Abstract
A new paradigm of epithelial tissue reconstitution has been suggested whereby circulating cells derived from bone marrow contribute to a variety of epithelial cell types. With regard to the lung, several recent reports have used immunofluorescence microscopy to demonstrate engraftment of bone marrow–derived cells as type II pneumocytes, the endogenous progenitors of the lung alveolus. We show here that immunofluorescence microscopy, as has been used in previous reports, cannot reliably identify rare engrafted cells in lung tissue sections after transplantation of bone marrow cells or purified hematopoietic stem cells tracked with ubiquitous labels. We have employed a lineage-specific reporter system based on transgenic mice that express the GFP reporter gene only in lung epithelial cells (surfactant protein C–GFP) to assay for engrafted cells by flow cytometry, histology, and molecular methods. Using this approach to evaluate transplant recipients, including those subjected to bleomycin-induced lung injury, we demonstrate that when autofluorescence, dead cells, and contaminating blood cells are excluded from analysis, there is no detectable reconstitution of lung alveolar epithelial cells by unfractionated bone marrow cells or purified hematopoietic stem cells.
Keywords: alveolar epithelium, bleomycin, bone marrow transplantation, stem cells, type II pneumocyte
Initial reports of transplanted hematopoietic stem cells (HSCs) able to engraft in a variety of nonhematopoietic tissues and assume epithelial phenotypes have suggested a new paradigm for epithelial tissue repair (1). Enthusiasm has been diminished, however, by subsequent studies that either failed to reproduce these initial findings (2) or concluded that engraftment events were due to transplanted cells fusing with endogenous somatic cells (3, 4). With regard to the lung, however, evidence of cell fusion was not found (3, 5), and multiple reports have now suggested that bone marrow–derived cells can become alveolar type II (AT2) pneumocytes (1, 5–10), the known progenitors of the lung alveolus (11). The implications of these controversial findings are profound, as the ability to exogenously deliver cells into the progenitor niche of the alveolus provides the first opportunity to reconstitute the injured lung via cell-based therapy.
At issue in previous reports of engrafted epithelia is the almost exclusive reliance on immunostaining and fluorescence microscopy, by which fluorescing antibodies or DNA probes have been used to track and phenotype transplanted cells. This technique has been described as prone to artifact, as antibodies may react with cells other than their intended targets, and microscopy is by nature a subjective assay (12). Particularly problematic is the intrinsic structure of the lung, where each alveolus is comprised of septae containing capillaries filled with circulating blood cells that can be misinterpreted as hematopoietic derivatives “engrafted” in the lung (2). Furthermore, dead cells, fixative exposure, and the extensive elastin content of the lung may contribute to high background autofluorescence that can be difficult to distinguish from true fluorescent labeling.
Using mice that express transgene reporters under lineage-specific control, we sought to objectively test the hypothesis that HSCs or unfractionated bone marrow can form AT2 cells in normal or injured lungs. We avoided assays that depended on antibody or probe binding to track transplanted cells, and ensured objective analysis of only live lung cells by fluorescence-activated cell sorter (FACS) to reach conclusions that were supported by both histologic and molecular biology techniques. We report here that, when autofluorescence and dead cells are excluded from analysis, there is no detectable reconstitution of lung alveolar epithelial cells by unfractionated bone marrow or purified hematopoietic stem cells after transplantation.
MATERIALS AND METHODS
Mice
Transplantation studies involving lineage-specific reporters used 8-wk-old donor mice that express enhanced green fluorescent protein (GFP) under regulatory control of a 3.7-kb human surfactant protein C promoter (SP-C–GFP mice [13]; generous gift of Dr. Michael O'Reilly, University of Rochester, Rochester, NY). Transplants involving ubiquitous reporters used 8-wk-old donor mice that express GFP under control of the chicken β-actin (B-actin) promoter with cytomegalovirus enhancer (C57BL/6j-Tg[ACTB-EGFP]1Osb/J; Jackson Labs, Bar Harbor, ME). Recipient mice were congenic 8- to 10-wk-old C57BL/6j-Ly5.2 (CD45.2; for B-actin transplants) or C57BL/6-Ly5.1 (CD45.1; B6.SJL-Ptprca Pep3b/BoyJ; for SP-C–GFP transplants). All recipients were obtained from Jackson Labs. Mice were maintained in a pathogen-free animal facility, and all animal studies were approved by the Standing Committee on Animals of Harvard Medical School.
HSC Purification
HSCs were purified from the bone marrow of mice by the Hoechst dye efflux method originally developed in our laboratory (14) with the following modifications (15). This method identifies the bone marrow side population (SP) that is highly enriched in hematopoietic stem cell activity. Bone marrow single cell suspensions were prepared by crushing femurs, tibiae, and iliac crests with a pestle. A quantity of 4.5 million cells/ml in calcium- and magnesium-free Hanks' Balanced Salt Solution supplemented with 2% heat-inactivated fetal bovine serum (FBS), 1% penicillin/streptomycin, and 10 mM HEPES (HBSS+; Invitrogen, Carlsbad, CA) were incubated with 8.8 mcg/ml of Hoechst 33342 dye (Molecular Probes, Eugene, OR) for 90 min at 37°. After Hoechst staining, cell suspensions were washed in HBSS+, and red blood cells were depleted by centrifugation over Ficoll-Paque Plus (830 × g for 20 min; Amersham Biosciences, Piscataway, NJ). The buffy coat layer was collected and stained with 2 μg/ml of propidium iodide (PI) and filtered through a 40-μm Falcon cell strainer (BD Biosciences, San Jose, CA) for purification by flow cytometry.
Flow Cytometry
Cell sorting was performed on a MoFlo triple laser instrument (DakoCytomation, Fort Collins, CO) using Summit 3.1 software (Dako-Cytomation). Analysis of raw data was completed with FlowJo software (Treestar, Ashland, OR). The laser emissions were 488, 350, and 647 nm. Fluorescence was detected with the following bandpass filters; 530/40 for FITC and GFP, 580/30 for phycoerythrin (PE), 670/30 for PI, 405/30 for Hoechst Blue, 570/20 for Hoechst Red, and 670/20 for APC (Omega Optical Inc., Brattleboro, VT). Hoechst blue and red fluorescence was detected in linear scale acquisition. First a live cell gate was created excluding cell fragments (low forward scatter) or events that contained high PI fluorescence. Cells within this live cell gate were displayed on a Hoechst Red–Hoechst Blue histogram, and 200 SP cells per recipient were sorted for transplantation studies.
Competitive Long-Term Blood Repopulation and Analysis of Peripheral Blood Chimerism
CD45.1 recipient mice were lethally irradiated with either 11 Gy or 12 Gy of radiation in a single dose, or 14 Gy delivered as two doses of 7 Gy given 3 h apart on the day before transplantation. Recipients were maintained on standard chow and water supplemented with 2 mg/ml of neomycin sulfate antibiotic (Sigma-Aldrich, St. Louis, MO) for 14 d. Two hundred donor hematopoietic stem cells obtained from SP-C–GFP or B actin–GFP transgenic donor mice (both of CD45.2 genotype) were mixed with 2 × 105 unfractionated CD45.1 competitor bone marrow cells and intravenously injected retro-orbitally into CD45.1 congenic recipient mice (or CD45.2 wild-type mice recipients for β-actin GFP transplants). Peripheral blood was obtained from the retro-orbital plexus every 4 wk, and red blood cells were depleted by lysis (Red Blood Cell Lysing Buffer; Sigma-Aldrich). White blood cells were then stained using either fluorescence-conjugated anti–CD45.1-PE and –CD45.2-APC antibodies (for recipients of CD45.1 transplants) or pan-anti–CD45-PE (for recipients of B-actin GFP+ transplants). All FACS antibodies were purchased from BD Pharmingen (San Diego, CA). Levels of blood chimerism were calculated as the proportion of CD45 labeled white blood cells that expressed the donor CD45.2 or GFP marker gene. For whole marrow transplantation, 10 million unfractionated donor bone marrow cells were injected without competitor into recipients. Robust long-term hematopoietic reconstitution (> 50% blood chimerism for > 3 mo after transplantation) was documented in all recipients before further analysis.
Single Hematopoietic Stem Cell Transplantation
Single bone marrow SP cells from a donor GFP+ transgenic mouse (B actin–GFP; Jackson Labs) were sorted into separate wells of a 96-well plate that contained 100 μl of HBSS+ and 3 × 105 Sca-1–negative competitor marrow cells from a wild-type mouse. The entire contents of each well were then injected into the retro-orbital venous plexus of each recipient. A separate syringe was used for each well to avoid cross-contamination. An additional 96-well plate sorted with single cells by this method was examined by microscope to confirm that 100% of wells contained only 1 sorted cell. Eighteen mice that received single cell transplants were then followed to select two recipients with > 40% GFP+ blood chimerism. These highly chimeric recipients were then harvested for further analysis of bone marrow, peripheral blood and lung engraftment at 4 mo and 1 yr after transplantation as detailed in the text. Self-renewal of the transplanted single stem cells was documented by serial transplantation of bone marrow from these recipients into secondary recipients.
Lung Histology
Lung tissue was perfused free of blood by saline irrigation via the right ventricle. The left mainstem bronchus was ligated and the left lung excised for RNA, DNA extraction, and enzyme digestion for FACS. The right lung was then inflated with 4% paraformaldehyde for overnight fixation followed by embedding in optimal tissue compound (OCT) for frozen tissue sectioning. Five-micrometer-thick frozen sections from each of the four lobes of the right lung were examined. For recipients of SP-C–GFP marrow transplants, frozen sections were treated with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI)-supplemented mounting medium (Vectashield; Vector Labs, Burlingame, CA), coverslipped, and examined for GFP expression by fluorescence microscopy. No antibody labeling was employed in analysis of SP-C–GFP recipient mice. Frozen tissue sections from recipients of B actin–GFP transplants were processed for pro–SP-C immunostaining by incubation with a polyclonal rabbit anti-pro–SP-C antibody (Chemicon, Temecula, CA) followed by Cy3-conjugated secondary goat anti-rabbit IgG antibody (Jackson Immunoresearch Laborotories, West Grove, PA). Two negative control sections stained with nonspecific rabbit IgG in place of the primary antibody were included in each analysis. Background autofluorescence was determined through examination of 10 simultaneously prepared negative control sections that were stained with DAPI alone. All sections were examined using a Zeiss Axioplan 2 fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with GFP (green), Cy3 (red), and DAPI (blue) filter set. Digital images were captured and processed using Axiovision 3.1 software (Carl Zeiss, Inc.).
Preparation of Lung Single Cell Suspensions for FACS
Saline-perfused lung samples were finely minced with razor blades, and enzymatically digested for 1 h at 37°C in a solution of 0.1% Collagenase A in 2.4 U/ml Dispase II (Roche, Indianapolis, IN) with 2.5 mM CaCl2. The resulting digest was then filtered through a 70-μm Falcon cell strainer (BD Biosciences) to remove debris and washed with HBSS+. Where indicated in the text, lung suspensions from B actin–GFP transplants were stained with Fc Block and monoclonal PE-conjugated rat anti-mouse CD45 or isotype control antibody (BD Pharmingen). All single cell suspensions were stained with 2 μg/ml of PI, filtered through a 40 um Falcon cell strainer, and analyzed by flow cytometry (laser and filter settings listed above; MoFlow, Fort Collins, CO). Raw data were then processed with FlowJo software (Treestar). Forward/side scatter properties were used to exclude cell fragments and red blood cells. PI staining was used to exclude dead cells. The live lung cell subgate was then analyzed for GFP fluorescence and red (PE) autofluorescence.
RT-PCR and PCR
RNA was extracted from snap-frozen tissue excised from the left lung according to the manufacturer's instructions (RNEasy Kit; Qiagen, Valencia, CA) and all samples were treated with DNase to minimize contamination with genomic DNA. cDNA was prepared by reverse transcription under standard conditions using random hexamer priming (First-Strand cDNA Synthesis Kit; Amersham Biosciences). PCR amplification of GFP and β-actin cDNA was performed using FastStart Taq DNA Polymerase (Roche) and the following primer pairs: GFP forward, ATGGTGAGCAAGGGCGAGGAGCTG; GFP reverse, CTTGTACAGCTCGTCCATGCCGAG; B-actin forward, AAGAGAGGTATCCTGACCCT; B-actin reverse (intron spanning) GCTCGTTGCCAATAGTGATG. RNA aliquots that were not exposed to reverse transcriptase (−RT) were included as negative controls in all RT-PCR analyses. PCR conditions were: (GFP) 95°C × 1 min, 60°C × 1 min, 72°C × 1 min, 30 cycles; (β-actin) 95°C × 1 min, 55°C × 1 min, 72°C × 1 min, 30 cycles. For detection of GFP and β-actin in genomic DNA, DNA was extracted from lung tissue according to the manufacturer's instructions (DNeasy; Qiagen) and subjected to PCR using the above primer pairs and conditions. All RT-PCR and PCR assays were analyzed qualitatively to screen for engraftment of donor-derived cells.
Lung Injury
Mice were subjected to bleomycin-induced lung injury as previously described (16). After long-term hematopoietic reconstitution (> 3 mo after transplantation), recipient mice were anesthetized with isoflurane, and a blunt-end canula was used to intratracheally instill 0.05 U bleomycin sulfate in 100 μl sterile PBS. One month after bleomycin exposure, recipients' lungs were harvested for analysis.
RESULTS
Lung Engraftment after Transplantation of Ubiquitously Labeled Bone Marrow Cells
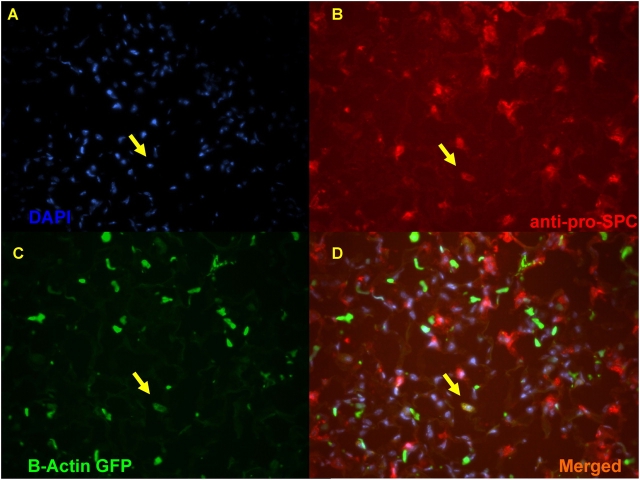
Initial transplant experiments were aimed at assessing the capacity of unfractionated bone marrow cells, highly purified HSCs, or single bone marrow stem cells to engraft as lung AT2 cells. Donor marrow populations were obtained from donor mice that express the GFP reporter gene under control of ubiquitously active promoter elements (B-actin–GFP). After long-term hematopoietic reconstitution of lethally irradiated recipients with unfractionated marrow (n = 3), HSCs (n = 3), or single stem cells (n = 2), the vast majority (> 99%) of lung cells that immunostained red with a AT2 marker (pro–SP-C) did not exhibit GFP fluorescence. Conversely, the vast majority of GFP+ cells within the lung (> 99% of 300 GFP+ cells per section) did not express pro–SP-C (Figure 1). This quantification was based on analysis of eight frozen sections per recipient with ∼ 2,000 nucleated cells present on each section. FACS-based phenotyping of 25,000 live GFP+ cells present in each recipient's lungs demonstrated that > 99% were hematopoietic cells that expressed CD45 (Figure 2). By immunofluorescence microscopy, 1.39 cells ± 0.79 (mean ± SD) per tissue section did appear to exhibit both green (GFP) and red (pro–SP-C immunostaining) fluorescence (Figures 1 and 2); however, this number was no different than background (Figure 3), as negative control sections that were not exposed to primary anti-pro–SP-C antibody exhibited similar rare cells that fluoresced in both green and red channels.
Figure 1.
Transplantation of unfractionated bone marrow ubiquitously labeled with GFP (B-actin–GFP). Representative lung frozen tissue section from a recipient analyzed 1 yr after transplantation of 10 million unfractionated GFP+ bone marrow cells. At the time of analysis this recipient had 88% GFP+ peripheral blood chimerism. (A) DAPI nuclear counterstaining (blue); (B) immunostaining with an antibody against a specific alveolar type II (AT2) pneumocyte cell marker (Cy3 anti-pro–SP-C; red); and (C) fluorescence of GFP (B-actin–GFP; green) are merged in D, illustrating the 1 cell (of 200 AT2 cells examined on this section) that appeared to express both markers (arrows). The number of cells that are “double-labeled” with GFP fluorescence and AT2-specific red immunostaining in this recipient lung is no different than the number of cells per section that show background autofluorescence on negative control sections (shown in Figure 3 below).
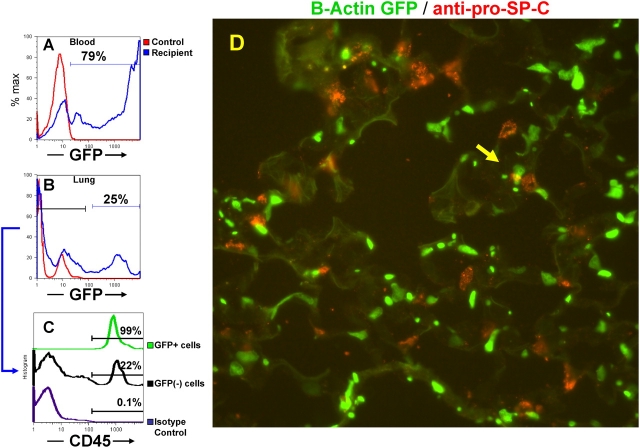
Figure 2.
Hematopoietic reconstitution by a single GFP+ bone marrow stem cell. A single hematopoietic stem cell was purified from the bone marrow of a B-actin–GFP donor mouse, and 1 yr later recipient blood and lung tissue were harvested for analysis. (A) FACS histogram illustrates robust hematopoietic reconstitution of 79% of the recipient's peripheral blood with donor-derived GFP+ cells. (B) FACS-analysis of live cells (PI-negative) from the left lung of this recipient, shows the presence of GFP+ marrow-derived cells (25% of the lung cell suspension). (C) FACS-analysis of the GFP+ and GFP(−) subgates of the lung cell suspension from B demonstrates that 99% of the GFP+ cells (green histogram) express the panhematopoietic marker CD45. In contrast, 22% of GFP(−) cells in this sample are CD45+ (black histogram). An aliquot of this sample stained with a negative control antibody of identical isotype illustrates specificity of the CD45 antibody staining (purple histogram). Similar engraftment results were obtained from recipients that received either unfractionated marrow or 200 purified HSC transplants. (D) A frozen tissue section prepared from the right lung of this recipient was immunostained with Cy3-conjugated anti–pro-SP-C antibody showing that AT2 cells (red) and donor marrow–derived cells (green) are distinct, with the possible artifactual exception of two cells whose tips appear to overlap (arrow).
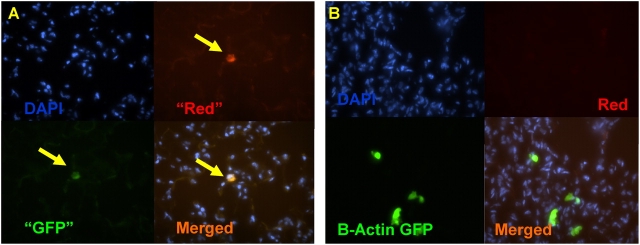
Figure 3.
Distinguishing autofluorescent artifact from engraftment is not possible with dual channel immunofluorescence microscopy. (A) Lung frozen tissue section from a wild-type mouse that has not been exposed to antibody staining shows rare cells that exhibit equal red and green autofluorescence (arrows). (B) Recipient lung after bone marrow transplantation from a B-actin–GFP mouse (no antibody exposure). Donor-derived cells in this section fluoresce green but not red, indicating true GFP+ fluorescence. Nuclei are counterstained with DAPI.
We found that the majority of this rare background signal was due to autofluorescent cells in the lung parenchyma, because unstained sections from wild-type mice also revealed 1.30 ± 1.16 cells per section that exhibited both green and red fluorescence (Figure 3A). In contrast, true GFP+ cells on recipient sections could be distinguished from rare autofluorescing cells by ensuring that the GFP+ cells exhibited green but not red fluorescence (Figure 3B). Even 1 yr after bone marrow transplantation and reconstitution of > 80% of recipients' hematopoietic compartments with donor cells, no recipient exhibited cells within the lung that exhibited both GFP and AT2 marker fluorescence at frequencies above negative control background (Figures 1–3). We concluded that immunofluorescence microscopy could not determine with certainty whether rare engraftment of AT2 cells was occurring after transplantation of bone marrow cells that ubiquitously express the GFP marker.
Use of a Lineage-Specific Transgenic Reporter Shows No Reconstitution of AT2 Cells by Bone Marrow Cells
Next, a lineage-specific reporter system was employed to track the fate of donor marrow populations. Donor unfractionated marrow or highly purified HSCs for transplantation were obtained from transgenic mice that express the GFP reporter gene under regulatory control of the SP-C promoter (SP-C–GFP mice) (13); generous gift of Dr. Michael O'Reilly, University of Rochester). By transplanting marrow from these donors into wild-type recipients, our goal was to develop a sensitive engraftment assay that would exclude background autofluorescence and would avoid any possibility of nonspecific binding of antibodies. The lineage-specific reporter system employed would also avoid mistakenly counting donor-derived blood cells “engrafted” in the lung and would allow the detection of the fluorescent reporter gene only in AT2 cells.
Specific expression of GFP in AT2 cells of donor mice (13) was confirmed to be at a high level (10- to 1,000-fold above background) in 25% of all AT2 cells or 3.5% of total lung cell suspensions obtained by enzyme digestion (Figure 4). GFP-expressing AT2 cells in these transgenic mice are thus easily distinguishable from background lung autofluorescence on frozen tissue sections as well as FACS analyses without the use of antibody-based labeling (Figure 4). As previously described (13), we found that this transgene was not expressed in lung endothelium, peripheral blood, or bone marrow (data not shown).
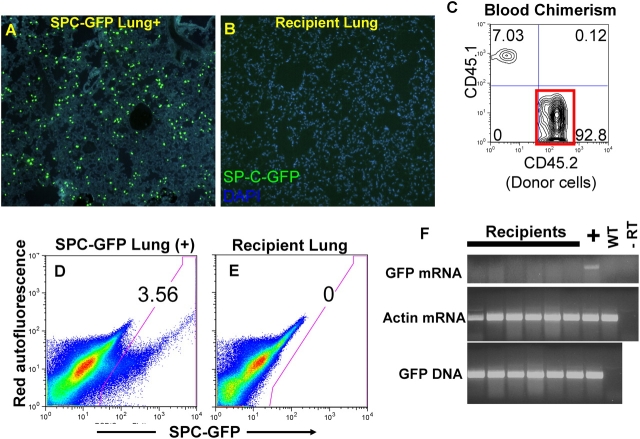
Figure 4.
Assessment of bone marrow–derived lung engraftment after transplantation of bone marrow cells from the SP-C–GFP lineage-specific reporter mouse. (A) Bright GFP fluorescence only in alveolar type II (AT2) cells is seen in lung frozen tissue sections from the transgenic (positive control) mouse. (B) Representative lung section from a recipient mouse 4 mo after transplantation of 10 million unfractionated bone marrow cells obtained from a donor SP-C–GFP mouse. No GFP+ engrafted cells are seen. (C) Peripheral blood analyzed from this same recipient shows 93% blood chimerism (red box) arising from the donor (CD45.2) transplanted marrow cells (recipient is a CD45.1 congenic mouse). (D) FACS analysis of 1 million live (propidium iodide excluding) lung cells from a SP-C–GFP transgenic mouse (positive control) shows GFP+ fluorescence in 3.56% of cells. (E) Representative FACS of 1 million live lung cells from a recipient transplanted with SP-C–GFP bone marrow shows no GFP+ events. (F) Representative analysis of RNA and DNA extracts from six recipients' lungs shows donor-derived cells are present in recipients' lungs (GFP DNA+), but are not AT2 cells because they do not express GFP mRNA. Controls are labeled as: “+” (SP-C-GFP transgenic lung); WT (wild-type lung); −RT (RNA extract from SP-C–GFP lung minus reverse transcription). RT-PCR of the housekeeping gene β-actin illustrates that cDNA is present in each sample.
To test the ability of bone marrow cells to form AT2 cells, unfractionated bone marrow or purified HSCs from SP-C–GFP donors were transplanted into congenic Ly5.1 wild-type recipient mice that had undergone lethal irradiation at escalating doses of ionizing radiation (11Gy, 12Gy, or 14Gy). After long-term robust blood reconstitution (70–93% Ly5.2+ blood chimerism for > 3 mo), the lungs of each recipient were analyzed for engraftment of donor-derived cells by three antibody independent assays: (1) FACS analysis of 106 live lung cells for engrafted AT2 cells (defined as cells that excluded PI and were GFP+ but not autofluorescent; i.e. PI negative/PE negative); (2) fluorescence microscopy of frozen tissue sections from each of four lung lobes for GFP+ cells (defined as green positive/red negative cells); and (3) RT-PCR-based assessment of GFP mRNA expression.
As shown in Table 1, Figure 4, and Figures E1–E3 in the online supplement, in 28 recipients studied, we found no donor-derived AT2 cells on any recipient tissue section or in any FACS sample. At the molecular level, we found no evidence of AT2 cell engraftment, as GFP mRNA was not expressed in any recipient lung. As expected, DNA from donor-derived cells, predominantly of hematopoietic lineage, was easily detected by PCR for the GFP transgene.
TABLE 1.
Summary of lung engraftment after transplantation of bone marrow populations from donor sp-c–gfp reporter mice
| Cells Transplanted | No. of Recipients | Radiation Dose | Lung Injury | Engrafted AT2 cells out of no. of cells analyzed (FACS) |
Engrafted AT2 cells/sections analyzed (microscopy) |
|---|---|---|---|---|---|
| Whole marrow (107) | 3 | 11 Gy | No | 0 of 3 × 106 | 0/60 |
| Whole marrow (107) | 4 | 12 Gy | No | 0 of 4 × 106 | 0/80 |
| Whole marrow (107) | 9 | 14 Gy | No | 0 of 9 × 106 | 0/180 |
| Whole marrow (107) | 4 | 14 Gy | Bleomycin | 0 of 4 × 106 | 0/80 |
| HSCs (200) | 4 | 14 Gy | No | 0 of 4 × 106 | 0/80 |
| HSCs (200) | 4 | 14 Gy | Bleomycin | 0 of 4 × 106 | 0/80 |
| TOTAL | 28 | 0 of 28 × 106 | 0/560 |
Definition of abbreviations: FACS, fluorescence-activated cell sorter; GFP, green fluorescent protein; HSCs, hematopoietic stem cells; SP-C, surfactant protein C.
Because the lung is normally a quiescent organ, it is possible that lung injury might be required to facilitate engraftment of bone marrow-derivatives in lung tissue. For this reason, 3 mo after hematopoietic reconstitution with SP-C–GFP donor marrow cells, we exposed eight recipients to intratracheal bleomycin, which is known to cause epithelial lung injury (17, 18) (see Figure E4 for hematoxylin and eosin–stained lung sections). After a 1-mo recovery period after bleomycin exposure, recipients of unfractionated marrow (n = 4) or HSC transplants (n = 4) showed no AT2 cell reconstitution by bone marrow–derived cells (Table 1).
Sensitivity of FACS-Based Assays of Engraftment
To test the ability of the cell sorter used in these experiments to detect very rare engraftment events, five GFP+ cells from the lungs of SP-C–GFP mice were added to a sample of 8 × 105 wild-type lung cells. Four of five GFP+ AT2 cells were successfully identified by cell sorting; thus engraftment rates as low as 1 in 640,000 cells should have been detectable using this assay. As an average of 35,000 brightly GFP fluorescent AT2 cells were present in each transgenic control sample, we estimated that 980,000 AT2 cells were present in the 28 million total lung cells from 28 recipients that were analyzed by FACS in these studies, and no engrafted GFP+ cells were detected.
DISCUSSION
Our finding that neither unfractionated bone marrow nor hematopoietic stem cells reconstitute the pulmonary alveolar epithelium is in contrast to previous reports. We demonstrated that immunofluorescence microscopy alone is not a reliable assay for bone marrow engraftment, because rare autofluorescent cells can be mistaken for “engraftment.” In contrast, the use of multiple methods of analysis (based on histology, FACS, and molecular biology) and a lineage-specific reporter system ensures that nonspecifically labeled cells, hematopoietic cells, as well as dead or autofluorescent cells were not included as “engraftment” events. When these background events were excluded from analysis, there was no longer evidence of bone marrow–derived cells contributing to the lung epithelium, even after bleomycin-induced injury. Although we cannot entirely exclude the possibility that epithelial engraftment in these studies could have occurred without activation of the SP-C promoter, this seems unlikely, as transgenic lineage-specific reporter systems have been widely employed to trace the differentiation of cell lineages in vivo (19, 20).
These findings suggest that the differentiative repertoire of hematopoietic stem cells likely does not include AT2 cells and calls into question whether other epithelial cell types are, in fact, reconstituted to any degree by HSCs or their progeny. As HSCs derive from embryonic mesoderm, transdifferentiation or de-differentiation into endodermal lineages, such as lung epithelium, may not be possible for this hematopoietic cell type. Although it appears increasingly likely that HSCs are more “tissue-committed” than recently claimed, the availability of lineage-specifc reporter systems should allow more rigorous testing of the differentiative potential of transplanted HSCs. For example, recent reports that other mesodermal derivatives, such as endothelial cells and their circulating progenitors, may be derived from HSCs (21, 22) should be extensively tested with lineage-specific reporter methodology. In addition, the question of whether pulmonary mesenchymal cell types may be derived from bone marrow populations, as has been reported (23–25), similarly warrants further testing. Indeed, endothelial or mesenchymal engraftment may account for the 0.1–1% of cells in our studies that appeared to be B-actin–GFP positive and CD45-deficient by FACS.
It is important to note that the reported epithelial engraftment potential of cultured marrow populations (such as mesenchymal stem cells [26] or plastic-adherent cultured marrow [16, 27]) was not tested in our experiments. The epithelial reconstituting potential of these bone marrow subsets is therefore still possible. However, these plastic-adherent marrow cell types should be present in the large doses of unfractionated bone marrow transplanted in our experiments, and the lack of AT2 engraftment we observed suggests that they either form other cell types in the lung, do not truly engraft, or obtain an epithelial differentiative repertoire only after culturing.
Fusion of donor-derived hematopoietic cell types with differentiated somatic cells has been proposed as one mechanism whereby marker gene expression can be transferred to recipient somatic cells, such as hepatocytes and muscle fibers (3, 4). However, our failure to detect derivatives of unfractionated bone marrow or HSCs contributing to surfactant-expressing cells in the lung suggests that AT2 marker gene expression arising from bone marrow cells, whether by fusion or transdifferentiation, does not readily occur.
Supplementary Material
Acknowledgments
The authors are grateful to Alan Fine, Jacqueline Chang, Xi Sun, and Ross Summer of the Boston University Pulmonary Center for input and assistance with pro–SP-C immunostaining methods, and to Beverly Neugeboren of Harvard Medical School for technical support. They thank Michael O'Reilly of the University of Rochester for helpful input and for generously providing SP-C–GFP transgenic mice. We are also grateful for valuable intellectual input from Alex Balazs, Gustavo Mostoslavsky, George Murphy, and Jonathan Walsh at Harvard Medical School.
Supported by NIH 5PO-HL54785 (R.C.M.) and 1 KO8 HL071640–01 (D.N.K.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Conflict of Interest Statement: None of the authors have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- 2.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 2002;297:2256–2259. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature 2003;425:968–973. [DOI] [PubMed] [Google Scholar]
- 4.Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, Nakano Y, Meyer EM, Morel L, Petersen BE, Scott EW. Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 2002;416:542–545. [DOI] [PubMed] [Google Scholar]
- 5.Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science 2004;305:90–93. [DOI] [PubMed] [Google Scholar]
- 6.Grove JE, Lutzko C, Priller J, Henegariu O, Theise ND, Kohn DB, Krause DS. Marrow-derived cells as vehicles for delivery of gene therapy to pulmonary epithelium. Am J Respir Cell Mol Biol 2002;27:645–651. [DOI] [PubMed] [Google Scholar]
- 7.Theise ND, Henegariu O, Grove J, Jagirdar J, Kao PN, Crawford JM, Badve S, Saxena R, Krause DS. Radiation pneumonitis in mice: a severe injury model for pneumocyte engraftment from bone marrow. Exp Hematol 2002;30:1333–1338. [DOI] [PubMed] [Google Scholar]
- 8.Suratt BT, Cool CD, Serls AE, Chen L, Varella-Garcia M, Shpall EJ, Brown KK, Worthen GS. Human pulmonary chimerism after hematopoietic stem cell transplantation. Am J Respir Crit Care Med 2003;168:318–322. [DOI] [PubMed] [Google Scholar]
- 9.Kleeberger W, Versmold A, Rothamel T, Glockner S, Bredt M, Haverich A, Lehmann U, Kreipe H. Increased chimerism of bronchial and alveolar epithelium in human lung allografts undergoing chronic injury. Am J Pathol 2003;162:1487–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattsson J, Jansson M, Wernerson A, Hassan M. Lung epithelial cells and type II pneumocytes of donor origin after allogeneic hematopoietic stem cell transplantation. Transplantation 2004;78:154–157. [DOI] [PubMed] [Google Scholar]
- 11.Mason RJ, Williams MC. Type II alveolar cell: defender of the alveolus. Am Rev Respir Dis 1977;115:81–91. [DOI] [PubMed] [Google Scholar]
- 12.Chien KR. Stem cells: lost in translation. Nature 2004;428:607–608. [DOI] [PubMed] [Google Scholar]
- 13.Roper JM, Staversky RJ, Finkelstein JN, Keng PC, O'Reilly MA. Identification and isolation of mouse type II cells on the basis of intrinsic expression of enhanced green fluorescent protein. Am J Physiol Lung Cell Mol Physiol 2003;285:L691–L700. [DOI] [PubMed] [Google Scholar]
- 14.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996;183:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kotton DN, Fabian AJ, Mulligan RC. A novel stem cell population in adult liver with potent hematopoietic reconstitution activity. Blood 2005;106:1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kotton DN, Ma BY, Cardoso WV, Sanderson EA, Summer RS, Williams MC, Fine A. Bone marrow-derived cells as progenitors of lung alveolar epithelium. Development 2001;128:5181–5188. [DOI] [PubMed] [Google Scholar]
- 17.Adamson IYR, Bowden DH. Bleomycin-induced injury and metaplasia of alveolar type 2 cells. Am J Pathol 1979;96:531–544. [PMC free article] [PubMed] [Google Scholar]
- 18.Chandler DB, Hyde DM, Giri SN. Morphometric estimates of infiltrative cellular changes during the development of bleomycin-induced pulmonary fibrosis in hamsters. Am J Pathol 1983;112:170–177. [PMC free article] [PubMed] [Google Scholar]
- 19.Motoike T, Loughna S, Perens E, Roman BL, Liao W, Chau TC, Richardson CD, Kawate T, Kuno J, Weinstein BM, et al. Universal GFP reporter for the study of vascular development. Genesis 2000;28:75–81. [DOI] [PubMed] [Google Scholar]
- 20.Vassar R, Rosenberg M, Ross S, Tyner A, Fuchs E. Tissue-specific and differentiation-specific expression of a human K14 keratin gene in transgenic mice. Proc Natl Acad Sci USA 1989;86:1563–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med 2002;8:607–612. [DOI] [PubMed] [Google Scholar]
- 22.Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, Wong MH, Fleming WH. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood 2004;103:13–19. [DOI] [PubMed] [Google Scholar]
- 23.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol 2003;171:380–389. [DOI] [PubMed] [Google Scholar]
- 26.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA 2003;100:8407–8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002;418:41–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.