density.
Morphometric analysis was performed on lung tissue to determine the VDs of airspace and tissue components. VD values expressed in the table represent mean ± SEM (n = 3–8).
P < 0.05 versus control.
Alveolar Surface Area
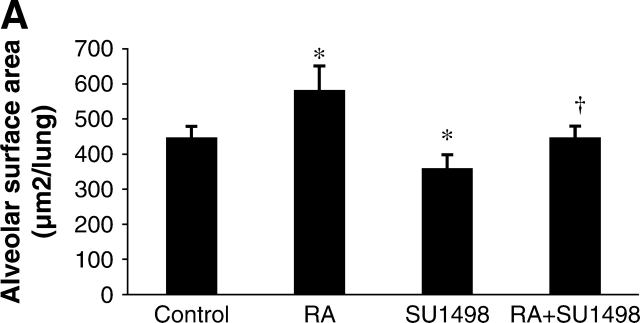
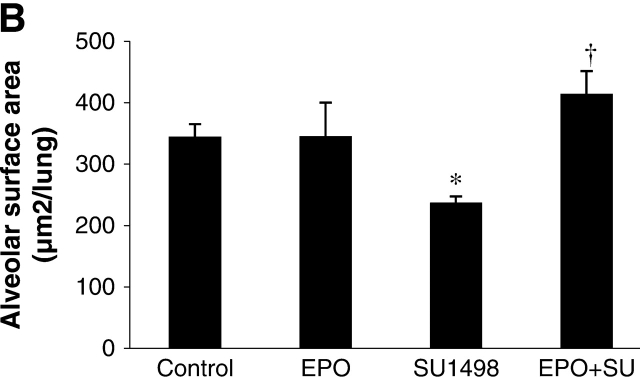
Alveolar surface area represents the total area in the lung tissue capable of gas exchange, thus it is a measure of potential lung function. In the RA experiment (Figure 2A), the alveolar surface area of SU1498-treated mice was significantly decreased by 27% when compared with the surface area in control mice. RA alone significantly increased the surface area compared with the surface area of control mice. Surface area in RA plus SU1498-treated animals was significantly increased compared with the surface area of the SU1498-treated mice and was similar to control values. In the EPO experiment, the alveolar surface area from SU1498-treated mice was significantly decreased by 31% when compared with the surface area of control mice (Figure 2B). EPO alone did not alter the surface area compared with the surface area of control mice. However, surface area in EPO plus SU1498-treated animals was significantly increased when compared with the surface area of the SU1498-treated mice.
Figure 2.
Alveolar surface area in Day 21 mouse lung tissue. Graphical demonstration of alveolar surface area determinations after morphometric analysis of lungs from control, RA-treated, SU1498-treated, RA + SU1498–treated (A), or EPO-treated alone or in combined with SU1498 (B). Values represent mean ± SEM (n = 3–8). *P < 0.05 versus control, † P < 0.05 versus SU1498.
Mean Alveolar Volume
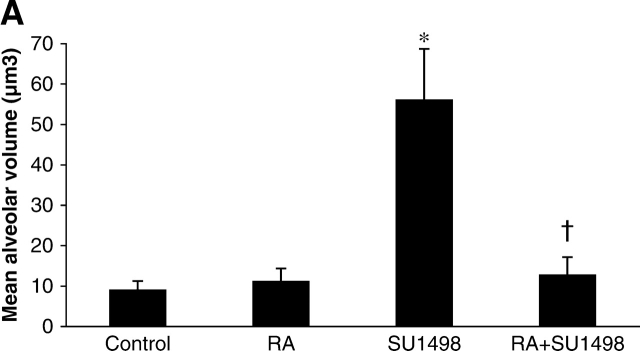
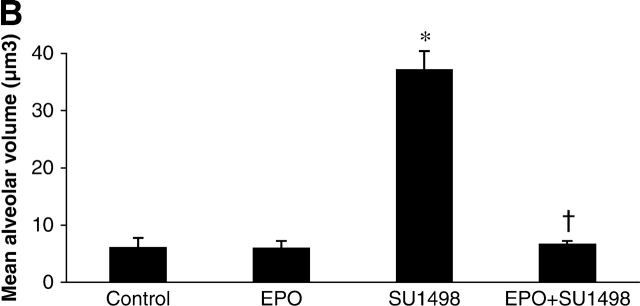
The mean alveolar volume is an estimate of the volume of individual alveoli. When compared with control mice, the mean alveolar volume was 3-fold greater in the SU1498-treated mice. RA alone had no effect on alveolar volume; however, in combination with SU1498, it reversed the effects of SU1498 to control levels (Figure 3A). Similarly, EPO significantly decreased the mean alveolar volume when coadministered to SU1498-treated animals as compared with animals that received SU1498 only (Figure 3B).
Figure 3.
Mean alveolar volumes in Day 21 mouse lung tissue. Graphical representations of calculated values for mean alveolar volumes of lungs of mice in the RA experiment (A) and in the EPO experiment (B). Mice were treated with vehicle only (Control), RA, SU1498, RA + SU1498, EPO, or EPO + SU1498. Values represent mean ± SEM (n = 5–8). *P < 0.05 versus control, RA, RA + SU1498; † P < 0.05 versus SU1498.
Transmission Electron Microscopy
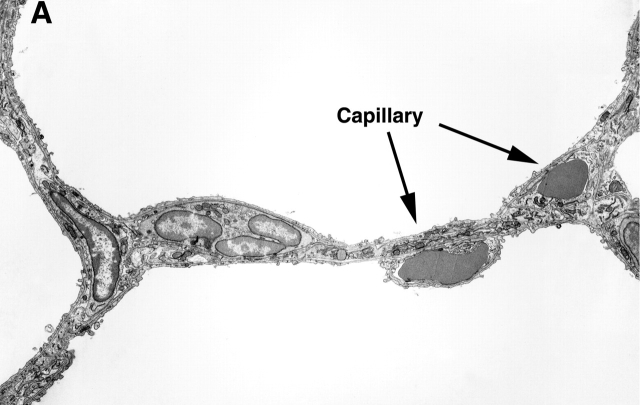
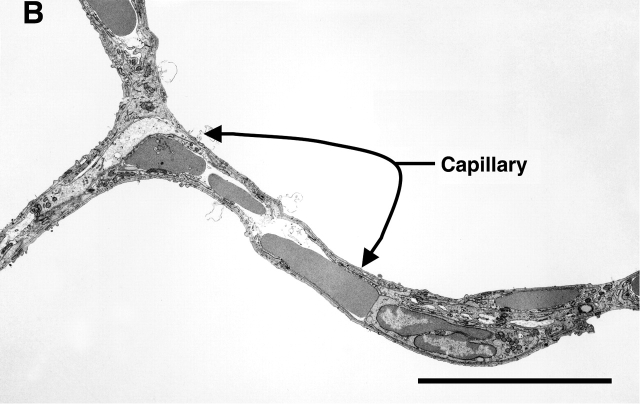
Transmission electron microscopic examination of the lungs was performed to compare the ultrastructure of the control versus SU1498-treated animals. Figure 4 includes representative photomicrographs of the two conditions. Capillaries were normal in structure in the distal lung of the control mouse (Figure 4A). In contrast, capillaries in the distal lung of the SU1498-treated mice were scarce, and capillaries that were present were dysmorphic and dilated, accommodating increased number of red blood cells in a single profile (Figure 4B). Type II cell ultrastructure appeared morphologically similar in all conditions (data not shown).
Figure 4.
Representative transmission electron micrographs of the lungs of Day 21 control (A) and SU1498-treated (B) mice. A demonstrates an alveolar wall with normal appearing capillaries that each contain a single red blood cell. In contrast, the alveolar wall of the SU1498-treated mice contains enlarged capillaries with multiple red blood cells demonstrated in the lumen (B). Representative capillaries are indicated by the arrows and labels. Original magnification: ×3,000; bar denotes 10 μm.
PECAM Immunoreactivity
We performed immunostaining for PECAM, an endothelial cell marker to identify capillaries in the distal lung. PECAM staining was observed as brown immunoreactivity under the light microscope. The VD of PECAM-positive tissue was significantly less in the SU1498-treated mice, suggestive of a decrease in the endothelial cell population in the developing lung. RA or EPO alone had no effect on the VD of the endothelial cell population; however, RA or EPO in combination with SU1498 tended to increase the PECAM-positive tissue when compared with SU1498 alone (not, however, to a statistically significant degree) (Tables 3A and 3B).
TABLE 3.
PECAM IMMUNOREACTIVITY IN THE LUNGS OF TREATED AND CONTROL MICE
| Groups | PECAM-Positive Tissue VD (%) |
|---|---|
| A. RA Experiment | |
| Control | 59.2 ± 0.0 |
| RA | 59.2 ± 0.2 |
| SU1498 | 48.7 ± 0.2* |
| RA+SU1498 | 51.5 ± 0.7 |
| B. EPO Experiment | |
| Control | 55.8 ± 1.5 |
| EPO | 56.1 ± 0.4 |
| SU1498 | 43.3 ± 0.8* |
| EPO+SU1498 | 50.3 ± 0.1 |
Definition of abbreviations: EPO, eryhtropoietin; PECAM, platelet endothelial cell adhesion molecule; RA, retinoic acid; VD, volume density.
Morphometric analysis was performed to determine the VD of lung tissue immunoreactive for PECAM, a marker for endothelial cells. Values are expressed in the table as the mean ± SEM (n = 3–8).
P < 0.05 versus control, RA, or EPO.
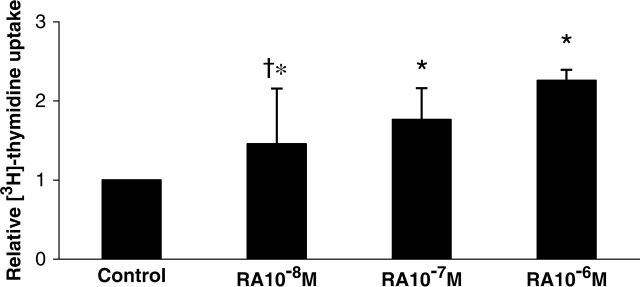
Proliferation Assay for Endothelial Cells
To determine the effect of RA on endothelial cell growth, human fetal lung capillary endothelial precursor cells were incubated in the absence or presence of different concentrations of RA. A concentration-dependent response in proliferation was demonstrated with RA (Figure 5). When cells were incubated in the presence of SU1498 alone or in combination with RA, there was no detectable uptake of [3H]-thymidine by the cells.
Figure 5.
Effect of RA on the proliferation of human fetal endothelial cells. Human fetal lung endothelial precursor cells were incubated in the absence or presence of RA (10−6 M, 10−7 M, 10−8 M). A dose response of increased [3H]-thymidine uptake with increasing concentrations of RA was observed. Values represent mean ± SEM (n = 5). *P < 0.05 versus control, † P < 0.05 versus 10−8 M.
DISCUSSION
This study introduces and characterizes a novel model of inhibited alveolarization in the newborn mouse created with a single dose of the angiogenesis/VEGFR2 inhibitor, SU1498. VEGF is a trophic factor that is indispensable for the survival of endothelial cells. Recently, decreased levels of VEGF have been associated with the development of chronic lung disease in prematurely born infants. For example, autopsy specimens of infants who died with BPD exhibit reductions in VEGF mRNA and protein and in the angiogenic receptors Flt-1 and Tie-2 (23). These reductions were associated with a decrease in PECAM-1 endothelial cell staining and an abnormal distribution of alveolar capillaries. Additionally, Lassus and colleagues observed that infants who later developed BPD had lower levels of VEGF in tracheal aspirates during their first days of life (24).
Although the conducting airways are formed by 20 wk of gestation, alveoli begin to appear only after ∼ 32 wk of gestation. Premature infants born at a gestational age of 24–27 wk are in the late canalicular stage of lung development and after treatment can have a high incidence of BPD (23). Simplified large alveoli, decreased secondary crest formation, and diminished PECAM staining representing a decrease in capillary density are observed in “new” BPD lung tissue and are consistent with an arrest of the canalicular phase of lung development in BPD (2). Dysmorphic dilated capillaries were observed in an autopsy study of infants with BPD performed by Thibeault and coworkers (25). Thus, our SU1498-treated mouse model reflects many of the characteristics of the “new BPD,” including large, simplified alveolar structures and sparse, dysmorphic, and dilated capillaries.
We evaluated the effects of RA and EPO on alveolar development in this BPD model. Treatment with either RA or EPO maintained alveolar surface area, mean alveolar volume, and volume density of endothelial cells in our SU1498-treated mouse model of inhibited alveolar development. Previous work by Massaro and colleagues demonstrated a decrease in alveolar surface area with dexamethasone treatment of newborn rats that was not reversed with RA treatment (12). The authors speculated that the absence of difference in intergroup surface area in rats treated with dexamethasone and RA was from RA inducing eruption of alveolar septa and regulating the distance between septa but not the ultimate size of the alveoli, which is determined by the length of the septa. In contrast, we demonstrated the maintenance of alveolar surface area with either RA or EPO. This difference is likely the result of the manner by which the two models of inhibited alveolar development were generated. Inhibition of angiogenesis is the mechanism most likely responsible for the phenotype of our model. Therefore, we speculate that both RA and EPO may have effects that maintain pulmonary vascular development in this model of impaired alveolarization. Treatment with dexamethasone may result in a more complex interruption of development that is not as readily reversed or maintained. Considering the restraints on lung volume conferred by the bony thorax, it is ideal to increase the surface area for greater gas exchange without changing the lung volume itself (26). RA and EPO achieve this requirement by inducing an increased number of smaller alveoli. Similar changes in the mean alveolar volume were evident with RA and EPO treatment in this study.
RA affects the gene expression of more than 300 gene products involved in rat lung morphogenesis and in extracellular matrix synthesis (27). Administration of teratogenic doses of RA or RA deficiency can cause marked lung dysmorphogenesis in rat embryos (28). Lung tissue RA is found in high concentrations at the time of maximum branching activity, and RA is crucial for rat lung bud initiation and distal-proximal patterning (26). RA regulates a number of signaling pathways involved in cell proliferation and development (29) and RA can act as an antioxidant, protecting the lungs from oxygen free radicals generated through direct oxygen exposure and from inflammatory cells (30). Daily treatment with RA in dexamethasone-treated rats results in a 50% increase in the number of alveoli formed (26). Newborn rats exposed to hyperoxia and treated with RA had increased alveolar septation after 4 wk of recovery when compared with animals exposed to hyperoxia alone (30). This demonstrates a role for RA in the preservation of the potential for septation beyond the normal developmental period.
RA also influences endothelial cells in mammalian blood vessel formation. Mice with deletion of retinaldehyde dehydrogenase 2, the enzyme required to produce active RA in the embryo, exhibit decreased endothelial cell maturation, disrupted vascular plexus remodeling, and lack of later stages of vessel assembly including mural cell differentiation (31). Such dilation and inappropriate patterning of blood vessels is associated with a lack of endothelial cell cycle progression from G1 to S phase in vivo (31). Pulmonary microvascular endothelial cells increase the expression of mRNA for cellular retinol binding protein-I in response to RA released by the lipid-laden interstitial fibroblasts (32). However, the ability of RA to act directly as a mitogenic factor for small vessel endothelial cells in the developing lung has not been previously described.
Our lab has previously isolated and characterized a population of capillary endothelial precursor cells from developing human lung (22). In the present study, we demonstrated that RA increases the proliferation of human fetal lung capillary endothelial precursor cells in a concentration-dependent manner. This finding is suggestive that the ability of RA to induce alveolar development may be mediated by direct effects on the endothelium of the developing lung.
We also evaluated the effects of EPO on the SU1498-treated mice. EPO is a known endothelial cell growth factor that is widely used clinically for the treatment and prevention of anemia in chronic renal diseases and anemia of prematurity (13). EPO also participates in other functions such as neuroprotection and development (13). We have recently demonstrated the presence of EPO receptors in the epithelium and mesenchyme of the developing human lung (15). We further demonstrated a role for EPO in promoting the differentiation of type II cells in the mid-trimester human lung. We showed that EPO can stimulate the proliferation of endothelial precursor cells derived from human fetal lung in a manner similar to that observed for RA in the present investigation (15). A decrease in mean alveolar volume in SU1498-treated mice comparable to the levels observed in the control animals with subsequent EPO treatment demonstrates the ability of EPO to maintain alveolar development in the face of VEGFR2 inhibition.
A limitation of our studies, particularly apparent in the EPO experiments, is the relatively small number of animals studied. The number did not allow us to analyze lung weights or to measure dynamic compliance as indicators of the effects of the various agents on lung growth or function. In addition, the small sample size puts some of our results at risk for type II errors where actual differences between study conditions may not be determined. One example is the body weight determinations in Table 1, where decreased body weight in the SU1498-treated animals compared with the controls is suspected but not supported statistically.
Though VEGF is widely regarded to be a growth factor specific for endothelial cells, we and others have demonstrated important roles for VEGF in epithelial cell function in the developing lung (33, 34). VEGFR2 is abundant in the epithelium of the human fetal lung and VEGF stimulates the proliferation of epithelial cells in human fetal lung in vitro (34). VEGF also induces the conversion of glycogen to surfactant in premature mice and exogenous VEGF will rescue prematurely born mice from respiratory distress (33). The importance of VEGF in type II cell function during recovery from oxygen injury has also been noted (35). These data demonstrate roles for VEGF in lung development beyond its proangiogenic properties.
Therefore, it is possible that inhibition of VEGFR2 with SU1498 inhibits alveolar development via effects on endothelial and epithelial cells in the developing lung. Although it is tempting to assume that RA and EPO independently act to maintain alveolar development in the face of VEGFR inhibition via maintenance of the endothelial cell population, each agent may also act to preserve the function of the developing epithelium. Additional investigation is warranted to discern the specific roles of these factors in lung development.
In conclusion, we are the first to demonstrate that RA and EPO can support the maintenance of alveolar development in the face of exposure to the antiangiogenic compound, SU1498, a VEGFR2 inhibitor. Each growth factor maintained the mean alveolar volume and endothelial cell volume densities in our model of inhibited alveolarization. Our data support the speculation that the endothelium plays a major role in alveolar development and that strategies aimed at maintaining the health of the endothelium in the developing lung may result in the prevention or treatment of BPD in premature infants.
Acknowledgments
The authors thank Melissa Darling for her technical analysis of the morphometric data, Aditya Mahajan for his technical assistance, and Paul Reimann for his superb assistance with the figures.
This work was supported by NIH grants R01-HL63228 (M.J.A.) and R01-HL62861 (J.M.S.).
Originally Published in Press as DOI: 10.1165/rcmb.2005-0050OC on September 1, 2005
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Korhonen PTO, Koivisto AM, Laippala P, Ikonen S. Frequency and risk factors in bronchopulmonary dysplasia in a cohort of very low birth weight infants. Early Hum Dev 1999;54:245–258. [DOI] [PubMed] [Google Scholar]
- 2.Coalson JJ. Pathology of new bronchopulmonary dysplasia. Semin Neonatol 2003;8:73–81. [DOI] [PubMed] [Google Scholar]
- 3.Abman SH. Bronchopulmonary dysplasia: “a vascular hypothesis.” Am J Respir Crit Care Med 2001;164:1755–1756. [DOI] [PubMed] [Google Scholar]
- 4.Gerber HP, Dixit V, Ferrara N. Vascular endothelial growth factor induces expression of the antiapoptotic proteins Bcl-2 and A1 in vascular endothelial cells. J Biol Chem 1998;273:13313–13316. [DOI] [PubMed] [Google Scholar]
- 5.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 2003;9:669–676. [DOI] [PubMed] [Google Scholar]
- 6.Dumont DJ, Fong GH, Puri MC, Gradwohl G, Alitalo K, Breitman ML. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev Dyn 1995;203:80–92. [DOI] [PubMed] [Google Scholar]
- 7.Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH. Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L600–L607. [DOI] [PubMed] [Google Scholar]
- 8.Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 1999;340:1962–1968. [DOI] [PubMed] [Google Scholar]
- 9.Biesalski HK, Nohr D. Importance of vitamin-A for lung function and development. Mol Aspects Med 2003;24:431–440. [DOI] [PubMed] [Google Scholar]
- 10.Maden M, Hind M. Retinoic acid in alveolar development, maintenance and regeneration. Philos Trans R Soc Lond B Biol Sci 2004;359:799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 2000;23:162–167. [DOI] [PubMed] [Google Scholar]
- 12.Massaro GD, Massaro D. Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol 2000;278:L955–L960. [DOI] [PubMed] [Google Scholar]
- 13.Juul SE. Nonerythropoietic roles of erythropoietin in the fetus and neonate. Clin Perinatol 2000;27:527–541. [DOI] [PubMed] [Google Scholar]
- 14.Juul SE, Yachnis AT, Christensen RD. Tissue distribution of erythropoietin and erythropoietin receptor in the developing human fetus. Early Hum Dev 1998;52:235–249. [DOI] [PubMed] [Google Scholar]
- 15.Acarregui MJRJ, Littig JL. Erythropoietin increases SP-A protein levels and capillary endothelial cell proliferation in human fetal lung in vitro. Pediatr Res 2003;53:35A. [Google Scholar]
- 16.Strawn LM, McMahon G, App H, Schreck R, Kuchler WR, Longhi MP, Hui TH, Tang C, Levitzki A, Gazit A, et al. Flk-1 as a target for tumor growth inhibition. Cancer Res 1996;56:3540–3545. [PubMed] [Google Scholar]
- 17.Young TE, Mangum B. Neofax: a manual of drugs used in neonatal care, 14th ed. Raleigh, NC: Acorn; 2001.
- 18.Amy RW, Bowes D, Burri PH, Haines J, Thurlbeck WM. Postnatal growth of the mouse lung. J Anat 1997;124:131–151. [PMC free article] [PubMed] [Google Scholar]
- 19.Beckstead JH. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J Histochem Cytochem 1994;42:1127–1134. [DOI] [PubMed] [Google Scholar]
- 20.Snyder JM, Jenkins-Moore M, Jackson SK, Goss KL, Dai HH, Bangsund PF, Giguere V, McGowan SE. Alveolarization in retinoic acid receptor-beta-deficient mice. Pediatr Res 2005;57:384–391. [DOI] [PubMed] [Google Scholar]
- 21.McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM. Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 2000;23:162–167. [DOI] [PubMed] [Google Scholar]
- 22.Acarregui MJ, England KM, Richman JT, Littig JL. Characterization of CD34+ cells isolated from human fetal lung. Am J Physiol Lung Cell Mol Physiol 2003;284:L395–L401. [DOI] [PubMed] [Google Scholar]
- 23.Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM. Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;164:1971–1980. [DOI] [PubMed] [Google Scholar]
- 24.Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 2001;164:1981–1987. [DOI] [PubMed] [Google Scholar]
- 25.Thibeault DW, Mabry SM, Norberg M, Truog WE, Ekekezie II. Lung microvascular adaptation in infants with chronic lung disease. Biol Neonate 2004;85:273–282. [DOI] [PubMed] [Google Scholar]
- 26.Massaro GD, Massaro D. Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol Lung Cell Mol Physiol 1996;270:L305–L310. [DOI] [PubMed] [Google Scholar]
- 27.Cardoso WV, Mitsialis SA, Brody JS, Williams MC. Retinoic acid alters the expression of pattern-related genes in the developing rat lung. Dev Dyn 1996;207:47–59. [DOI] [PubMed] [Google Scholar]
- 28.Antipatis C, Ashworth CJ, Grant G, Lea RG, Hay SM, Rees WD. Effects of maternal vitamin A status on fetal heart and lung: changes in expression of key developmental genes. Am J Physiol Lung Cell Mol Physiol 1998;275:L1184–L1191. [DOI] [PubMed] [Google Scholar]
- 29.Dalvin S, Komatsuzaki K, Anselmo MA, Kling DE, Schnitzer JJ, Kinane TB. Retinoic acid decreases fetal lung mesenchymal cell proliferation in vivo and in vitro. Dev Growth Differ 2004;46:275–282. [DOI] [PubMed] [Google Scholar]
- 30.Veness-Meehan KA, Pierce RA, Moats-Staats BM, Stiles AD. Retinoic acid attenuates O2-induced inhibition of lung septation. Am J Physiol Lung Cell Mol Physiol 2002;283:L971–L980. [DOI] [PubMed] [Google Scholar]
- 31.Lai L, Bohnsack BL, Niederreither K, Hirschi KK. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development 2003;130:6465–6474. [DOI] [PubMed] [Google Scholar]
- 32.Dirami G, Massaro GD, Clerch LB, Ryan US, Reczek PR, Massaro D. Lung retinol storing cells synthesize and secrete retinoic acid, an inducer of alveolus formation. Am J Physiol Lung Cell Mol Physiol 2004;286:L249–L256. [DOI] [PubMed] [Google Scholar]
- 33.Compernolle V, Brusselmans K, Acker T, Hoet P, Tjwa M, Beck H, Plaisance S, Dor Y, Keshet E, Lupu F, et al. Loss of HIF-2alpha and inhibition of VEGF impair fetal lung maturation, whereas treatment with VEGF prevents fatal respiratory distress in premature mice. Nat Med 2002;8:702–710. [DOI] [PubMed] [Google Scholar]
- 34.Brown KR, England KM, Goss KL, Snyder JM, Acarregui MJ. VEGF induces airway epithelial cell proliferation in human fetal lung in vitro. Am J Physiol Lung Cell Mol Physiol 2001;281:L1001–L1010. [DOI] [PubMed] [Google Scholar]
- 35.Maniscalco WM, Watkins RH, Finkelstein JN, Campbell MH. Vascular endothelial growth factor mRNA increases in alveolar epithelial cells during recovery from oxygen injury. Am J Respir Cell Mol Biol 1995;13:377–386. [DOI] [PubMed] [Google Scholar]