Abstract
Host defenses against Histoplasma capsulatum require the action of several cytokines. Here, we explored the influence of interleukin (IL)–17 and IL-23 on immunity to H. capsulatum infection in mice. In lungs, synthesis of IL-17 was up-regulated during acute infection, and the cells producing it were predominantly CD3+. Neutralization of IL-17A blunted fungal clearance but did not promote progressive infection. Decreased inflammatory cell recruitment and increased levels of IL-6 and IL-10 were associated with impaired clearance. To determine whether the elevated cytokine levels were important in the action of IL-17A, IL-6−/− or IL-10−/− mice were treated with anti–IL-17A; neutralization of IL-17A did not alter fungal burden in either group of knockout mice. We explored the relationship between IL-17 and IL-23 because they have been reported to form a regulatory network. IL-23 transcription and protein level were increased in the lungs of infected mice. Mice producing IL-23 in the absence of IL-12 manifested prolonged survival that was IL-17 dependent. Thus, IL-17 is requisite for the generation of optimal inflammatory and protective responses. Generation of functional IL-17+ cells is dependent on IL-6 and IL-10. Our findings also establish the existence a regulatory IL-17/IL-23 axis in histoplasmosis.
Human infection with the eukaryotic pathogen Histoplasma capsulatum is accidental and develops when conidia and mycelial fragments become airborne from soil and are inhaled into the lungs. Transition from the mycelial to the yeast phase is a central event in pathogenesis. Once it occurs, yeasts seek intracellular residence and can be detected in several cell populations, including macrophages, dendritic cells, and neutrophils [1, 2]. Invasion of the lungs by this fungus initiates a complex inflammatory response that is regulated by cytokines and chemokines. The net result is a restriction of growth of the fungus, although tissues are not sterilized.
Perturbation of the cytokine response can lead to exacerbation of infection. Several cytokines are crucial for host defenses against H. capsulatum infection, including tumor necrosis factor (TNF)–α, interferon (IFN)–γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), and interleukin (IL)–1β [3–10]. In mice, the absence of any of these cytokines converts an otherwise nonlethal infection into a lethal one. An interconnection among these cytokines during the host response is often absent. Neutralization of one does not necessarily alter the production of the others that are needed for host protection. An exception is that neutralization of GM-CSF reduces TNF-α and IFN-γ levels in the lungs of infected mice [4].
The orchestration of an appropriate inflammatory response is an important component of protective immunity to H. capsulatum infection. The IL-17 family of cytokines (in particular, IL-17A) has been shown to possess proinflammatory properties and to regulate the balance between the type 1 T helper (Th1) response and the type 2 T helper response [11–13]. Recently, IL-17 has been detected in hepatic granulomas of mice with histoplasmosis [14].
Prompted by the above-mentioned studies, we postulated that IL-17 would be requisite for clearance of this fungus. We found that IL-17 was up-regulated in the lungs of mice during acute infection; that monoclonal antibody (MAb) to IL-17 perturbed the inflammatory response and was associated with a delayed clearance of the fungus during primary, but not secondary, infection; and that the absence of IL-17 was not associated with progressive infection. Additional experiments revealed that IL-23 could prolong the survival of mice in the absence of IL-12. This protective effect was dependent on IL-17. Thus, the IL-17/IL-23 axis promotes immunity to H. capsulatum infection.
METHODS
Mice
C57BL/6, IL-6−/−, IL-10−/−, IL-12p40−/−, and IL-12p35−/− mice were purchased from Jackson Laboratory. Mice were housed in isolator cages and maintained by the Department of Laboratory Animal Medicine, University of Cincinnati, which is accredited by the American Association for Accreditation of Laboratory Animal Medicine. All animal experiments were conducted in accordance with the Animal Welfare Act guidelines of the National Institutes of Health.
Preparation of H. capsulatum and infection of mice
H. capsulatum yeast cells (strain G217B) were prepared as described elsewhere [3]. To produce primary infection, mice were intranasally inoculated with 2 × 106 or 5 × 106 H. capsulatum yeast cells in 30 µL of Hanks’ balanced salt solution (HBSS). For secondary histoplasmosis, mice were intranasally inoculated with 1 × 104 yeast cells; 6–8 weeks later, mice were intranasally rechallenged with 2 × 106 yeast cells.
Organ culture for H. capsulatum
Recovery of H. capsulatum was performed as described elsewhere [3]. Fungal burden was expressed as the mean number (with standard errors [SEs]) of colony-forming units per organ. The limit of detection was 1 × 102 cfu.
MAb
Rat anti–mouse TNF-α (clone XT-22.1) was produced by the National Cell Culture. Rat anti–mouse IL-17A was purchased from R&D Systems.
Treatment with MAb
Mice were intraperitoneally injected with MAb on the day of infection and each week thereafter; 1 and 200 µg of MAb to TNF-α and IL-17A were administered, respectively. Control mice received rat IgG.
Preparation of lung leukocytes
Lungs were dissociated in 5 mL of HBSS by means of a gentleMACS Dissociator instrument (Miltenyi Biotec) and treated for 30 min with 1 mg/mL collagenase plus 30 µg/mL DNase at 37°C. The solution was filtered through 60-µm nylon mesh and washed 3 times with HBSS. Leukocytes (>97% CD45+) were isolated by gradient density centrifugation using Lympholyte-M medium (Cedar-lane Laboratories).
Flow cytometry
The following MAbs were purchased from BD Biosciences: fluorescein isothiocyanate–conjugated CD11c, CD69, CD8α, and I-Ab; phycoerythrin-conjugated Mac-3 and Ly6G; allophycocyanin-conjugated CD11c, CD3∊, CD62L, and CD25; and peridin-chlorophyll protein–conjugated CD4 and CD11b. Two million cells were incubated with 0.5 µg of MAb in staining buffer (1% bovine serum albumin in phosphate buffered saline) for 10 min at 4°C. Cells were washed in staining buffer, and fluorescence was measured using a FACScaliber flow cytometer (BD Biosciences). Between 50,000 and 100,000 events were counted.
Intracytoplasmic staining
Cells were stained with MAb to CD69, CD4, CD8α, and CD25, followed by permeabilization of cells and staining with phycoerythrin-conjugated anti–IL-17A (BD Pharmingen) as described elsewhere [15].
RNA isolation and cDNA synthesis
RNA was isolated from mouse lungs by means of TRIzol reagent (Invitrogen) and cDNA synthesized as described elsewhere [5].
Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
qRT-PCR for IL-17A, IL-17F, and IL-23 was performed using TaqMan primers (Applied Biosystems). Samples were analyzed on an ABI Prism 7500 instrument (Applied Biosystems). Transcript quantities were standardized to expression in an uninfected lung. In each experiment, the hypoxanthine phosphoribosyl transferase gene was analyzed, and data were expressed as the log10 of relative quantification. The conditions for amplification were: 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min.
Cytokine analysis of lung homogenates
Lung homogenates were prepared as described elsewhere [3], and levels of IL-4, IL-6, IL-10, IFN-γ, IL-17, IL-23, GM-CSF, and TNF-α were quantified by enzyme-linked immunosorbent assay (ELISA; R&D Systems).
Statistical analysis
Analysis of variance (ANOVA) was used to compare groups. The log rank sum test was used to analyze survival. Differences with P < .05 were considered statistically significant.
RESULTS
IL-17 in murine histoplasmosis
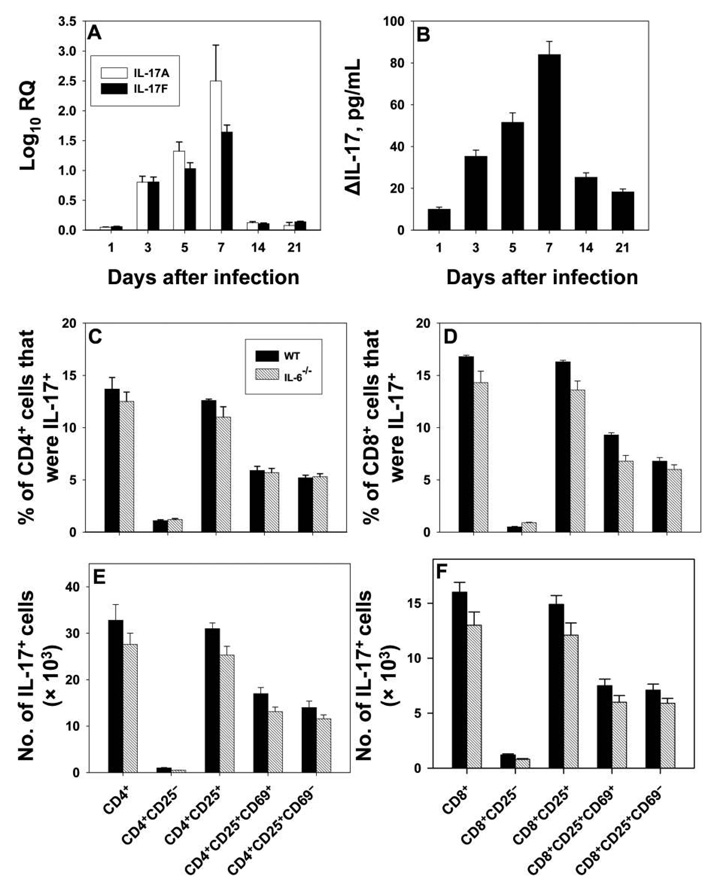
The generation of IL-17 in the lungs of mice infected with 2 × 106 H. capsulatum yeast cells was examined. Expression of IL-17A and IL-17F was detectable by qRT-PCR on day 3, peaked on day 7, and declined abruptly thereafter (figure 1A). IL-17 protein in lung homogenates was assessed by ELISA; peak production occurred on day 7 and declined rapidly afterward (figure 1B).
Figure 1.
Generation of interleukin (IL)–17 during the course of murine pulmonary histoplasmosis. Groups of mice were intranasally inoculated with 2 × 106 yeast cells, and lungs were analyzed at serial intervals for expression of IL-17A and IL-17F. Panel A shows the results of analysis by quantitative reverse-transcription polymerase chain reaction (qRT-PCR); data are expressed as the mean log10 relative quantification (RQ) for 6 mice. IL-17 was also assessed in lung homogenates by enzyme-linked immunosorbent assay, and results are shown in panel B. Data are expressed as ΔIL-17, which was calculated by subtracting the values for uninfected lungs from those for infected lungs (6–8 mice per group). Analysis of IL-17+ cells is shown in panels C–F. Cells from the lungs of wild-type (WT) and IL-6−/− mice were stained with monoclonal antibody to CD4, CD8, CD25, CD69, and IL-17. The proportion of cells expressing these markers for CD4+ or CD8+ cells is shown panels C and D, respectively; the absolute no. of cells in CD4+ or CD8+ cell subsets is shown in panels E and F, respectively. Results are means for 6–9 mice. In all panels, bars show standard errors.
Profile of IL-17+ cells in the lungs of mice with pulmonary histoplasmosis
Among lung leukocytes, 4.1% ± 0.7% were CD3+IL-17+, and between 10% and 15% of T cells generated IL-17. Non-CD3+ cells constituted a smaller fraction of the total, and all were Mac-3+CD11b+. The mean ± SE number of CD3+IL-17+ cells was 6.4 × 104 ± 0.5 × 104 (n = 12). IL-17 synthesis was equally distributed between CD4+ and CD8+ cells proportionally, although the absolute numbers differed (figure 1C–1F). In both subsets, the CD25+ population accounted for >90% of the IL-17+ cells, and these were equally distributed between CD25+CD69+ and CD25+CD69− cells.
Blunting of immunity by treatment with MAb to IL-17A
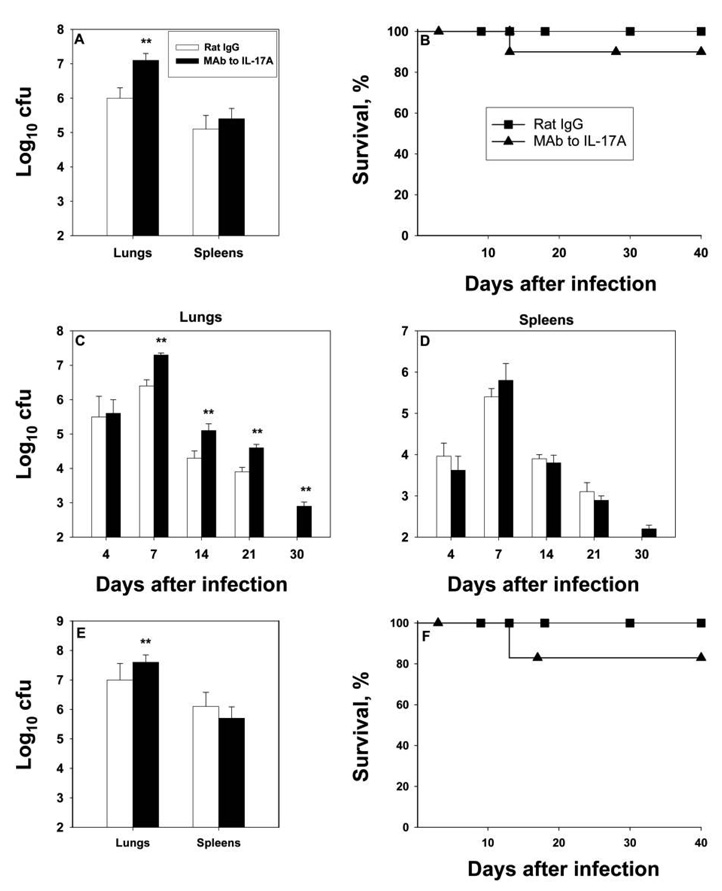
Infected mice were treated with anti–IL-17A or control antibody, and fungal burden was assessed on day 7. Neutralization of IL-17A blunted clearance (figure 2A). Because anti–IL-17A diminished the capacity to clear infection at its peak, we asked whether continued neutralization would affect survival. Ninety percent of mice given anti–IL-17A survived for up to 40 days (figure 2B). This finding prompted us to examine the fungal burden over time. Mice were infected and given MAb to IL-17A, and fungal burden was determined at intervals. The number of H. capsulatum in the lungs and spleens was similar between infected control mice and anti–IL-17A recipients on day 4. By day 7, the burden was higher in the lungs of mice whose IL-17A was neutralized and remained elevated relative to that in control mice on days 14, 21, and 30 (figure 2C and 2D). In this set of experiments, 1 mouse died on day 13. Surviving mice given MAb to IL-17A manifested a decrement in fungal burden over time. Despite the increase in colony-forming unit counts on day 7, the mice were able to contain the infection.
Figure 2.
Effect of monoclonal antibody (MAb) to interleukin (IL)–17A on fungal burden in murine pulmonary histoplasmosis. Groups of mice given 200 µg of MAb to IL-17A or control antibody (rat immunoglobulin [Ig] G]) were intranasally inoculated with 2 × 106 yeast cells, and lungs and spleens were cultured on day 7 of infection (A). Data are means for 6–8 mice; bars show standard errors (SEs). Panel B shows survival curves for 10 mice per group. These mice received antibodies on the day of infection and each week thereafter. Panels C and D depict the fungal burden during the course of murine histoplasmosis in mice that received 200 µg of rat IgG or MAb to IL-17A on the day of infection. Data are means for 6–12 mice; bars show SEs. Results are pooled from 2 independent experiments. Panel E illustrates the fungal burden in mice (6 per group) intranasally challenged with 5 × 106 yeast cells, and panel F shows survival curves for mice given this inoculum (6 per group; symbols are the same as for panel B). **P < .01 (analysis of variance).
To determine whether these results were a consequence of inoculum size, mice were challenged with a higher but nonlethal inoculum (5 × 106 yeast cells) and given control antibody or MAb to IL-17A. On day 7, neutralization of IL-17A caused a modest but statistically significant increase in colony-forming unit counts (P < .05, ANOVA) (figure 2E). There were no statistically significant differences in survival (P ≥ .05, log rank sum test) (figure 2F).
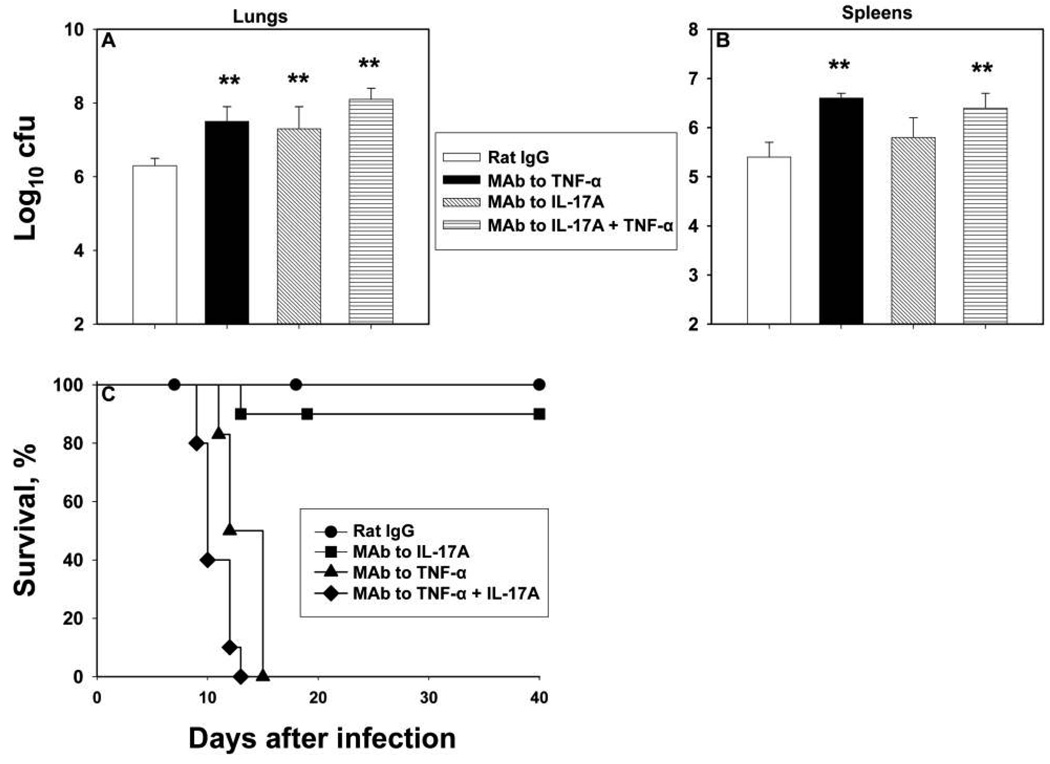
An elevation in colony-forming unit counts of the magnitude that we observed in IL-17A–neutralized mice is predictive of a lethal outcome. This supposition stems from observations made in mice given MAb to TNF-α, GM-CSF, or IFN-γ [3, 4, 16]. Therefore, we repeated the experiments using MAb to TNF-α and MAb to IL-17A. We also asked whether neutralization of IL-17A altered the response in mice given MAb to TNF-α. Treatment with MAb to IL-17A or to TNF-α induced a similar increase in colony-forming unit counts on day 7 (figure 3A and 3B); treatment with both caused a slight increase above that of each alone. Neutralization of TNF-α or of TNF-α plus IL-17A was lethal (figure 3C).
Figure 3.
Impact of neutralization of interleukin (IL)–17A and/or tumor necrosis factor (TNF)–α on murine histoplasmosis. Groups of mice were intranasally inoculated with 2 × 106 yeast cells and given rat immunoglobulin (Ig) G (control) or monoclonal antibody (MAb) to IL-17A, TNF-α, or both. Mice were killed on day 7, and mean colony-forming unit counts were determined in lungs (A) and spleens (B) from 6–8 mice per group; bars represent standard errors. Panel C shows survival curves for mice administered MAb to IL-17A, TNF-α, or TNF-α plus IL-17A (6–10 mice per group). **P < .01 (analysis of variance).
To determine whether IL-17 altered the host response in secondary histoplasmosis, previously infected mice were re-challenged with 2 × 106 yeast cells, and fungal burden and survival were assessed. On day 7 of infection, the log10 colony-forming unit counts in the lungs (mean ± SE, 5.1 ± 0.3) and spleens (mean ± SE, 3.6 ± 0.3) of infected control mice (n = 6) did not differ (P ≥ .05, ANOVA) from those in the lungs (mean ± SE, 4.9 ± 0.4) and spleens (mean ± SE, 3.7 ± 0.3) of mice treated with MAb to IL-17A (n = 6). All mice survived for 40 days.
Inflammatory response in the lungs of mice given MAb to IL-17A
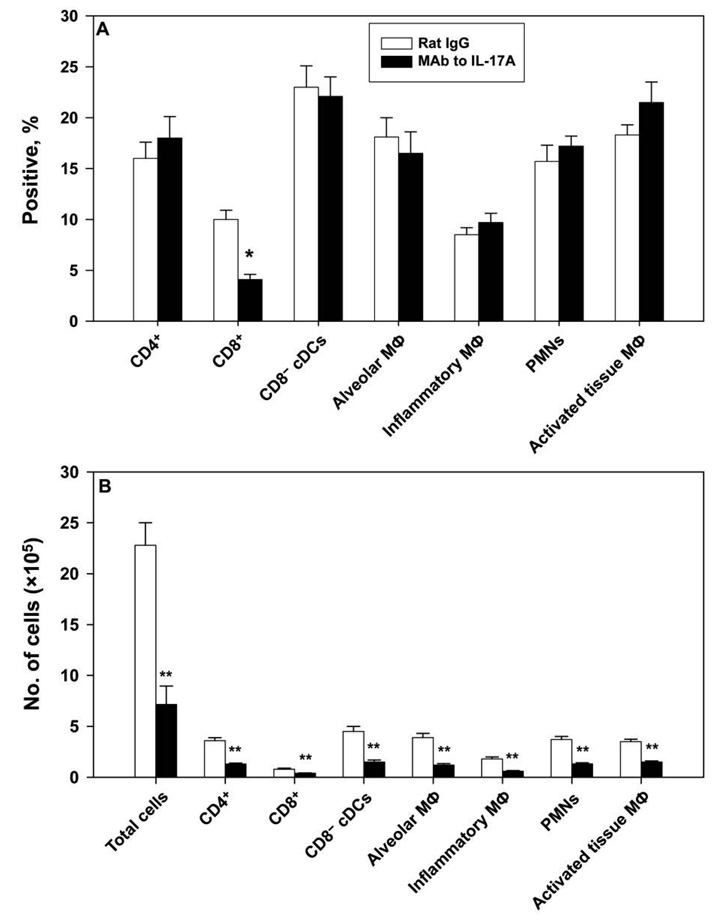
On day 7 of infection, we analyzed the inflammatory response in the lungs of mice given anti–IL-17A or control antibody. We chose this day because it represented the peak in the disparity in fungal burden. Proportionally, the CD8+ cell population in recipients of MAb to IL-17A was statistically significantly reduced, compared with that in control mice (P < .05, ANOVA) (figure 4A). Mice given MAb to IL-17A exhibited a markedly decreased number of total leukocytes in the lungs (P < .01, ANOVA). Consequently, the number of each of the inflammatory cell populations examined was statistically significantly depressed (P < .01, ANOVA with Bonferroni’s correction) (figure 4B).
Figure 4.
Analysis of the inflammatory cell infiltrate in mice given monoclonal antibody (MAb) to interleukin (IL)–17A. Leukocytes from lungs of mice infected with 2 × 106 yeast cells were analyzed on day 7 by flow cytometry. Panel A shows proportions of cells, and panel B shows absolute nos. of cells. Definitions of the inflammatory cell phenotypes are as follows: for polymorphonuclear leukocytes (PMNs), Ly-6GhighCD11b+CD11c−; for conventional CD8α− dendritic cells (CD8− cDCs), CD11c+CD11b+MHC-IIhigh; for alveolar macrophages, autofluorescent and CD11c+Mac-3+CD11b+ or CD11b−; for inflammatory macrophages, CD11bintermediateCD11c−CD62L+; and for activated tissue macrophages, CD11c−Mac3+CD11b− or CD11b+. Data are expressed as means for 6–7 mice per group; bars represent standard errors. **P < .01 (analysis of variance). Mϕ, macrophages.
Cytokine response in the lungs
We examined the generation of TNF-α, IFN-γ, GM-CSF, IL-4, IL-6, and IL-10 in the lungs of infected control mice and recipients of anti–IL-17A. There were no marked disturbances in GM-CSF, IFN-γ, or TNF-α production. However, statistically significant increases in IL-10 and IL-6 levels in mice receiving MAb to IL-17A were apparent (table 1).
Table 1.
Cytokine responses in the lungs of Histoplasma capsulatum–infected mice given monoclonal antibody (MAb) to interleukin (IL)–17 or control antibody.
| Cytokine | Rat IgG (control) | IL-17 MAb |
|---|---|---|
| GM-CSF | 313 ± 54 | 401 ± 54 |
| IFN-γ | 49,762 ± 7251 | 44,500 ± 6635 |
| TNF-α | 4014 ± 841 | 3501 ± 492 |
| IL-4 | 64 ± 5 | 82 ± 10 |
| IL-10 | 340 ± 52 | 509 ± 24a |
| IL-6 | 332 ± 87 | 748 ± 113a |
NOTE. Data are mean cytokine levels with standard errors, given in picograms per milliliter. Cytokine levels were assessed by enzyme-linked immunosorbent assay in homogenates of lungs from mice infected for 7 days (8 mice per group). GM-CSF, granulocyte-macrophage colony-stimulating factor; IFN, interferon; Ig, immunoglobulin; TNF, tumor necrosis factor.
P < .05 (t test).
To determine whether the increase in the IL-10 level was biologically relevant, IL-10−/− mice were given rat IgG or MAb to IL-17A and infected with H. capsulatum. Neutralization of IL-17A did not alter the fungal burden on day 7 of infection in IL-10−/− mice, although it did affect wild-type (WT) mice. The log10 colony-forming unit counts in the lungs (n = 6–7) of WT mice given rat IgG (mean ± SE, 6.3 ± 0.3) were statistically significantly lower than those in the lungs of WT mice given MAb to IL-17A (mean ± SE, 7.5 ± 0.5) (P < .01, ANOVA). In contrast, the fungal burden in the lungs of IL-10−/− mice administered rat IgG (mean ± SE, 6.4 ± 0.2) did not differ from that of IL-10−/− mice administered MAb to IL-17A (mean ± SE, 6.7 ± 0.5) (P ≥ .05, ANOVA). No differences were observed for spleens between the groups (data not shown).
IL-6 and IL-17
The generation of IL-17 by T cells is reported to be partially dependent on the presence of IL-6, although an alternate pathway of induction exists [17, 18]. Surprisingly, the proportion and number of cells in IL-6−/− mice that generated IL-17 was not much different than those in WT mice (figure 1C–1F). Because IL-6 levels were elevated in mice given anti–IL-17A, we ascertained whether IL-17 affected host resistance in IL-6−/− mice. MAb to IL-17A failed to exacerbate infection in IL-6−/− mice. Log10 colony-forming unit counts in IL-6−/− mice given control antibody (mean ± SE, 7.0 ± 0.4; n = 8) were similar to those in mice given MAb to IL-17A (mean ± SE, 7.2 ± 0.6). No difference was observed for spleens (data not shown).
IL-17/IL-23 axis
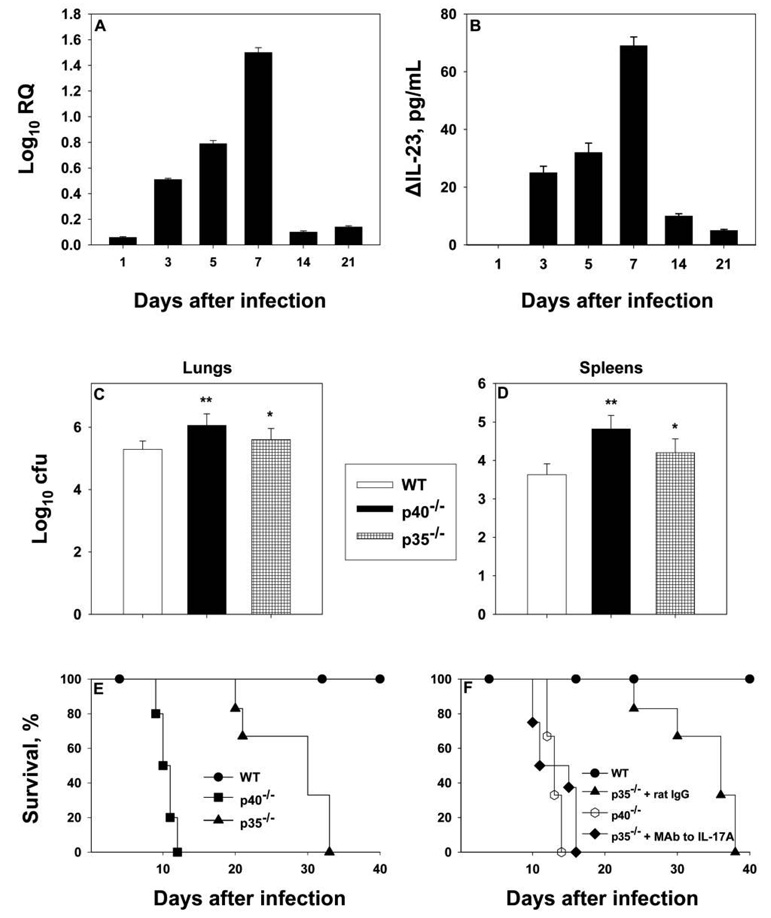
The IL-17/IL-23 network is important in the control of several infectious agents, including Candida albicans and Aspergillus fumigatus [19]. We initially asked whether IL-23 was produced during infection. In the lungs of infected mice, IL-23 levels peaked on day 7 of infection and decreased thereafter, as assessed by qRT-PCR (figure 5A). As assessed by ELISA, IL-23 levels were undetectable on day 1 but gradually increased on days 3–7 (figure 5B).
Figure 5.
Role played by interleukin (IL)–23. Groups of mice were intranasally inoculated with 2 × 106 yeast cells, and lungs were analyzed at serial intervals for expression of IL-23 by quantitative reverse-transcription polymerase chain reaction (qRT-PCR) (A) or enzyme-linked immunosorbent assay (ELISA) (B). Data for qRT-PCR and ELISA are expressed the mean log10 relative quantification (RQ) or the mean ΔIL-23 (calculated by subtracting the values for uninfected lungs from those for infected lungs), respectively; bars represent standard errors (5–6 mice per group). Mean colony-forming units were determined in lungs (C) and spleens (D) of 6–8 mice per group; bars represent standard errors. Panel E displays survival curves for wild-type (WT), IL-12p40−/−, and IL-12p35−/− mice (5 per group). Panel F illustrates the effect of MAb to IL-17A on IL-12p35−/− mice (6–8 per group). *P < .05 (analysis of variance); **P < .01 (analysis of variance).
Administration of neutralizing MAb to the p40 subunit of IL-12 impairs protective immunity to H. capsulatum infection [9, 20]. This finding was interpreted to signify the importance of IL-12 in host defenses. However, these studies were conducted before the discovery of IL-23, and it is now apparent that neutralizing the activity of p40 impairs both IL-12 and IL-23. Therefore, we examined whether IL-23 was important to the host defense. IL-12p35−/− mice (lacking IL-12) and IL-12p40−/− mice (lacking IL-12 and IL-23) were intranasally inoculated with H. capsulatum, and fungal burden on day 7 of infection and survival were analyzed. Both groups had elevated colony-forming unit counts in lungs and spleens on day 7 of infection (figure 5C and 5D), but p35−/− mice exhibited prolonged survival, compared with p40−/− mice (P < .01, log rank sum test) (figure 5E). To examine whether IL-17 was involved in the prolonged survival of mice that could generate IL-23, infected p35−/− mice were treated with MAb to IL-17A. Survival was statistically significantly truncated in mice given MAb to IL-17A (P < .01, log rank sum test) (figure 5F).
DISCUSSION
Here, we have demonstrated that IL-17 is necessary for generation of an optimal inflammatory and protective immune response in murine pulmonary histoplasmosis. However, neutralization of 17A did not abolish immunity in the intact host, suggesting that it may be dispensable for protective immunity. In contrast, IL-17 was requisite for the protective effect induced by IL-23 in the absence of IL-12. The inability to profoundly impact host resistance was independent of inoculum size. Mice given anti–IL-17A and a greater number of yeast cells manifested an elevated fungal burden but not higher mortality. We did not assess lethal inocula in these experiments because neutralization was shown only to exacerbate, rather than ameliorate, fungal burden. The detrimental effect of anti–IL-17A was accompanied by increases in IL-10 and IL-6 and a sharp decrease (~70%) in the number of leukocytes in the lungs. Despite alterations, the overwhelming majority of mice survived.
IL-17 constitutes a cytokine family that includes A, B, C, D (IL-27), E (IL-25), and F. As assessed by qRT-PCR, the temporal expression of IL-17A and IL-17F in infected lungs was similar, with a peak on day 7. IL-17A and IL-17F can exist as A or F homodimers or as A-F heterodimers [21]. The majority of biological activity resides with the A homodimers or A-F heterodimers; both the A homodimers and the A-F heterodimers are inactivated by MAb to IL-17A [21]. An explanation for the finding that mice did not die of infection is that IL-17A and IL-17F cooperate to confer protection against H. capsulatum. If both cytokines were antagonized, the effect may have been more pronounced. This assertion is unlikely, given that the effect of anti–IL-17A on day 7 of infection was as striking as that induced by MAb to TNF-α, yet mice given the latter did not recover. Moreover, anti–IL-17A would attenuate the action of IL-17A and A-F heterodimers [21]. The role played by IL-17F in histoplasmosis requires additional investigation.
IL-17 influences protective immunity to numerous pathogens, including bacteria, fungi, and protozoa [19, 22–28]. Our model contrasts with many infectious models in which the absence of IL-17 induces a profound loss in immunity. The influence of IL-17A neutralization on fungal burden was not observed earlier than day 7, when cellular immunity is engaged [29]. On day 4, colony-forming unit counts were similar between mice given MAb to IL-17A and control mice. However, these data do not exclude the possibility that neutralization of IL-17A affects inflammatory cell recruitment before day 7. The findings indicate that innate immunity does not rely on IL-17 to control H. capsulatum infection, whereas cellular immunity does. In contradistinction, neutralization of IL-17A did not thwart the efficacy of protective immunity in secondary infection. The absence of IL-17 is dispensable when Th1 immunity preexists. These results contrast with the requirement for IL-17 in vaccine-induced immunity to Mycobacterium tuberculosis and Bordetella pertussis infection [30, 31].
As a molecular effector, IL-17 possesses a number of functions. Recognized for its ability to mobilize neutrophils, this cytokine also enhances the development of osteoclasts, the expression of β-defensins, and the antifungal activity of neutrophils [12, 32, 33]. It is a central component of pathologic inflammation manifest in experimental autoimmune encephalomyelitis, rheumatoid arthritis, and inflammatory bowel disease [17, 34–36]. Neutralization of IL-17A in mice infected with H. capsulatum was accompanied by an unequivocal perturbation in the inflammatory response, but this decrement was not accompanied by rapidly progressive infection. Thus, the host is capable of withstanding a significant loss of inflammatory cells without a concomitant major impairment in immunity.
IL-17 was originally identified as a product of CD4+ activated memory T cells, but the number of sources has subsequently grown [37]—γ/δ T cells, CD8+ T cells, and alveolar macrophages also produce this cytokine [23, 38–42]. In our model, T cells were the predominant, although not exclusive, origin of IL-17. Moreover, activated CD4+ and CD8+ T cells, as denoted by the expression of CD25 and/or CD69, were IL-17+. It is unlikely that CD4+CD25+ cells are regulatory T cells, given that Foxp3, the transcription factor that drives the development of these cells, is reciprocally expressed with the transcription factor RORγt, which promotes the generation of IL-17+ cells [43]. Although the number of CD4+IL-17+ cells exceeded that of CD8+IL-17+ cells, the functional differences between these 2 populations remain to be determined.
Both IL-6 and transforming growth factor–β chiefly dictate the induction of type 17 T helper cells from naive T cells, although IL-21 can substitute for IL-6 [17, 18]. IL-17A neutralization caused IL-6 levels to increase, indicating that IL-17 influenced the generation of IL-6 in histoplasmosis. Surprisingly, the absence of IL-6 did not alter the emergence of IL-17+ cells in the lungs of mice with histoplasmosis; the number of these cells was only slightly reduced in the knockout mice. The emergence of IL-17+ cells in infected IL-6−/− mice is possibly a consequence of IL-21, the alternative cytokine known to evoke these cells [18]. Despite their presence, IL-17+ cells appear to be nonfunctional in an IL-6–deficient environment. The findings suggest that alternate mediators may induce IL-17+ cells in histoplasmosis but that they do not contribute to immunity. More importantly, the findings indicate that IL-6–dependent development of functional IL-17+ cells, in conjunction with Th1 cells, promote protective immunity in pulmonary histoplasmosis.
Neutralization of IL-17A did not induce a dramatic alteration in the cytokine profile in the lungs of infected mice. The only major disturbance was an increase in the IL-10 level, which is known to impair protective immunity to H. capsulatum [44]. The levels of IFN-γ, TNF-α, and GM-CSF, all effectors that are necessary for protective immunity, were not different from those in control mice. This finding contrasts with that found using models of C. albicans and A. fumigatus infection, in which IL-17A negatively regulates Th1 responses [19, 45, 46]. The elevation in the IL-10 level was functionally important, given that neutralization of IL-17A in IL-10−/− mice failed to increase fungal burden. Although IL-10 is known to depress IL-17 levels, scant literature on the influence of IL-17 on IL-10 exists [47]. The paucity of data intimates that these 2 cytokines may counterregulate one another.
IL-17 interacts with TNF-α to regulate the generation of cytokines or chemokines [48]. We endeavored to determine whether IL-17 altered the host response in H. capsulatum–infected mice given MAb to TNF-α. We hypothesized that neutralization of IL-17A might mitigate the severity of infection found in mice lacking TNF-α. However, the converse occurred, albeit slightly. Thus, TNF-α and IL-17 are not antagonistic but rather work in concert in host defenses.
Previous studies had shown that neutralization with a MAb to the p40 protein in mice produces progressive histoplasmosis [9, 20]. The original interpretation was that IL-12 was essential, but this was asserted before the discovery of IL-23, which shares p40 with IL-12 and contains a unique p19 protein [49]. We revisited our earlier results to determine whether IL-23 contributed to the protective immune response to H. capsulatum. Although mice lacking p40 or p35 died, the survival time in mice lacking p35 was extended, compared with that in mice lacking p40. This finding demonstrates that IL-23 can promote immunity in mice. Another key finding was that prolonged survival in IL-23–producing mice was critically dependent on IL-17A. These results unequivocally indicate that the IL-17/IL-23 axis influences the host response to H. capsulatum. The results extend previous findings regarding the significance of the IL-17/IL-23 pathway to another fungal pathogen, C. albicans [19].
In summary, IL-17 and IL-23 influence the protective response to this fungus. IL-17 regulates the inflammatory response and optimizes host control of infection. IL-17 contributes to immunity only when IL-10 and IL-6 are present. Although a lack of IL-17 did not lead to unrestrained infection, it is possible that deficiencies in IL-17 and other cytokines (such as TNF-α) may tip the balance of immunity in favor of the fungus. Our findings emphasize the complexity of the host response to this eukaryotic pathogen and provide insights into the molecular effectors that confer an effective immune response.
Acknowledgments
Financial support: Department of Veterans Affairs (merit review); National Institute of Allergy and Infectious Diseases (grants AI-73337, AI-34361, and AI-61298).
Footnotes
Potential conflicts of interest: G.S.D. has been a consultant for Amgen and Centorcorp. R.S.G. reports no potential conflicts.
References
- 1.Deepe GS, Jr, Gibbons RS, Smulian AG. Histoplasma capsulatum manifests preferential invasion of phagocytic subpopulations in murine lungs. J Leukoc Biol. 2008;84:669–678. doi: 10.1189/jlb.0308154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deepe GS., Jr . Principles and practices of infectious diseases. 6th ed. Vol 2. In: Mandell GL, Bennett JE, Dolin R, editors. Histoplasma capsulatum. Philadelphia: Elsevier Churchill Livingstone; 2004. pp. 3012–3026. [Google Scholar]
- 3.Allendoerfer R, Deepe GS., Jr Blockade of endogenous TNF-α exacerbates primary and secondary pulmonary histoplasmosis by differential mechanisms. J Immunol. 1998;160:6072–6082. [PubMed] [Google Scholar]
- 4.Deepe GS, Jr, Gibbons R, Woodward E. Neutralization of endogenous granulocyte-macrophage colony-stimulating factor subverts the protective immune response to Histoplasma capsulatum. J Immunol. 1999;163:4985–4993. [PubMed] [Google Scholar]
- 5.Deepe GS, Jr, McGuinness M. Interleukin-1 and host control of pulmonary histoplasmosis. J Infect Dis. 2006;194:855–864. doi: 10.1086/506946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu-Hsieh BA, Howard DH. Inhibition of the intracellular growth ofHistoplasma capsulatum by recombinant murine gamma interferon. Infect Immun. 1987;55:1014–1016. doi: 10.1128/iai.55.4.1014-1016.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu-Hsieh BA, Lee GS, Franco M, Hofman FM. Early activation of splenic macrophages by tumor necrosis factor alpha is important in determining the outcome of experimental histoplasmosis in mice. Infect Immun. 1992;60:4230–4238. doi: 10.1128/iai.60.10.4230-4238.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou P, Miller G, Seder RA. Factors involved in regulating primary and secondary immunity to infection with Histoplasma capsulatum: TNF-α plays a critical role in maintaining secondary immunity in the absence of IFN-γ. J Immunol. 1998;160:1359–1368. [PubMed] [Google Scholar]
- 9.Zhou P, Sieve MC, Bennett J, et al. IL-12 prevents mortality in mice infected withHistoplasma capsulatum through induction of IFN-γ. J Immunol. 1995;155:785–795. [PubMed] [Google Scholar]
- 10.Smith JG, Magee DM, Williams DM, Graybill JR. Tumor necrosis factor–α plays a role in host defense against Histoplasma capsulatum. J Infect Dis. 1990;162:1349–1353. doi: 10.1093/infdis/162.6.1349. [DOI] [PubMed] [Google Scholar]
- 11.Matsuzaki G, Umemura M. Interleukin-17 as an effector molecule of innate and acquired immunity against infections. Microbiol Immunol. 2007;51:1139–1147. doi: 10.1111/j.1348-0421.2007.tb04008.x. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romani L, Zelante T, De Luca A, Fallarino F, Puccetti P. IL-17 and therapeutic kynurenines in pathogenic inflammation to fungi. J Immunol. 2008;180:5157–5162. doi: 10.4049/jimmunol.180.8.5157. [DOI] [PubMed] [Google Scholar]
- 14.Heninger E, Hogan LH, Karman J, et al. Characterization of the Histoplasma capsulatum-induced granuloma. J Immunol. 2006;177:3303–3313. doi: 10.4049/jimmunol.177.5.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cain JA, Deepe GS., Jr Evolution of the primary immune response to Histoplasma capsulatum in murine lung. Infect Immun. 1998;66:1473–1481. doi: 10.1128/iai.66.4.1473-1481.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allendoerfer R, Deepe GS., Jr Intrapulmonary response to Histoplasma capsulatum in gamma interferon knockout mice. Infect Immun. 1997;65:2564–2569. doi: 10.1128/iai.65.7.2564-2569.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeachy MJ, Bak-Jensen KS, Chen Y, et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain Th-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 18.Korn T, Bettelli E, Gao W, et al. IL-21 initiates an alternative pathway to induce proinflammatory Th17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zelante T, De Luca A, Bonifazi P, et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007;37:2695–2706. doi: 10.1002/eji.200737409. [DOI] [PubMed] [Google Scholar]
- 20.Allendoerfer R, Biovin GP, Deepe GS., Jr Modulation of immune responses in murine pulmonary histoplasmosis. J Infect Dis. 1997;175:905–914. doi: 10.1086/513989. [DOI] [PubMed] [Google Scholar]
- 21.Liang SC, Long AJ, Bennett F, et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 22.Kelly MN, Kolls JK, Happel K, et al. Interleukin-17/interleukin-17 receptor-mediated signaling is important for generation of an optimal polymorphonuclear response against Toxoplasma gondii infection. Infect Immun. 2005;73:617–621. doi: 10.1128/IAI.73.1.617-621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamada S, Umemura M, Shiono T, et al. IL-17A produced by γδ T cells plays a critical role in innate immunity against Listeria monocytogenes infection in the liver. J Immunol. 2008;181:3456–3463. doi: 10.4049/jimmunol.181.5.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu YJ, Gross J, Bogaert D, et al. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000159. e1000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umemura M, Yahagi A, Hamada S, et al. IL-17-mediated regulation of innate and acquired immune response against pulmonary Mycobacterium bovis bacille Calmette-Guerin infection. J Immunol. 2007;178:3786–3796. doi: 10.4049/jimmunol.178.6.3786. [DOI] [PubMed] [Google Scholar]
- 26.Ye P, Rodriguez FH, Kanaly S, et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudner XL, Happel KI, Young EA, Shellito JE. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun. 2007;75:3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bozza S, Zelante T, Moretti S, et al. Lack of Toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J Immunol. 2008;180:4022–4031. doi: 10.4049/jimmunol.180.6.4022. [DOI] [PubMed] [Google Scholar]
- 29.Allendorfer R, Brunner GD, Deepe GS., Jr Complex requirements for nascent and memory immunity in pulmonary histoplasmosis. J Immunol. 1999;162:7389–7396. [PubMed] [Google Scholar]
- 30.Khader SA, Bell GK, Pearl JE, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 31.Higgins SC, Jarnicki AG, Lavelle EC, Mills KH. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J Immunol. 2006;177:7980–7989. doi: 10.4049/jimmunol.177.11.7980. [DOI] [PubMed] [Google Scholar]
- 32.Miossec P. Interleukin-17 in fashion, at last: ten years after its description, its cellular source has been identified. Arthritis Rheum. 2007;56:2111–2115. doi: 10.1002/art.22733. [DOI] [PubMed] [Google Scholar]
- 33.Weaver CT, Hatton RD, Mangan PR. Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 34.Yen D, Cheung J, Scheerens H, et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGeachy MJ, Cua DJ. The link between IL-23 and Th17 cell-mediated immune pathologies. Semin Immunol. 2007;19:372–376. doi: 10.1016/j.smim.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Lubberts E, Koenders MI, Oppers-Walgreen B, et al. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 2004;50:650–659. doi: 10.1002/art.20001. [DOI] [PubMed] [Google Scholar]
- 37.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 38.Da Silva CA, Hartl D, Liu W, Lee CG, Elias JA. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J Immunol. 2008;181:4279–4286. doi: 10.4049/jimmunol.181.6.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song C, Luo L, Lei Z, et al. IL-17-producing alveolar macrophages mediate allergic lung inflammation related to asthma. J Immunol. 2008;181:6117–6124. doi: 10.4049/jimmunol.181.9.6117. [DOI] [PubMed] [Google Scholar]
- 40.Burrell BE, Csencsits K, Lu G, Grabauskiene S, Bishop DK. CD8+ Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice. J Immunol. 2008;181:3906–3914. doi: 10.4049/jimmunol.181.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tzartos JS, Friese MA, Craner MJ, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L, Lopes JE, Chong MM, et al. TGF-β-induced Foxp3 inhibits Th17 cell differentiation by antagonizing RORγt function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deepe GS, Jr, Gibbons RS. Protective and memory immunity to Histoplasma capsulatum in the absence of IL-10. J Immunol. 2003;171:5353–5362. doi: 10.4049/jimmunol.171.10.5353. [DOI] [PubMed] [Google Scholar]
- 45.Zelante T, De Luca A, D’ngelo C, Moretti S, Romani L. IL-17/Th17 in anti-fungal immunity: what’s new? Eur J Immunol. 2009;39:645–648. doi: 10.1002/eji.200839102. [DOI] [PubMed] [Google Scholar]
- 46.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti–Candida albicans host defense in mice. J Infect Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 47.Gu Y, Yang J, Ouyang X, et al. Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol. 2008;38:1807–1813. doi: 10.1002/eji.200838331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAllister F, Henry A, Kreindler JL, et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: implications for airway inflammation in cystic fibrosis. J Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bettelli E, Kuchroo VK. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J Exp Med. 2005;201:169–171. doi: 10.1084/jem.20042279. [DOI] [PMC free article] [PubMed] [Google Scholar]