Abstract
Pulmonary tuberculosis (TB) has been characterized by inflammation with increased pro- or anti-inflammatory cytokines produced by macrophages. We have reported that IFN produces inhibitory C/EBPβ and represses transcription of the HIV-1 LTR in macrophages. STAT-1 and type I IFN receptor knockout mice have macrophages that are defective in IFN signaling, yet LPS stimulation induces inhibitory C/EBPβ, demonstrating that other cytokines can induce this repressor. LPS or Mycobacterium tuberculosis–derived lipoarabinomannan induce the anti-inflammatory cytokine interleukin (IL)-10, which represses the HIV-1 LTR in differentiated THP-1 macrophages by inducing inhibitory C/EBPβ. In contrast, in undifferentiated THP-1 monocytes, IL-10 did not inhibit HIV-1 replication or induce C/EBPβ. IL-10 signal transduction uses STAT-3, and macrophages from STAT-3−/− mice fail to produce inhibitory C/EBPβ after LPS or IL-10 stimulation. Transfection of STAT-3 into THP-1 cells enhances C/EBPβ promoter activity. THP-1 differentiation also increases STAT-3 protein, but not STAT-3 gene transcription, and induces a translational regulator, CUG-binding protein, that was essential for production of C/EBPβ. Differentiation induced post-transcriptional regulation is required to produce inhibitory C/EBPβ in response to IL-10. Only macrophages are able to repress HIV-1 LTR promoter activity and inhibit viral replication in response to IL-10 or type I IFN.
Keywords: infection, innate immunity, repression, transcription factors
The epidemic of tuberculosis (TB) in patients with AIDS is a major health crisis both in developing and industrialized countries (1, 2). Alveolar macrophages (AM) are the major source of HIV-1 replication during TB in co-infected patients (3, 4). The increase in viral replication and mutation induced by opportunistic infection likely causes acceleration of AIDS (5). The mechanisms regulating HIV-1 replication in AM are therefore central to the understanding of AIDS pathophysiology. This is especially true since AM are long-lived cells that may serve as a reservoir for HIV-1 during suppression of viral replication during highly active antiretroviral therapy (6).
Regulation of the HIV-1 LTR is an important step in the control of viral replication in infected cells. The HIV-1 LTR has three C/EBP sites in its negative regulatory element (7). These three sites are essential for control of viral replication in macrophages, but not lymphocytes (8). The C/EBP sites bind a number of transcription factors that regulate proinflammatory cytokine expression (9, 10) and viral replication in monocytes/macrophages (11, 12).
C/EBPβ is the predominant C/EBP transcription factor family member expressed in unstimulated AM (13). The C/EBPβ gene is intronless, but can produce both stimulatory and inhibitory isoforms depending on which translation start site is selected by the ribosome (14). In humans, there is a 37-kD isoform and an inhibitory 16-kD isoform produced from an internal AUG start codon (15). The 16-kD C/EBPβ is a dominant-negative transcription factor repressing promoters that contain C/EBP sites if expressed at 20% of the level of the stimulatory isoform (14). Translation of the inhibitory C/EBPβ isoform is induced by the RNA-binding protein, referred to as CUG (16). The inhibitory C/EBPβ likely recruits co-repressors to the promoter inhibiting transcription (17). Expression of a dominant-negative 16-kD C/EBPβ isoform strongly represses the HIV-1 LTR (18) and the TNF-α promoters in monocytes and macrophages (9). Resting AM strongly express inhibitory C/EBPβ, which maintains the baseline quiescent state of the lung (13). Production of the inhibitory C/EBPβ is an IFN effect in macrophages but not monocytes (19). Differentiation of monocytes to macrophages leads to IFN responsiveness through post-transcriptional induction of interferon response factor (IRF)-9. Targeted deletion of the Type I IFN receptor or STAT-1 can disrupt IFN signaling, with loss of IFN effects and dissemination of intracellular infectious agents (20, 21).
Interleukin (IL)-10 is induced during TB and appears to be associated with anergy and failure to produce a TH-1 response (22, 23). The IL-10 and TGF-β interact with one another to suppress the immune response (24, 25). IL-10 polymorphisms are associated with alteration of active disease in humans, supporting a clinically relevant role of this cytokine in TB (26). Lymphocytes from anergic patients can suppress HIV-1 replication in vitro (27). In vitro models, however, suggest that IL-10 can contribute to control of mycobacterial growth in dendritic cells (28). The anti-inflammatory effects of IL-10 are mediated by STAT-3, which initiates signaling after phosphorylation and dimerization. For in vitro study, a chimeric STAT-3/estrogen receptor is activated upon exposure to the estrogen analog 4-hydroxy-tamoxifen (29). In contrast, targeted deletion of STAT-3 in macrophages leads to hyperresponsiveness to inflammatory stimuli such as LPS. Even in the absence of infection these mice develop a colitis similar to that observed in IL-10 knockout mice (30). In addition, disruption of STAT-3 leads to proliferation of neutrophils, demonstrating that STAT-3 impacts differentiation of the myeloid lineage (31).
We evaluated the anti-inflammatory role of IL-10 in macrophages. IL-10 induced inhibitory C/EBPβ in macrophages, but deletion of the signaling molecule STAT-3 abrogated this effect. IL-10 was unable to induce inhibitory C/EBPβ in monocytes, but maturation of monocytes to macrophages gained this ability, probably through induction of the post-translational effect of CUG-binding protein. These findings provide a basis of the anti-inflammatory arm of the host response to Mycobacterium tuberculosis after therapy is initiated.
MATERIALS AND METHODS
Lipoarabinomannan Stimulation of Monocyte-Derived Macrophages
Peripheral blood mononuclear cells (PBMC) were obtained from blood by Ficoll-Hypaque sedimentation, and buffy coats were washed four times in PBS. PBMC were suspended in RPMI 1640 with 10% FCS and allowed to adhere to plastic plates overnight; cells were then incubated with LPS (2 μg/ml) or lipoarabinomannan (LAM; 1 μg/ml). LPS-free Man-LAM was obtained from John Belisle (Colorado State University, NIAID, NIH Contract NO1-AI-75320). Supernatants were sampled at Day 1 and at 2-d intervals thereafter. ELISA (R&D Systems, Minneapolis, MN) were used to assay IL-10 concentrations.
Cell Culture
Murine bone marrow–derived macrophages were prepared as previously described (31). Briefly, tibial/fibial bone marrow from CO2-killed mice were suspended in DMEM +10% FCS + 20% LCCM growth medium. Adherent cells were harvested by gentle scraping after 7 d, washed, and resuspended in RPMI at a density of 106 cells/ml. The cells were treated with 2 μg/ml and harvested 24 h after treatment. THP-1 and HL-60 cells were cultured in RPMI 1640 with 10% FCS. Cells were differentiated with 20 ng/ml phorbol 12-myristate 13-acetate (PMA) for 24 h or as indicated in each experiment. For experiments to examine the effect of IFN or IL-10 on C/EBPβ, cells were either left undifferentiated or differentiated with PMA for 24 h and then treated with IFN-β or IL-10 as indicated. Peritoneal macrophages were isolated from STAT3 knockout mice or WT controls 3 d after injection with 3 ml of 3% brewers thioglycolate by peritoneal cavity lavage with 10 cc of PBS after mice were killed. Cells were suspended in RPMI containing 10% FCS + fungizone + penicillin/streptomycin at a density of 106 cells/ml and plated in 6-well plates with 106 cells/well.
HIV-1 Promoter Assay
For the model of activation of latent HIV-1 from monocytes or macrophages, we used BF 24 cells (AIDS Research and Reference Reagent Program #1296). Protein extracts for chloramphenicol acetyl transferase (CAT) ELISA (Roche Molecular Biochemicals, Mannheim, Germany) were processed according to the manufacturer's instructions. For pseudotyped virus infection experiments, the virus contained the envelope of HIV-1JR-FL and the luciferase reporter gene replacing the envelope gene. Infection were performed as reported with an MOI of 0.5. Protein extracts for luciferase (Roche Molecular Biochemicals) were processed according to manufacturer's instructions (13).
Transfection
Transfections were done using a Gene Gun (Biorad) with 0.5 μg of each plasmid (for 1 × 107 cells) mixed with 0.5 mg of gold. Transfections were performed using 50 and 100 psi to drive the gun using STAT-3-ER (29) and cotransfected with C/EBP β Luc (32). 4HT (1 μmol/ml) was added for 24 h before cells were harvested; values were normalized to protein concentration.
Whole-Cell and Nuclear Extract Preparation and Immunoblot
Cells were washed twice in PBS. Whole-cell extracts were prepared for immunoblot analysis by incubation in RIPA buffer (PBS, 1% NP40, 0.5% deoxycholate) containing 3 μg/ml aprotinin, 1 mM PMSF, and 1 mM Na orthovanadate for 30 min and disruption by passage through a 21-g needle. Proteins were separated by SDS-PAGE. For each gel, equal amounts of protein were loaded in each lane. From gel to gel, the amount of protein per lane was 30–100 μg for detection of endogenous proteins. For detection of C/EBPβ 10–20% linear-gradient gels were used. For detection of STAT-3, 10% gels were used. After electrophoresis, protein was electrotransferred to nylon membranes for detection of C/EBPβ or STAT-3. Membranes were blocked with nonfat dry milk, then probed with antibodies against C/EBPβ, STAT-3, or CUG-binding protein from Santa Cruz Biotechnology Inc. (Santa Cruz, CA), visualized with anti-rabbit HRP and ECL (Amersham Inc., Arlington Heights, IL). Multiple film exposures were scanned by laser densitometry and analyzed with Imagequant software (Molecular Dynamics; Amersham Biosciences, Piscataway, NJ) for quantitation of immunoreactive STAT-3. Both the anti-C/EBPβ and anti–STAT-3 antisera reacted with nonspecific bands, which confirmed equal protein loading from lane to lane.
Determination of Transcription Rates
Cells were treated as described. Run-on assays were performed as previously described (19). Radiolabeled nascent RNA was isolated and hybridized to excess plasmid DNA fixed to nitrocellulose. A β-actin clone was used as an internal standard. Bluescript KS- (Stratagene, La Jolla, CA) and RcCMV (Invitrogen, Carlsbad, CA) were used as negative controls and to determine background signal. The results were visualized and quantitated with a phosphorimager and Imagequant software (Molecular Dynamics).
RESULTS
IFN and IL-10 Are Members of a Redundant Cytokine Network that Induces a C/EBPβ Repressor in Macrophages but Not Monocytes
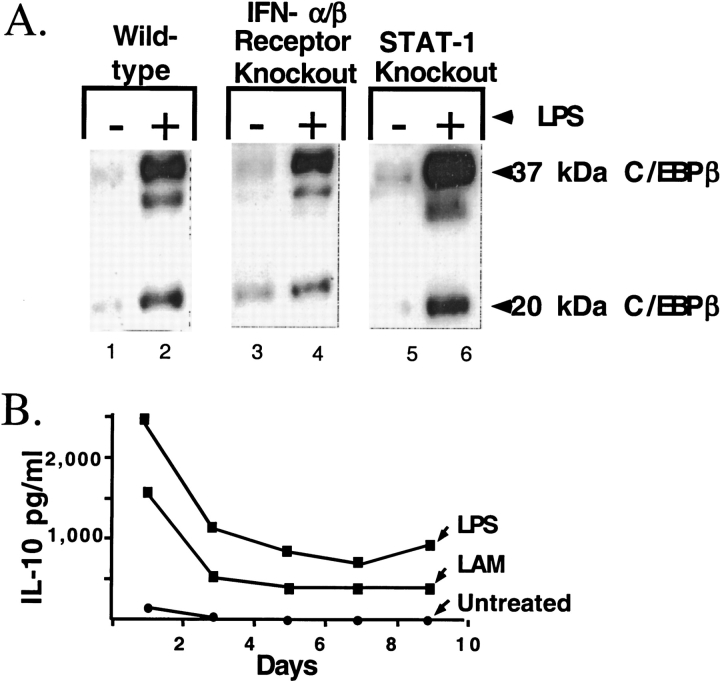
We have previously sown that production of the inhibitory transcription factor C/EBPβ is an IFN effect that represses HIV-1 replication in macrophages after an inflammatory stimulus (13). Macrophages with targeted deletion of STAT-1 and the type I IFN receptor were used to define the role of IFN signaling in production of the inhibitory C/EBPβ. Similar to human macrophages, wild-type murine bone marrow–derived macrophages stimulated with LPS upregulated both long-form stimulatory and short-form inhibitory C/EBPβ (Figure 1, lane 2). Therefore, bone marrow–derived macrophages from mice are a good model to study regulation of inhibitory C/EBPβ.
Figure 1.
The effect of stimulation with LPS or infection with M. tuberculosis on C/EBPβ expression and IL-10 production in macrophages. (A) C/EBPβ expression in bone-marrow-derived macrophages from wild-type and mutant mice. The mutations in the IFN pathways are labeled above the immunoblot. Lanes 1, 3, and 5 are without LPS stimulation. Lanes 2, 4, and 6 are with LPS stimulation for 72 h. (B) Monocyte-derived macrophages were treated with LPS or M. tuberculosis LAM. The increase IL-10 was sustained over nine days of cell culture. LPS induced 2-fold more IL-10 than did infection with M. tuberculosis.
The C/EBPβ repressor is induced by LPS in macrophages from mice deficient in the p100 type-I IFN (Figure 1, lanes 3 and 4) and in macrophages deficient in STAT-1 (Figure 1, lanes 5 and 6). IFN signaling is therefore not necessary for expression of the C/EBPβ repressor in macrophages. This suggests that other cytokines released in response to LPS will also induce the repressor. Since IL-10 is a well-characterized anti-inflammatory cytokine that is induced by LPS or M. tuberculosis LAM, we measured IL-10 production in our system. Stimulation with LPS or LAM strongly induced IL-10. Of note high levels of IL-10 were maintained for 9 d in cell culture (Figure 1B). LPS induced higher levels of IL-10 than did LAM, and so subsequent experiments were conducted with LPS stimulation.
IL-10 Produces Inhibitory C/EBPβ in Macrophages but Not Monocytes
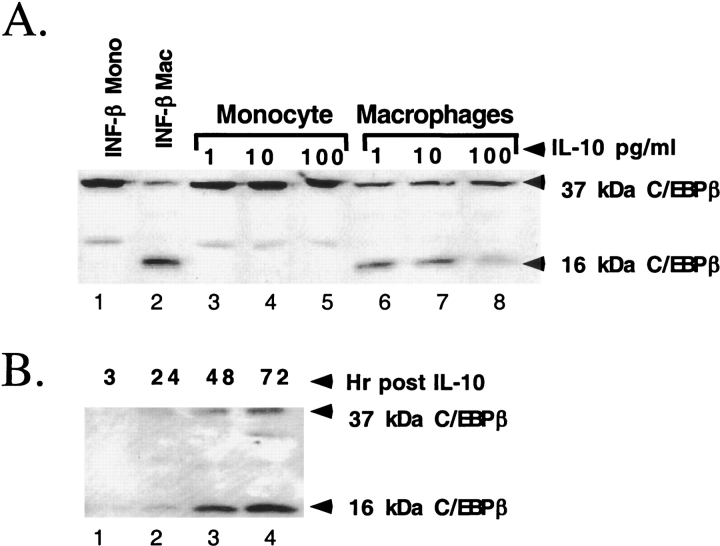
HL-60 cells, a transformed monocytic cell line, were treated with IL-10 or IFN-β. IFN-β did not alter C/EBPβ expression in monocytes but induced the inhibitory C/EBPβ after differentiation to macrophages (Figure 2A, lanes 1 and 2). IL-10 ranging in concentration from 1–100 pg/ml does not alter C/EBPβ expression in HL-60 monocytes (Figure 2A, lanes 3–5), but induced inhibitory C/EBPβ in macrophages (lanes 6–8). Of note, IFN-β induced the inhibitory C/EBP isoform more strongly than did IL-10. Similar results were obtained with THP-1 and U937 cells demonstrating the effect of differentiation was not cell line specific (data not shown).
Figure 2.
The effect of IL-10 on C/EBPβ expression in monocytes and macrophages. (A) Dose response of undifferentiated and differentiated HL-60 cells. A Western blot demonstrates that macrophages produce inhibitory 16-kD C/EBPβ after stimulation with either IFN-β or IL-10. Undifferentiated HL-60 monocytes fail to produce inhibitory 16-kD C/EBP. (B) Time course of C/EBPβ expression in differentiated THP-1 cells. There is significant induction of inhibitory C/EBPβ 24 h after stimulation of THP-1 macrophages with 100 pg/ml of IL-10. The level of inhibitory C/EBPβ continues to increase until 72 h after stimulation.
In the absence of cytokine treatment, there is little C/EBPβ protein expression. Treatment of differentiated THP-1 macrophages with 100 pg/ml of IL-10 led to significant induction of inhibitory C/EBPβ in 24 h (Figure 2B, lane 2). There was continued induction of C/EBPβ up to 72 h after stimulation (lanes 3 and 4). Taken together, these results demonstrate that only differentiated macrophages produce inhibitory C/EBPβ in response to cytokine stimulation.
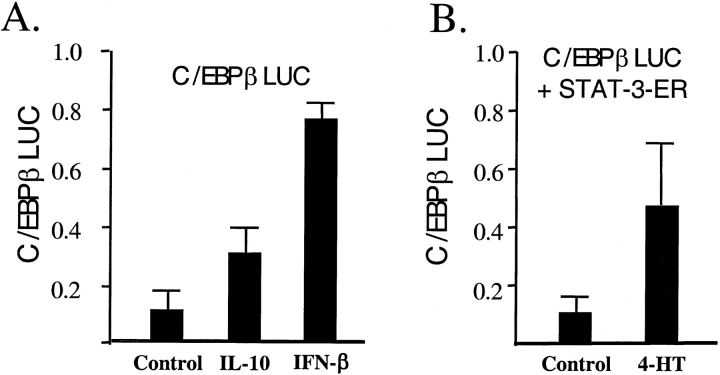
A C/EBPβ promoter reporter construct was used to test the transcriptional effects of IL-10 stimulation and STAT-3 activation on C/EBPβ transcription. IL-10 increased C/EBPβ promoter activity 3-fold, while IFN-β produced a 7-fold increase in macrophage-like cells (Figure 3A). To confirm that activated STAT-3 is capable of inducing C/EBPβ transcription, we used the C/EBPβ reporter construct co-transfected with an expression plasmid expressing STAT-3-ER. STAT-3-ER is a chimeric protein containing the estrogen binding domain of the estrogen receptor and the DNA binding domain of STAT-3. 4-hyrdoxy-tamoxifen activates the fusion protein leading to induction of promoters with STAT-3 binding sites. C/EBPβ promoter activity increased 5-fold when undifferentiated monocytic cells containing the STAT-3-ER construct were treated with 4-hydroxy-tamoxifen (Figure 3B).
Figure 3.
Stimulation of C/EBPβ. (A) IFN-β or IL-10 stimulate C/EBPβ promoter to produce luciferase in differentiated macrophages. IFN-β is more effective than IL-10. (B) Transfection of STAT-3/estrogen receptor (ER) plasmid results in dimerization of STAT-3/ER fusion protein after treatment with 4-hydroxy-tamoxifen. This results in stimulation of the C/EBPβ promoter in undifferentiated THP-1 cells.
IL-10 Inhibits HIV-1 Promoter Function and Replication in Macrophages but Not Monocytes
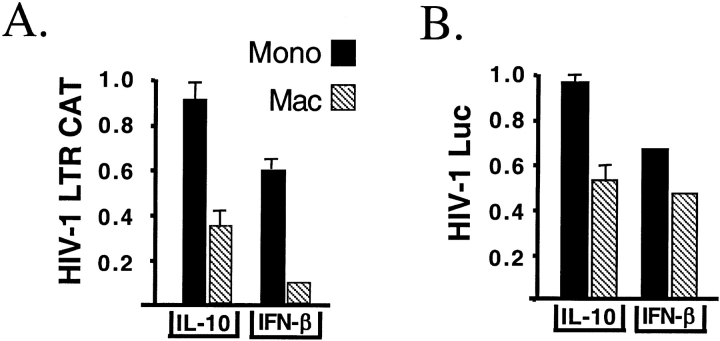
HIV-1 LTR promoter activity was used to test the functional effect of stimulation with IL-10. Initial experiments used BF-24 cells, which are THP-1 cells stably transfected with a CAT construct under the control of the HIV-1 LTR. In the absence of PMA differentiation, IL-10 had no effect on LTR activity in monocytic BF-24 cells, whereas IFN-β produced a 40% reduction. After differentiation, IL-10 inhibited HIV-1 LTR activity by 65%, while IFN-β produced a 90% reduction in CAT reporter activity (Figure 4A).
Figure 4.
The effect of IL-10 on HIV-LTR activity and HIV-1 replication in monocytes and macrophages. (A) IL-10 has no effect on LTR activity in undifferentiated monocytic cells, while IFN-β produces a 40% reduction of LTR activity (black bars). IFN-β or IL-10 inhibit HIV-1 LTR–driven CAT production in differentiated BF-24 cells 24 h after treatment (hatched bars). IL-10 produces a 60% inhibition, while IFN-β produces a 90% inhibition. The reporter activity in the absence of stimuli is defined as 1. (B) IL-10 has no effect on mRNA expression after infection of monocytic cells with pseudotyped HIV-1 with luciferase substituted for the envelope gene. IFN-β produces a 40% reduction in undifferentiated cells. IL-10 and IFN-β produce a 50% reduction in reporter expression in differentiated THP-1 cells. The reporter activity in the absence of stimuli is defined as 1.
A pseudotyped HIV-1, carrying HIV-1JR-FL envelope with luciferase in place of the envelope gene, was used to confirm the conclusion of the LTR reporter construct. IL-10 did not alter HIV-1 LTR–driven luciferase reporter activity when pseudotyped HIV-1 infected undifferentiated THP-1 cells. IFN-β did produce a 40% reduction. Once THP-1 cells were differentiated by PMA into macrophages, both IL-10 and IFN-β produced a 50% decline in HIV-1 LTR luciferase reporter activity (Figure 4B). These results demonstrate that IL-10 has antiviral activity only in differentiated macrophage like cells.
STAT-3 Regulates C/EBPβ Production after IL-10 Stimulation
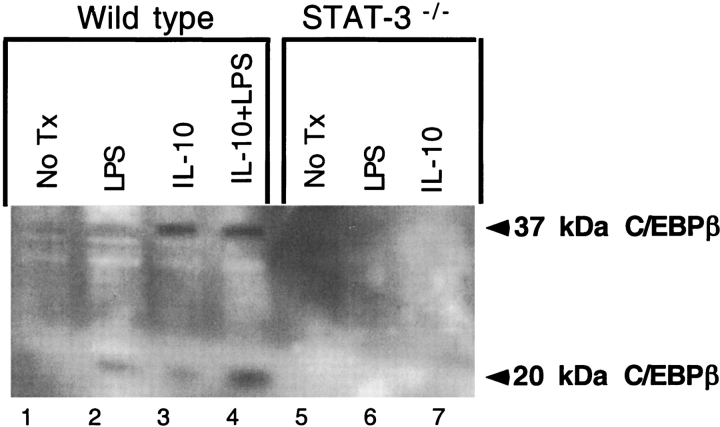
Since STAT-3 is a transcription factor critical for IL-10 signaling, macrophages with targeted deletion of STAT-3 were used to test if STAT-3 was necessary for production of inhibitory C/EBPβ after stimulation with IL-10 or LPS. Cultured wild-type mouse peritoneal macrophages do not express inhibitory C/EBPβ (Figure 5A, lane 1). IL-10, LPS or a combination of these two stimuli induces the inhibitory isoform (Figure 5A, lanes 2–4). Peritoneal macrophages with targeted deletion of STAT-3 no longer produce the inhibitory isoform after stimulation with LPS or IL-10 (Figure 5A, lanes 6 and 7).
Figure 5.
The effect of alteration of STAT-3 level and activity on C/EBPβ expression in monocytes and macrophages. Wild-type peritoneal macrophages produce inhibitory C/EBPβ after treatment with LPS or IL-10 (lanes 2 and 3). The combination is synergistic (lane 4). The STAT-3 knockout macrophages fail to induce C/EBPβ after stimulation with both LPS and IL-10 (lanes 6 and 7).
Differentiation Leads to Post-Transcriptional Upregulation of STAT-3 and Gain of IL-10 Response
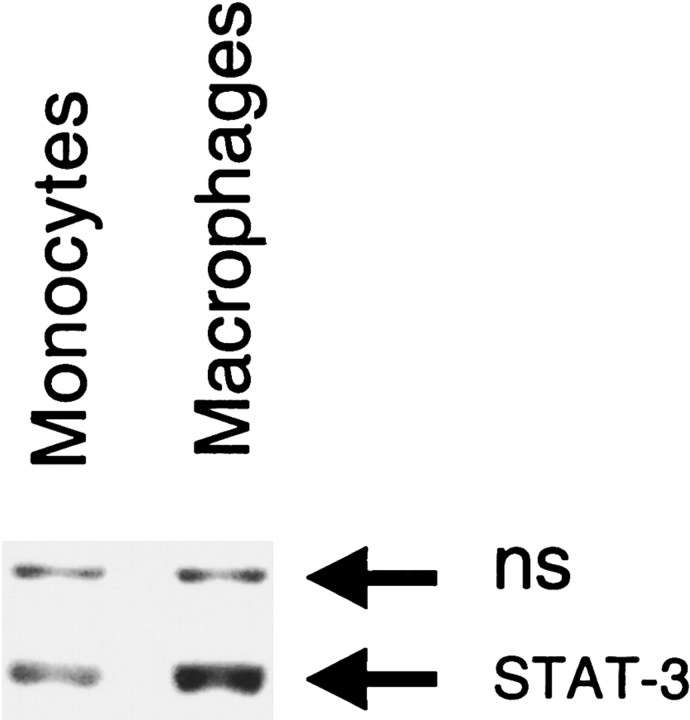
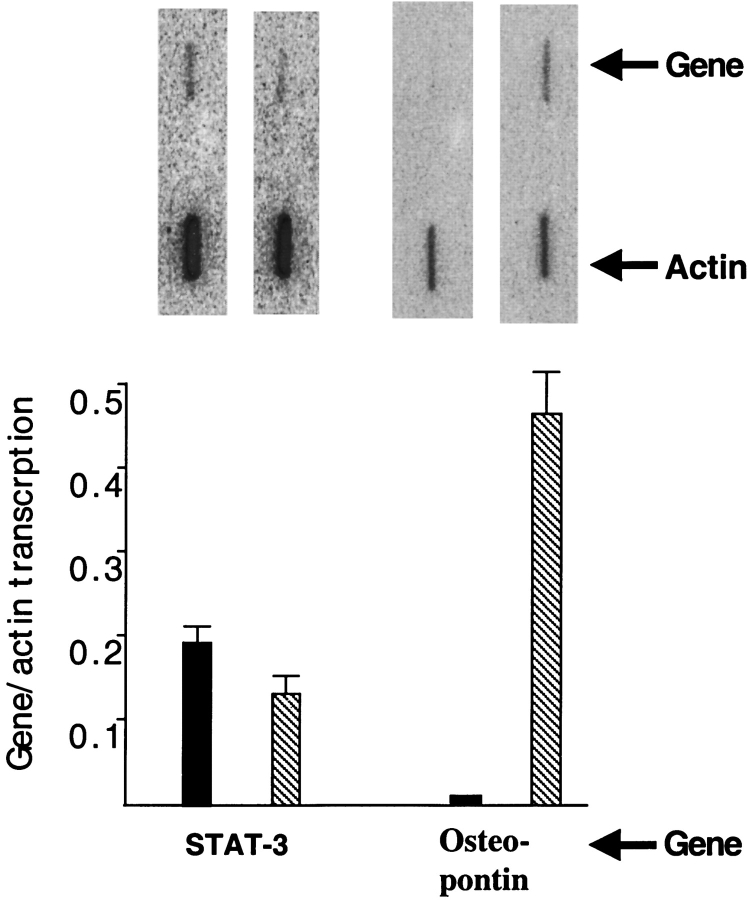
To test if differentiation altered the level of STAT-3 protein, an immunoblot of extracts from monocytic and macrophage-like cells was performed. Differentiation produced a 3-fold increase in STAT-3 protein (Figure 6, compare lanes 1 and 2). To test if this was due to induction of STAT-3 transcription, a nuclear run was performed. Differentiation did not alter STAT-3 transcription (Figure 7). Transcription of osteopontin was induced over 50-fold, demonstrating that differentiation did alter transcription of some genes (Figure 7). The discrepancy between transcription and protein suggests that differentiation leads to post-transcriptional induction of STAT-3.
Figure 6.
The effect of differentiation on STAT-3 protein. Differentiation of THP-1 monocytes to macrophages increased STAT-3 protein expression 3-fold. NS represents a nonspecific band used as an internal control for loading.
Figure 7.
The effect of differentiation on STAT-3 transcription. Differentiation of THP-1 monocytes to macrophages does not increase transcription of STAT-3. Differentiation led to over a 50-fold increase in osteopontin transcription. Blot of run-on experiment presented at the top and quantitation presented at the bottom of the figure.
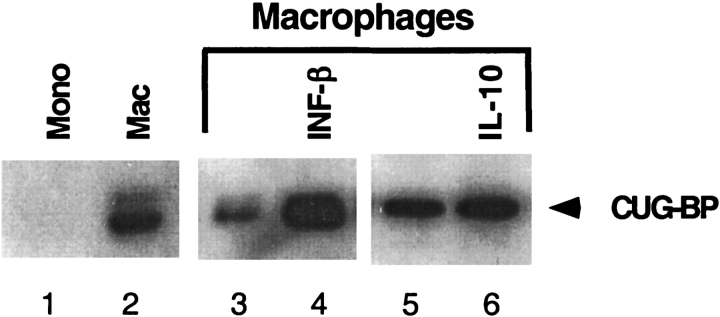
CUG-binding protein leads to production of inhibitory C/EBPβ during in vitro translation (16). Since only macrophages produced inhibitory C/EBPβ, nuclear extracts of monocytes and macrophages were tested for CUG-binding protein expression. In the absence of differentiation, no CUG-binding protein was detected (Figure 8, lane 1). After differentiation, macrophages strongly expressed CUG-binding protein (lane 2). There was further induction of CUG-binding protein six hours after treatment with IFN-β (compare lanes 3 and 4), while IL-10 treatment produced little induction of CUG-binding protein (compare lanes 5 and 6). The ability to produce inhibitory C/EBPβ therefore correlates with the presence of CUG-binding protein.
Figure 8.
The effect of differentiation, IFN-β, and IL-10 on CUG-binding protein. Undifferentiated THP-1 cells do not express CUG-binding protein (lane 1). PMA differentiation strongly induces CUG-binding protein (lane 2). Six hours after treatment with 1 U/ml of IFN-β, there is strong induction of CUG-binding protein (compare lane 3 to lane 4). IL-10 treatment did not produce significant alteration of CUG-binding protein in macrophages (lanes 5 and 6).
DISCUSSION
We have demonstrated that induction of the inhibitory C/EBPβ is an interferon effect in macrophages but not monocytes (19). Induction of this transcriptional repressor inhibits HIV-1 LTR promoter activity and viral replication (8, 11). When expression of the inhibitory C/EBPβ is specifically inhibited by an antisense oligonucleotide, HIV-1 replication in macrophages is enhanced (33, 34). In vivo TB infection accelerates HIV-1 replication in alveolar macrophages through downregulation of inhibitory C/EBPβ and activation of NF-κB (3). We now report that like IFNs, the anti-inflammatory cytokine IL-10 induces the C/EBPβ repressor in macrophages but not monocytes. Induction of C/EBPβ by IL-10 may explain the inhibition of HIV-1 replication after PPD stimulation of CD8 cells in vitro (27). Further, IFN-γ, the type II IFN, is able to induce inhibitory C/EBPβ in macrophages, and this may inhibit HIV-1 replication observed after aerosolized IFN-γ (35). The state of differentiation appears to be the critical parameter controlling the ability of cells to produce this repressor in response to cytokine stimulation.
We used transformed monocytic cell lines THP-1 U937 and HL-60 to model the regulation of C/EBPβ in monocytes. After treatment with PMA, these cells differentiate and regulate the C/EBPβ and NF-κB transcription factor expression like primary monocytes and macrophages (3, 13, 19). Undifferentiated THP-1 monocytes are unresponsive to IL-10. This cytokine does not affect C/EBPβ expression or expression of transcription factors mediating IFN effect in undifferentiated cells. After differentiation, IL-10 induces transcription of STAT-3 in a positive autoregulatory loop and also increases STAT-3 protein. Evidence that supports this conclusion includes: (1) differentiated THP-1 cells express 3-fold higher STAT-3 protein concentration than do undifferentiated THP-1 cells, (2) murine macrophages deficient in STAT-3 are unable to induce the C/EBPβ repressor after stimulation with IL-10 or LPS, and (3) engineered activation of a chimeric STAT-3-ER protein leads to enhanced C/EBPβ promoter activity in undifferentiated THP-1 cells. These data demonstrate that activated STAT-3 is both necessary and sufficient for induction of C/EBPβ after stimulation with IL-10.
Similar to the type I interferon system, the effect of differentiation is mediated by post-transcriptional induction of a transcription factor critical for cytokine signaling. In the case of IL-10, the signaling intermediate is STAT-3. In the case of type I interferon, the signaling intermediate is IRF-9. Since signaling by IFN-γ does not require IRF-9, monocytes are capable of responding to this cytokine. The effectiveness of the STAT-3-ER fusion in driving C/EBPβ transcription in undifferentiated THP-1 cells demonstrates that other components of the transcriptional machinery required for expression of C/EBPβ are present in monocytic cells. The CUG-binding protein was induced by differentiation. The presence of this RNA-binding protein led to production of inhibitory C/EBPβ during in vitro translation (16). It is likely that upregulation of this protein during differentiation contributes to the ability of differentiated cells to produce the inhibitory C/EBPβ in response to IL-10 and perhaps other cytokines.
The data reported in this investigation emphasize the importance of the state of differentiation in determining the response to cytokine stimulation. Differentiation induces latent transcription factors required for type I IFN and IL-10 responsiveness. This increased signaling capacity is complemented by a gain in the capacity to produce a C/EBPβ repressor protein from an intronless mRNA. Formation of STAT-3 dimer or ISGF-3 would increase transcription of the C/EBPβ gene. In monocytes, translation of this mRNA produces a stimulatory C/EBPβ isoform and activate transcription of proinflammatory genes (19).
In macrophages, induction of C/EBPβ transcription would lead to translation of a repressor, inhibiting transcription of the same proinflammatory genes that were stimulated in monocytes (9, 10). Repression of cytokine genes that contain C/EBP sites may explain the deleterious effect of high levels of IL-10 production on the inflammatory response during tuberculosis (23–27).
In active inflammation in the lower respiratory tract in TB, sarcoidosis, asbestosis, etc., there is recruitment of monocytes, which can produce cytokines that amplify inflammation. As the inflammation declines, IL-10 and differentiated macrophages can further dampen inflammation by induction of inhibitory C/EBPβ protein. Also, since C/EBP sites exist in the promoters of many viral genes (36), expression of the C/EBPβ repressor in resting alveolar macrophages will affect many viral types and may be one mechanism of resistance to viral infection of the lung.
It is likely that the alterations in RNA processing and translation induced by differentiation will affect other pathways. Expression of the repressor is abolished when activated lymphocytes contact the macrophage (3). This contact-mediated derepression then produces a macrophage capable of producing proinflammatory cytokines, supporting high-level HIV-1 replication. The interplay of repression and derepression will influence the large number of inflammatory genes containing promoters with C/EBP sites. This interplay will likely be pivotal in determining if a given stimulus leads to up- or downregulation of inflammation and HIV-1 replication.
Acknowledgments
The authors thank Dr. Littman for providing the pseudotyped HIV-1, Dr. Yokota for providing the STAT-3 ER expression vector, Dr. Calame for providing the C/EBP reporter construct, Tatsushi Matsumura for assistance with the luminometer, and Dr. Borkowsky for useful discussion.
Supported by NIH/NCRR MO1 RR00096, NIH HL 57879, NIH HL 59832, the American Lung Association, the Center for AIDS Research, and the Japanese Foundation for AIDS Prevention.
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, Dye C. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med 2003;163:1009–1021. [DOI] [PubMed] [Google Scholar]
- 2.Toossi Z. Virological and immunological impact of tuberculosis on human immunodeficiency virus type 1 disease. J Infect Dis 2003;188:1146–1155. [DOI] [PubMed] [Google Scholar]
- 3.Hoshino Y, Nakata K, Hoshino S, Honda Y, Tse D, Shioda T, Rom WN, Weiden M. Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med 2002;195:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orenstein JM, Fox C, Whal SM. Macrophages as a source of HIV during opportunistic infections. Science 1997;276:1857–1861. [DOI] [PubMed] [Google Scholar]
- 5.Nakata K, Rom W, Honda Y, Condos R, Kanegasaki S, Cao Y, Weiden M. M. tuberculosis enhances human immunodeficiency virus-1 replication in the lung. Am J Respir Crit Care Med 1997;155:996–1003. [DOI] [PubMed] [Google Scholar]
- 6.Finzi D, Hermankova M, Pierson T, Carruth L, Buck C, Chaisson R, Quinn T, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 1997;278:1295–1300. [DOI] [PubMed] [Google Scholar]
- 7.Tesmer VM, Rajadhyaksha A, Babin J, Bina M. NF IL-6-mediated transcriptional activation of the long terminal repeat of the human immunodeficiency virus type 1. Proc Natl Acad Sci USA 1993;90:7298–7303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson AJ, Calame KL. CCAAT/enhancer binding protein (C/EBP) sites are required for HIV-1 replication in primary macrophages but not CD4+ T cells. Proc Natl Acad Sci USA 1997;94:8714–8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pope RM, Leutz A, Ness SA. C/EBP-Beta regulation of the tumor-necrosis-factor-alpha gene. J Clin Invest 1994;94:1449–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Broser M, Rom WN. Activation of the interleukin-6 gene by Mycobacterium tuberculosis or lipopolysaccharide is mediated by NF-IL6 and NF-κB. Proc Natl Acad Sci USA 1994;91:2225–2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henderson AJ, Connor RI, Calame KL. C/EBP activators are required for HIV-1 replication and proviral induction in monocytic cell lines. Immunity 1996;5:91–101. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Nakata K, Weiden M, Rom W. Mycobacterium tuberculosis enhances HIV-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest 1995;95:2324–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honda Y, Rogers L, Nakata K, Zhao B, Pine R, Nakai Y, Kurosu K, Rom WN, Weiden M. Type I interferon induces inhibitory 16 kDa C/EBP-β repressing the HIV-1 LTR in macrophages: pulmonary tuberculosis alters C/EBP expression enhancing HIV-1 replication. J Exp Med 1998;188:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 1991;67:569–579. [DOI] [PubMed] [Google Scholar]
- 15.An MR, Hsieh CC, Reisner PD, Rabek JP, Scott SG, Kunninger DT, Papaconstantinou J. Evidence for posttranscriptional regulation of C/EBP-α and C/EBP-β isoform expression during lipopolysaccharide-mediated acute-phase response. Mol Cell Biol 1996;16:2295–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldwin BR, Timchenko NA, Zahnow CA. Epidermal growth factor receptor stimulation activates the RNA binding protein CUG-BP1 and increases expression of C/EBPß-LIP in mammary epithelial cells. Mol Cell Biol 2004;24:3682–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J, Cantwell CA, Johnson PF, Pfarr CM, Williams SC. Transcriptional activity of CCAAT/enhancer-binding proteins is controlled by a conserved inhibitory domain that is a target for sumoylation. J Biol Chem 2002;277:38037–38044. [DOI] [PubMed] [Google Scholar]
- 18.Henderson AJ, Zou X, Calame K. C/EBP proteins activate transcription from human immunodeficiency virus type 1 long terminal repeat in macrophages/monocytes. J Virol 1995;69:5337–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiden M, Tanaka N, Qiao Y, Zhao BY, Honda Y, Nakata K, Canova A, Levy DE, Rom WN, Pine R. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and C/EBPβ expression. J Immunol 2000;165:2028–2039. [DOI] [PubMed] [Google Scholar]
- 20.Hwang S, Herzog PJ, Holland K, Sumarsono S, Tymms MJ, Hamilton JA, Whitty G, Bertoncello I, Kola I. A null mutation in the gene encoding a type I interferon receptor component eliminnates antiproliferative and antiviral responses to interferon α and β and alters macrophage responses. Proc Natl Acad Sci USA 1995;92:11284–11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Durbin J, Hackenmiller R, Simon M, Levy D. Targeted disruption of the mouse Stat-1 gene results in compromised innate immunity to viral disease. Cell 1996;84:443–450. [DOI] [PubMed] [Google Scholar]
- 22.Boussiotis VA, Tsai EY, Yunis EJ, Thim S, Delgado JC, Dascher CC, Berezovskaya A, Rousset D, Reynes JM, Goldfeld AE. IL-10-producing T cells suppress immune responses in anergic tuberculosis patients. J Clin Invest 2000;105:1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R, et al. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis 1999;180:2069–2073. [DOI] [PubMed] [Google Scholar]
- 24.Othieno C, Hirsch CS, Hamilton BD, Wilkinson K, Ellner JJ, Toossi Z. Interaction of Mycobacterium tuberculosis-induced transforming growth factor beta 1 and interleukin-10. Infect Immun 1999;67:5730–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonecini-Almeida MG, Ho JL, Boechat N, Huard RC, Chitale S, Doo H, Geng JY, Rego L, Lazzarini LCO, Kritski AL, et al. Down-modulation of lung immune responses by interleukin-10 and transforming growth factor beta (TGF-beta) and analysis of TGF-beta receptors I and II in active tuberculosis. Infect Immun 2004;72:2628–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delgado JC, Baena A, Thim S, Goldfeld AE. Ethnic-specific genetic associations with pulmonary tuberculosis Source. J Infect Dis 2002;186:1463–1468. [DOI] [PubMed] [Google Scholar]
- 27.Ranjbar S, Ly N, Thim S, Reynes JM, Goldfeld AE. Mycobacterium tuberculosis recall antigens suppress HIV-1 replication in anergic donor cells via CD8(+) T cell expansion and increased IL-10 levels. J Immunol 2004;172:1953–1959. [DOI] [PubMed] [Google Scholar]
- 28.Fortsch D, Rollinghoff M, Stenger S. IL-10 converts human dendritic cells into macrophage-like cells with increased antibacterial activity against virulent Mycobacterium tuberculosis. J Immunol 2000;165:978–987. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, Yokota T. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J 1999;18:4261–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takeda K, Clausen BE, Kaisho T, Tsujimura T, Terada N, Forster I, Akira S. Enhanced Th1 activity and development of chronic enterocolitis in mice devoid of Stat3 in macrophages and neutrophils. Immunity 1999;10:39–49. [DOI] [PubMed] [Google Scholar]
- 31.Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity 2002;17:63–72. [DOI] [PubMed] [Google Scholar]
- 32.Geijtenbeek TBH, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GCF, Middel J, Cornelissen ILMHA, Nottet HSLM, Kewal-Ramani VN, Littman DR, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 2000;100:587–597. [DOI] [PubMed] [Google Scholar]
- 33.Berrier A, Siu G, Calame K. Transcription of a minimal promoter from the NF-IL6 gene is regulated by CREB/ATF and SP1 proteins in U937 promonocytic cells. J Immunol 1998;161:2267–2275. [PubMed] [Google Scholar]
- 34.Komuro I, Yokota Y, Yasuda S, Iwamoto A, Akagawa KS. CSF-induced and HIV-1-mediated distinct regulation of Hck and C/EBP beta represent a heterogeneous susceptibility of monocyte-derived macrophages to M-tropic HIV-1 infection. J Exp Med 2003;198:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Condos R, Raju B, Canova A, Zhao B, Weiden M, Rom WN, Pine R. Recombinant interferon-gamma stimulates signal transduction and gene expression in alveolar macrophages in vitro and in tuberculosis patients. Infect Immun 2003;71:2058–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Akira S, Isshiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J 1990;9:1897–1906. [DOI] [PMC free article] [PubMed] [Google Scholar]