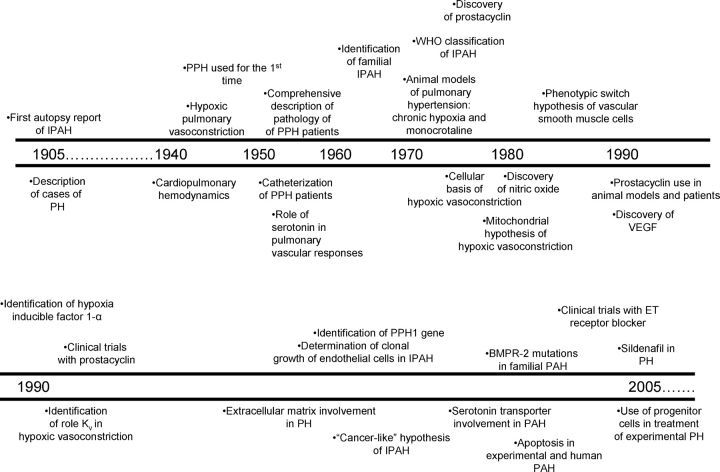
Pulmonary hypertension (PH) is defined hemodynamically as a systolic pulmonary artery pressure (PAP) greater than 30 mm Hg, or elevation of mean PAP in excess of 25 mm Hg. Behind this rather simple physiologic definition lie a large number of cellular and molecular alterations of the pulmonary arteries that ultimately account for the disease. The immense progress in the past 100 years of basic and clinical research of PH illustrates how biomedical science ultimately results in life-saving therapies, dramatically influencing patient morbidity and mortality (Figure 1).
Figure 1.
Timeline of significant achievements in the last 100 years of pulmonary hypertension research.
Since the autopsy observation of the first case of “pulmonary vascular sclerosis” by Romberg in 1891, our understanding of PH has evolved in parallel with the scientific achievements of the last century—each “small step” from the viewpoint of hindsight represents a significant achievement when framed in its historical context. Notwithstanding our tendency to consider only the most recent scientific advancements, we owe our current knowledge to those investigators dedicated to PH research over the past 100 years. PH is a syndrome that probably includes several diseases, all of which share increased pulmonary artery pressures. As such, any single clinical characterization or disease model used falls short of comprehensively addressing this complex syndrome, which varies with regards to its severity, the site of increased vascular resistance, and its association to underlying clinical conditions as outlined in a recently updated classification of pulmonary arterial hypertension (PAH [1]). Although this review emphasizes some of the most important human and animal experimental findings related to PH in general, a significant focus of the past 100 years of research has been the study of the rare, idiopathic form of PAH (IPAH, previously known as primary pulmonary hypertension or PPH). In this review, we will describe the early understanding of PH as a “novel disease” in the beginning of the century, based on the initial recognition of the clinical presentation in cohorts of patients with pulmonary hypertension, and the pathologic characterization of diseased pulmonary arteries. We will then emphasize the impact of the development of hemodynamic assessment of the pulmonary circulation, and highlight the modern age of cellular, molecular, and genetic milestones in the understanding of PH.
PULMONARY HYPERTENSION: THE EARLY AGES
As masterfully delineated by Fishman (2), the investigation of PH can be segregated into two “eras”: before and after the development of pulmonary arterial catheterization in the 1940s and '50s. Prior to pulmonary catheterization, PH was recognized in patients with cyanosis and/or unexplained right ventricular hypertrophy—possibly related to congenital heart malformations (3). From these early clinical descriptions of patients with potentially mixed clinical presentations, our understanding of PH evolved with a detailed description of pathologic specimens, and the clinical study of small cohorts of patients with unexplained right ventricular hypertrophy. In the 1900s PH was blamed on syphilis, a popular culprit for unknown vascular diseases at the turn of the century (3). Early attempts to identify relevant pulmonary vascular lesions led to notions such as the hypotheses that the pulmonary vascular lesions would arise from a congenital thinning of the pulmonary artery media (4). Interestingly, we came to realize much later that PH might indeed involve enhanced elastolytic activity as the preamble for the molecular mechanisms leading to medial hypertrophy (5).
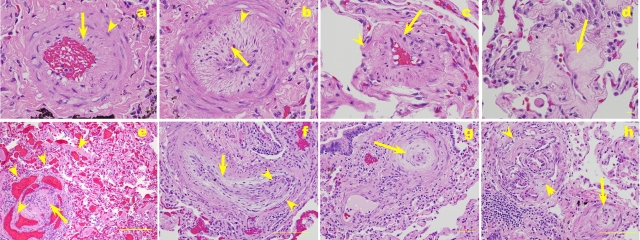
Strikingly detailed descriptions of the pulmonary vascular lesions in PPH highlighted the existence of intima lesions containing phenotypically altered endothelial cells (4), and predicted that these lesions might have arisen from proliferating endothelial cells. In a landmark report, Heath and Edwards detailed the vascular pathology of PH in an extensive study of 67 lungs of patients with PH associated with congenital heart malformations, and a single case of IPAH (6). This work proposed a structural classification for PH associated with congenital heart malformations, framing the pulmonary vascular remodeling into six grades based on the persistence of a fetal circulation pattern, variable degrees of medial and intima thickening, plexiform lesions, dilation lesions, and necrotizing arteritis. Although this classification became the standard characterization of all forms of PH, it was originally proposed solely for the classification of PH associated with congenital heart malformation, and not of other forms of PH, such as IPAH. In this detailed pathologic description, it is of interest that the authors speculated that some of the pathologic alterations were due to raised pulmonary artery pressures or that the high pulmonary vascular resistance led to the more complex forms of vascular remodeling such as plexiform and dilation lesions (Figure 2).
Figure 2.
Pulmonary vascular remodeling in a lung of a patient with IPAH. (a) Pulmonary artery with medial hypertrophy (arrowhead), with intima thickening by longitudinally oriented smooth muscle cells (arrow). (b) Pulmonary artery with marked medial and intima thickening, with crenated internal elastic layer (dark arrow), suggestive of vasoconstriction. (c) Peripheral muscularization of a small precapillary pulmonary artery (arrowhead), also showing intima thickening. (d) Peripheral pulmonary artery with collagenous intima thickening (arrow). (e) Plexiform lesion (arrow) surrounded by markedly vascular dilation compromising both vessels draining the lesion and in alveolar capillaries (arrowheads). (f) Longitudinal section of a pulmonary artery with a concentric arrangement of elongated smooth muscle cell–like cells (arrow) and a plexiform lesion (flanked by arrowheads). (g) Concentric lesion cross-sectioned (arrow). (h) Plexiform lesion (arrowheads) and an adjoining pulmonary artery with marked intima thickening leading to reduction of vascular lumen (bar = 20 μm in a–d and g; 200 μm in e, f, and h).
The fundamental importance of pathology was evident in the first classification scheme devised by the World Health Organization (2). The term primary pulmonary hypertension included chronic thromboembolic disease, pulmonary venoocclusive disease, and primary plexogenic arteriopathy (when plexiform lesions were present). This classification relied on the interpretation of lung pathology as critical in defining the disease process. However, the limitations of this classification became readily apparent as the vascular lesions that underlie this classification (e.g., plexiform lesions) could not help discriminate the underlying conditions associated with PAH, since they are not pathognomonic of any particular form of PH (with the exception that plexiform lesions are only present in severe PH). Currently, PH is classified hemodynamically and clinically, rather than based on the pulmonary vascular pathology to direct diagnosis and treatment (7). Although variations of this classification eventually emerged (8), all have suffered from a lack of insight into how the lesions are caused. Whether the etiologic agents of PH produce specific pathologic lesions, what the relationship is between the underlying clinical conditions associated with PH (such as HIV infection, scleroderma, etc.) and the vascular injury, and how the vascular lesions relate to the hemodynamic impact on pulmonary artery pressures, remain unclear. This sets the backdrop of our current interpretation of the pathologic alterations in PH (9). Interrogation of the nature of these structural elements began about 40–50 years later, as for example, in studies that led to the determination of clonal growth and somatic mutations involving endothelial cells from these lesions (also known as plexiform lesions) (10).
THE HEMODYNAMIC ERA: THE AGE OF HYPOXIC PULMONARY VASOCONSTRICTION AND CELLULAR AND MOLECULAR BASIS OF PH
Hypoxic Pulmonary Vasoconstriction and Hypoxic Pulmonary Hypertension
Our modern understanding of PH, particularly IPAH, started with the advent of pulmonary catheterization (11) as a tool to assess pulmonary vascular hemodynamics. This “physiological revolution” fundamentally changed our understanding of the pulmonary circulation and treatment of PH (2), as it forced us to move beyond the finding of unexplained right ventricular failure or descriptive pathologic assessments of pulmonary vascular lesions (12). Primary pulmonary hypertension, a term that until recently galvanized the interest in PH, was introduced in the 1950s to categorize patients who had isolated elevation of pulmonary artery pressures.
One of the major contributions of the introduction of cardiac and pulmonary artery catheterization was the landmark discovery of hypoxic vasoconstriction (13, 14). As the terminal airway and alveolar oxygen (but not necessarily pulmonary artery Po2) levels decrease below 60 mm Hg, the pulmonary artery contracted potentially improving V̇/Q̇ mismatch by redirecting blood flow to more oxygenated alveoli. The understanding of the molecular mechanisms underlying hypoxic pulmonary vasoconstriction (HPV) progressed toward elucidating whether local cellular mediators would trigger HPV or, alternatively, the HPV response would be directly transduced on vascular smooth muscle cells. The first evidence in support of a direct effect of alveolar hypoxia in HPV was the demonstration that Ca+2 channel blockers inhibited smooth muscle contractility and HPV (15).
The hypoxia sensing mechanisms are one of the most fascinating ongoing areas of study of the pulmonary circulation field. Most of the evidence accumulated thus far points to the mitochondrial electron transport as a source of free radicals (acting as mediators) that might give rise to hypoxia-triggered vasoconstrictive (effector) mechanisms (16). Among these mechanisms, we have learned that hypoxia inhibits the family of voltage-dependent potassium channels, Kv (17), resulting in depolarization of the resting membrane potential and calcium entry through voltage-dependent calcium channels. The increase in intracellular calcium activates calcium/calmodulin myosin light chain kinase, leading to enhanced cell tension and cellular proliferation, all of which account for the smooth muscle cell hypertrophy and hyperplasia. Pulmonary artery smooth muscle cells from patients with IPAH have decreased potassium current correlating with a decreased mRNA/protein level of the potassium channel Kv1.2 and Kv1.5 (18). In aggregate, the research centered on the identification and elucidation of HPV has influenced significantly our understanding of the effects of alveolar hypoxia on diseases such as atelectasis, emphysema, obstructive sleep apnea, and cystic fibrosis, all of which may be complicated to some degree by PH. Most importantly, HPV has provided the underpinnings of the “excessive vasoconstriction hypothesis” of PH (12, 19).
Chronic hypoxia could then lead to chronic PH (20). Although a clear mechanistic connection between the acute (e.g., HPV) and chronic hypoxic responses of the pulmonary circulation has not been established up to the present time, a (somewhat weak) link has evolved between vasoconstriction and chronic pulmonary vascular remodeling in the setting of PH (21). Although chronic hypoxia continues to serve as a model of human PH and certainly models some forms of PH associated with respiratory disorders, its relation to human idiopathic PH remains unclear, since hypoxic remodeling does not show marked luminal reduction by intimal growth and complex vascular lesions as is the case in severe PAH.
Cellular Basis of Pulmonary Hypertension
While the first 50 years of PH research defined most of our understanding of the pathology of human PH, the last 50 years of PH research has benefited from the development of animal models, such those involving exposure to chronic hypobaric or nitrogen-diluted normobaric hypoxia and endothelial cell injury by the pyrrole liver metabolite of seeds of Crotalaria spectabilis (monocrotaline) (20, 22). These models have helped us to understand the role of vascular cells in pulmonary vascular remodeling, to elucidate the role of mediators in pulmonary vessel control, and to develop new therapies.
Although the early pathologic descriptions of the human vascular remodeling were remarkably thorough, the complexity of the cellular responses in pulmonary vessel remodeling continues to be uncovered. Initially believed to serve a mere barrier, the endothelial cells were found to be both sensors and effectors able to transmit changes in flow and pressure through the release of vasoregulator and growth factors. Although all endothelial cells were initially considered a phenotypically homogeneous population, it has become apparent that endothelial cells from different organs are indeed uniquely fitted to perform specific functions in the context of organ specialization. There is increasing recognition that there are at least two forms of endothelial cells within the lung—the micro- and macro-vascular cells that can be distinguished by lectin affinity (23). Similarly, the medial smooth muscle layer is at least as complex. A minimum of four phenotypically distinct SMC subpopulations that respond differently to stress exist within the media of the adult bovine main and proximal pulmonary arteries (24). While not as well studied, the adventitial layer appears to be heterogeneous as well.
A major debate in the dispute regarding the most relevant vascular cell in the pathogenesis of PH has centered on the “abnormal” cell type in the development of PH. Pathologic changes occur in all three layers of the pulmonary arteries. Given the uniformity of medial hypertrophy in all forms of PH, many have believed that the abnormality rested in the vascular smooth muscle cells and, therefore, much effort was placed in understanding the biology of these cells. Although we learned that the proximal pulmonary arteries are composed of several different smooth muscle cell phenotypes which uniquely respond to stress, questions still remain about the cellular fate of the remodeled smaller pulmonary arteries. A conceptual advance in the field was the realization that pulmonary vascular smooth muscle cells would undergo a phenotypic switch in PH, from a contractile normal phenotype to a synthetic phenotype leading to cell growth and matrix deposition (25). We learned that the abnormal smooth muscle cell phenotype would also include the overexpression of endothelin (26) or serotonin transporters (27), which play a role in PH as discussed below. Furthermore, the altered smooth muscle cells in PH may arise from other pathways than the phenotypic switch from a resident smooth muscle cell, since there is recent evidence that endothelial cells might transdifferentiate into smooth muscle cells (28). With the increased understanding of the role of progenitor and stem cells in organ repopulation, there might be indeed a role for progenitor/stem cell in giving rise to smooth muscle or endothelial cells in the remodeled pulmonary arteries.
Endothelial cells have occupied a center role in the current understanding of the pathogenesis of IPAH (29). The past 15 years have witnessed the rise of the endothelial cell as a central player in the pathogenesis of severe PAH. Endothelial cells from IPAH patients have decreased expression of prostacyclin synthase, suggesting that they might be the main determinant of the decreased ratio of prostacyclin metabolite 2,3-dinor-6-keto prostaglandin F1α to thromboxane in the urine of patients with IPAH when compared with normal individuals (30). Furthermore, decreased expression of endothelial cell nitric oxide synthase was documented in IPAH vessels (31), whereas endothelin expression was increased (26). The investigation of the molecular nature of the endothelial cells in the characteristic plexiform lesion revealed that these endothelial cells undergo a clonal growth in IPAH pulmonary arteries (10), suggesting for the first time that mutations in tumor suppressor genes might underlie the pathogenesis of IPAH (32), and that the abnormal cell proliferation in IPAH might follow pathogenetic paradigms similar to cancer growth (33).
The past two decades witnessed an enhanced understanding of the role of the extracellular matrix in the pathobiology of PH. This paradigm is based on the identification of a serum-derived elastase that, when able to permeate through the vascular wall, leads to enhanced smooth muscle cell production of a matrix protein, tenascin, capable of stimulating smooth muscle cell growth (5). However, due to discrepant results, we still lack a clear understanding of the pathophysiologic roles of metalloproteases in PH.
We have now come to consider the balance of cell proliferation and apoptosis as ultimately accounting for the remodeling of pulmonary arteries. Excessive endothelial and smooth muscle cell proliferation was detected in experimental models of PH (34). However, the potential pathogenetic role of apoptosis in PH was documented by the finding that IPAH lungs showed a remarkable low level of apoptotic cells (35). Apoptosis may play discrepant roles in the induction and the resolution stages of experimental pulmonary vascular remodeling. Early endothelial cell apoptosis was required for the induction of pulmonary vascular remodeling and severe PH (36), whereas smooth muscle cell apoptosis followed the inhibition of vascular elastase activity and the de-remodeling of hypertensive pulmonary arteries (37). Furthermore, K+ channels not only regulate hypoxic vasoconstriction, but also allow for smooth muscle cell survival because K+ activation causes smooth muscle cell apoptosis (38).
THE ERA OF MEDIATORS OF PULMONARY VASCULAR TONE, AND SMOOTH MUSCLE CELL AND ENDOTHELIAL CELL FUNCTION
The convergence of increased ability to access physiologically the pulmonary circulation and the explosion of our understanding of pharmacologic mediators resulted in a significant advance of our understanding of cell signaling underlying PH. The combination of these achievements and the availability of animal models allowed for the development of presently available therapies.
Prostacyclin
Prostacyclin was originally isolated from rabbit or pig aorta as a prostaglandin able to inhibit potently human platelet aggregation (39) and dilate the pulmonary circulation (40). These observations led to the proposed use of prostacyclin in patients with IPAH (41). Subsequent studies, which demonstrated decreased ratio of 6-keto PG1α (a metabolite of prostacyclin) to thromboxane in the urine of patients with IPAH, lent further support for the clinical use of prostacyclin in IPAH (30). Decreased expression of prostacyclin synthase was demonstrated in pulmonary arteries of patients with severe idiopathic and secondary PAH (42), supporting the hypothesis that PAH resulted from an imbalance of factors that act as vasodilators/cell growth suppressors and those that act as vasoconstrictors/cell growth promoters. A major development in PH research was the initial clinical studies that culminated with the remarkable success of this drug in the treatment of this disease (43).
Nitric Oxide
Originally identified as the reactive intermediate by which nitroprusside caused smooth muscle cell relaxation, it was not until nitric oxide (NO) was identified as the endothelium-derived relaxing factor that its role was explored in PH (44). NO was found to be a critical vasodilator that could also inhibit platelet aggregation and proliferation of vascular smooth muscle cells. Human studies reported variable production of NO in patients with idiopathic PPH. However, important mechanistic insights into the role of NO in the control of pulmonary vascular tone and remodeling originated from animal models, which showed NO-mediated protection against HPV in lungs, inhibition of smooth muscle proliferation and platelet aggregation, and downregulation of endothelin-1 production (45). NO has broader effects in that it contributes to angiogenesis, endothelial cell survival, and mobilization of bone marrow progenitor cells.
The hypothesized role of endothelial NO deficiency in contributing to PH was further strengthened by the modest but salutary effects of inhaled NO and NO donors such as l-arginine in patients with PH. NO stimulates the production of cGMP starting a regulatory cascade resulting in pulmonary vasodilation. While inhaled NO is not used as a clinical therapy in patients with severe PH, sildenafil, an oral inhibitor of phosphodiestarase 5, has beneficial effects on IPAH, probably by means of increasing cyclic GMP through decreased breakdown (46).
Endothelin-1
First identified as a small peptide secreted from endothelial cells, endothelin (ET)-1 is a multifunctional molecule serving as a potent vasoconstrictor as well as a smooth muscle cell mitogen (47). ET-1 expression is elevated in animal models of PH and in patients with PH. It signals through two distinct G protein–coupled receptors, endothelin-A (ETA) and endothelin-B (ETB). Activation of ETA results in vasoconstriction, whereas the effects of ETB stimulation vary depending on cell type: on smooth muscle cells it causes vasoconstriction while on endothelial cells it leads to vasodilatation. ET-1 receptor antagonists, such as Bosentan, improves patient's functional status and other indices of PH-related morbidity, and are currently being used clinically for this condition (48).
Vascular Endothelial Growth Factor
The discovery of vascular endothelial growth factor (VEGF) in the late 1980s and early '90s led to the realization of its critical role in lung morphogenesis, growth, regulation of pulmonary artery structure, and alveolar integrity. As with NO, we still do not fully understand the role of VEGF in PH, since the animal and human data appear to contradict each other. Normal endothelial cells do not characteristically secrete VEGF. However, endothelial cells in PH were shown to express VEGF (49) and platelet levels of VEGF were also elevated in PH. On the other hand, increases in VEGF led to improvement of pulmonary hemodynamics in hypoxic PH in rats (50). In the same line, VEGF blockade resulted in severe PH (37).
The conundrum of the role of VEGF in the pulmonary circulation may be explained by the survival and differentiation role of VEGF in the normal endothelial cells; its overexpression is protective against the disease. However, in the diseased setting, VEGF may be primarily growth promoting, thus contributing to endothelial cell clusters or plexiform lesion formation.
Hypoxia-Inducible Factors 1α and 2α
Hypoxia sensing and hypoxia-dependent gene expression represent exciting areas of investigation, with broad impact on tumor and physiologic angiogenesis. The transcription factor hypoxia-inducible factor (HIF)-1α was discovered on the basis of its ability to upregulate the erythropoietin gene by binding to the 3′prime end of the erythropoietin coding region (51), while HIF-2α was cloned based on sequence homology with HIF-1α (52). Both were shown to activate hypoxia-inducible genes such as VEGF. Remarkably, mice deficient in HIF-1α (53)or HIF-2α (54) were protected against hypoxic PH, and HIF-1α and its binding partner localize to plexiform lesions, concordant with the expression pattern of VEGF (49). Although the role of HIF in human pulmonary vascular disease is unclear, it appears that HIF-1α has broader roles in hypoxia sensing by the carotid body (55).
Serotonin
Serotonin and acetylcholine were among the first molecules found to alter the pulmonary vasomotor tone. The mechanism by which serotonin causes pulmonary vasoconstriction and smooth muscle cell proliferation is complex, as there are both multiple isoforms of the cell surface receptor and an active transporter that internalizes serotonin (56).
Numerous animal studies have demonstrated a role for serotonin in the development of hypoxic PH. Hypoxia has been demonstrated to induce the secretion of serotonin from intact pulmonary neuroepithelial bodies and expression of the 5-hydroxytryptamine transporter (5-HTT) (57). In addition, patients with IPAH have elevated levels of serotonin and a polymorphism within the 5-HTT transporter, which renders cultured pulmonary artery smooth muscle cells more susceptible to the growth-promoting effects of serotonin (27). The role of fluoxetine or other similar drug class inhibitors in the treatment of human PAH awaits further clinical trials.
THE GENETIC AGE OF PH RESEARCH
The work on mediators of vasomotor control or vascular cell growth could not address why a fraction of patients with IPAH belonged to families with a higher incidence of the disease. Indeed, genetic clustering of patients with IPAH had been known for decades. This unique feature of the disease led two groups to characterize these patients and search for the candidate genes via linkage analysis. This effort succeeded in identifying a region in the short arm of chromosome 2 in the region 2p32 with a clear association to familial IPAH (58, 59). The breakthrough was not sufficient to indicate the candidate gene, as it covered approximately 5 million bases. Coincidental with these studies in the mid-1990s, it was uncovered that endothelial cells in plexiform lesions in patients with IPAH were monoclonal as assessed by X chromosome silencing by methylation. On the other hand, similar lesions in lungs of patients with PH due to congenital heart malformations were all polyclonal, that is, showing changes consistent with a reactive population as seen after cell injury and disrepair (10). The most important implications of these findings were the indication that mutations in either growth-promoting or tumor suppressor genes might represent the underpinning of the monoclonal cell growth in IPAH. The years that followed culminated with the discovery that mutations in the transforming growth factor β family of receptors represented the genetic link to IPAH (33, 60–62).
In the past four years, we learned that the genetic mutations in bone morphogenetic protein receptor (BMPR)-2 distribute randomly along the gene with no preferential sites for mutations or so-called hot spots (60, 61). Furthermore, BMPR-2 mutations account for ∼ 70% of the family members with IPAH; therefore, a second locus probably exists. Perhaps, the most striking development in the investigation of BMPR-2 mutations was that up to 10% of the sporadic cases carry the mutation. Several critical questions still linger in the field. How does a heterozygous mutation cause the disease? Do mutations in BMPR-2 cause the disease by haplotype insufficiency, suggesting that replenishing BMPR-2 could prevent the disease? Or that it is by a dominant-negative effect on the preserved copy of the gene product? Or is it a marker of predisposition and second hits are necessary to produce the disease? If the mutation is germline (i.e., passed along to all somatic cells from the germinative cells), why is the lung the only organ affected by the disease?
CONCLUSIONS
Although there have been many important achievements in the past 100 years of PH research, our understanding of PH and the pulmonary circulation remains incomplete. Arguably, understanding the natural history of the different forms and severities of PH remains our most profound limitation. At the present time, patients are diagnosed relatively late in their disease, and the sampling of lung tissue is avoided given the risks of morbidity and mortality, the lack of clinical utility, the advances in hemodynamic monitoring, and improved noninvasive imagining of the pulmonary circulation. Undoubtedly, the next 100 years will continue our mechanistic understanding of this disease and provide better treatment for our patients. Novel insights will be gained into etiologic agents associated with PH, as recently indicated by the potential association of Kaposi's Sarcoma herpes virus-8 with IPAH (63) or the unraveling of immunologic mechanisms underlying scleroderma associated severe PAH or anorectic drugs such as dexfenfluramine (which, as with aminorex in the 1960s and early '70s, has been linked with IPAH in the past decade). Currently, extensive efforts to elucidate the genetic basis of the disease continue along with the search for surrogate markers, such as genetic expression studies using peripheral blood cells (64), and identification of additional candidate and disease-modifying genes. As novel techniques are adapted and applied to the study of PH biomarkers will be developed allowing for early diagnosis, phenotypic stratification of patients, and tailored therapies. Further understanding of the genetic, molecular and cellular mechanisms of PH will hopefully provide us with more refined and relevant animal models. Finally, novel therapies are already on the horizon, as for instance, progenitor cell infusion aimed at reconstituting a diseased pulmonary circulation (65).
Acknowledgments
The authors thank Dr. Ivan McMurtry for helpful discussions. The authors apologize for not being able to reference relevant contributions by several investigators due to editorial constraints.
This work was supported by the NIH awards K08-HL076297 (to A.Z.), R01-HL049441 (to P.M.H.), and P01-HL66254 (to R.M.T.).
References
- 1.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004;43:S5–S12. [DOI] [PubMed] [Google Scholar]
- 2.Fishman AP. A century of primary pulmonary hypertension. In: Rubin LJ, Rich S, editors. Primary pulmonary hypertension, 1st ed. New York: Marcel Decker; 1997. pp. 1–17.
- 3.Arrilaga FC. Sclerose de l'artere pulmonaire a certains etats pulmonaries chroniques. Archives Maladie Coeur 1913;6:518–529. [Google Scholar]
- 4.Gilmour JR, Evans W. Primary pulmonary hypertension. J Pathol Bacteriol 1946;58:687–697. [DOI] [PubMed] [Google Scholar]
- 5.Zhu L, Wigle D, Hinek A, Kobayashi J, Ye C, Zuker M, Dodo H, Keeley FW, Rabinovitch M. The endogenous vascular elastase that governs development and progression of monocrotaline-induced pulmonary hypertension in rats is a novel enzyme related to the serine proteinase adipsin. J Clin Invest 1994;94:1163–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heath D, Edwards JE. The pathology of pulmonary hypertensive disease: a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal changes. Circulation 1958;18:533–547. [DOI] [PubMed] [Google Scholar]
- 7.Haworth SG, Rabinovitch M, Meyrick B, Michel R, Pietra GG, Polak JM, Reid LM, Tuder RM. Primary Pulmonary Hypertension: Executive Summary from the World Symposium-Primary Pulmonary Hypertension. Rich S. 2–5. 1998. World Health Organization.
- 8.Palevsky HI, Schloo BL, Pietra GG, Weber KT, Janicki JS, Rubin E, Fishman AP. Primary pulmonary hypertension: vascular structure, morphometry, and responsiveness to vasodilator agents. Circulation 1989;80:1221. [DOI] [PubMed] [Google Scholar]
- 9.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, Reid LM, Tuder RM. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 2004;43:25S–32S. [DOI] [PubMed] [Google Scholar]
- 10.Lee SD, Shroyer KR, Markham NE, Cool CD, Voelkel NF, Tuder RM. Monoclonal endothelial cell proliferation is present in primary but not secondary pulmonary hypertension. J Clin Invest 1998;101:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cournand A, Ranges HA. Catheterization of the right auricle in man. Proc Soc Exp Biol Med 1941;46:462. [Google Scholar]
- 12.Dresdale DT, Schultza M, Michton RJ. Primary pulmonary hypertension: I. Clinical and hemodynamic study. Am J Med 1951;11:686–705. [DOI] [PubMed] [Google Scholar]
- 13.Euler USv, Liljsjestrand G. Observations of the pulmonary arterial blood pressure on the cat. Acta Physiol Scand 1946;12:301–320. [Google Scholar]
- 14.Hales CA. Physiological function of hypoxic pulmonary hypertension. In: Yuan JX, editor. Hypoxic pulmonary vasoconstriction: cellular and molecular mechanisms. Norwell, MA: Kluwer Academic Publishers; 2002. pp. 3–32.
- 15.McMurtry IF, Davidson AB, Reeves JT, Grover RF. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ Res 1976;38:99–104. [DOI] [PubMed] [Google Scholar]
- 16.Rounds S, McMurtry IF. Inhibitors of oxidative ATP production cause transient vasoconstriction and block subsequent pressor responses in rat lungs. Circ Res 1981;48:393–400. [DOI] [PubMed] [Google Scholar]
- 17.Post JM, Hume JR, Archer SL, Weir EK. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol 1992;262:C882–C890. [DOI] [PubMed] [Google Scholar]
- 18.Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet 1998;351:726–727. (letter). [DOI] [PubMed] [Google Scholar]
- 19.Wood P, Besterman EM, Towers MK, Mcilroy MB. The effect of acetylcholine on pulmonary vascular resistance and left atrial pressure in mitral stenosis. Br Heart J 1957;19:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barer GR, Cook C, Clegg EJ, Shaw JW. Pulmonary vascular and cardiac changes in rats and mice exposed to chronic hypoxia. J Pathol 1970;101:i–ii. [DOI] [PubMed] [Google Scholar]
- 21.Voelkel NF, Tuder RM. Hypoxia-induced pulmonary vascular remodeling: a model for what human disease? J Clin Invest 2000;106:733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carillo L, Aviado DM. Monocrotaline-induced pulmonary hypertension. Lab Invest 1968;20:243–248. [PubMed] [Google Scholar]
- 23.Kelly JJ, Moore TM, Babal P, Diwan AH, Stevens T, Thompson WJ. Pulmonary microvascular and macrovascular endothelial cells: differential regulation of Ca2+ and permeability. Am J Physiol 1998;274:L810–L819. [DOI] [PubMed] [Google Scholar]
- 24.Frid MG, Aldashev AA, Dempsey EC, Stenmark KR. Smooth muscle cells isolated from discrete compartments of the mature vascular media exhibit unique phenotypes and distinct growth capabilities. Circ Res 1997;81:940–952. [DOI] [PubMed] [Google Scholar]
- 25.Mecham RP, Whitehouse LA, Wrenn DS, Parks WC, Griffin GL, Senior RM, Crouch EC, Stenmark KR, Voelkel NF. Smooth muscle-mediated connective tissue remodeling in pulmonary hypertension. Science 1987;237:423–426. [DOI] [PubMed] [Google Scholar]
- 26.Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, Kimura S, Masaki T, Duguid WP, Stewart DJ. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 1993;328:1732–1739. [DOI] [PubMed] [Google Scholar]
- 27.Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, Adnot S. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J Clin Invest 2001;108:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res 2002;90:1189–1196. [DOI] [PubMed] [Google Scholar]
- 29.Loscalzo J. Endothelial dysfunction in pulmonary hypertension. N Engl J Med 1992;327:70–75. [DOI] [PubMed] [Google Scholar]
- 30.Christman BW, McPherson CD, Newman JH, King GA, Bernard GR, Groves BM, Loyd JE. An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 1992;327:70–75. [DOI] [PubMed] [Google Scholar]
- 31.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995;333:214–221. [DOI] [PubMed] [Google Scholar]
- 32.Yeager ME, Halley GR, Golpon HA, Voelkel NF, Tuder RM. Microsatellite instability of endothelial cell growth and apoptosis genes within plexiform lesions in primary pulmonary hypertension. Circ Res 2001;88:e8–e11. [DOI] [PubMed] [Google Scholar]
- 33.Voelkel NF, Cool CD, Lee SD, Wright L, Geraci MW, Tuder RM. Primary pulmonary hypertension between inflammation and cancer. Chest 1999;114:225S–230S. [DOI] [PubMed] [Google Scholar]
- 34.Meyrick B, Reid L. Hypoxia and incorporation of 3H-thymidine by cells of the rat pulmonary arteries and alveolar wall. Am J Pathol 1979;96:51–70. [PMC free article] [PubMed] [Google Scholar]
- 35.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med 2001;163:737–744. [DOI] [PubMed] [Google Scholar]
- 36.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon GG, Waltenberger J, Voelkel NF, Tuder RM. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J 2001;15:427–438. [DOI] [PubMed] [Google Scholar]
- 37.Cowan KN, Heilbut A, Humpl T, Lam C, Ito S, Rabinovitch M. Complete reversal of fatal pulmonary hypertension in rats by a serine elastase inhibitor. Nat Med 2000;6:698–702. [DOI] [PubMed] [Google Scholar]
- 38.Krick S, Platoshyn O, Sweeney M, Kim H, Yuan JX. Activation of K+ channels induces apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol 2001;280:C970–C979. [DOI] [PubMed] [Google Scholar]
- 39.Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature 1976;263:663–665. [DOI] [PubMed] [Google Scholar]
- 40.Hyman AL, Chapnick BM, Kadowitz PJ, Lands WE, Crawford CG, Fried J, Barton J. Unusual pulmonary vasodilator activity of 13,14-dehydroprostacyclin methyl ester: comparison with endoperoxides and other prostanoids. Proc Natl Acad Sci USA 1977;74:5711–5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watkins WD, Peterson MB, Crone RK, Shannon DC, Levine L. Prostacyclin and prostaglandin E1 for severe idiopathic pulmonary artery hypertension. Lancet 1980;1:1083. [DOI] [PubMed] [Google Scholar]
- 42.Tuder RM, Cool CD, Geraci MW, Wang J, Abman SH, Wright L, Badesch DB, Voelkel NF. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am J Respir Crit Care Med 1999;159:1925–1932. [DOI] [PubMed] [Google Scholar]
- 43.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 1996;334:296–302. [DOI] [PubMed] [Google Scholar]
- 44.Gruetter CA, Barry BK, McNamara DB, Gruetter DY, Kadowitz PJ, Ignarro L. Relaxation of bovine coronary artery and activation of coronary arterial guanylate cyclase by nitric oxide, nitroprusside and a carcinogenic nitrosoamine. J Cyclic Nucleotide Res 1979;5:211–224. [PubMed] [Google Scholar]
- 45.Perrella MA, Edell ES, Krowka MJ, Cortese DA, Burnett JC Jr. Endothelium-derived relaxing factor in pulmonary and renal circulations during hypoxia. Am J Physiol 1992;263:R45–R50. [DOI] [PubMed] [Google Scholar]
- 46.Sastry BK, Narasimhan C, Reddy NK, Raju BS. Clinical efficacy of sildenafil in primary pulmonary hypertension: a randomized, placebo-controlled, double-blind, crossover study. J Am Coll Cardiol 2004;43:1149–1153. [DOI] [PubMed] [Google Scholar]
- 47.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411–415. [DOI] [PubMed] [Google Scholar]
- 48.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002;346:896–903. [DOI] [PubMed] [Google Scholar]
- 49.Tuder RM, Chacon M, Alger LA, Wang J, Taraseviciene-Stewart L, Kasahara Y, Cool CD, Bishop AE, Geraci MW, Semenza GL, et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: evidence for a process of disordered angiogenesis. J Pathol 2001;195:367–374. [DOI] [PubMed] [Google Scholar]
- 50.Partovian C, Adnot S, Raffestin B, Louzier V, Levame M, Mavier IM, Lemarchand P, Eddahibi S. Adenovirus-mediated lung vascular endothelial growth factor overexpression protects against hypoxic pulmonary hypertension in rats. Am J Respir Cell Mol Biol 2000;23:762–771. [DOI] [PubMed] [Google Scholar]
- 51.Wang GL, Semenza GL. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc Natl Acad Sci USA 1993;90:4304–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 1997;11:72–82. [DOI] [PubMed] [Google Scholar]
- 53.Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 1999;103:691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P. Heterozygous deficiency of hypoxia-inducible factor-2{alpha} protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest 2003;111:1519–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci USA 2002;99:821–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SL, Fanburg BL. Serotonin uptake by bovine pulmonary artery endothelial cells in culture. II. Stimulation by hypoxia. Am J Physiol 1986;250:C766–C770. [DOI] [PubMed] [Google Scholar]
- 57.Eddahibi S, Fabre V, Boni C, Martres MP, Raffestin B, Hamon M, Adnot S. Induction of serotonin transporter by hypoxia in pulmonary vascular smooth muscle cells: relationship with the mitogenic action of serotonin. Circ Res 1999;84:329–336. [DOI] [PubMed] [Google Scholar]
- 58.Morse JH, Jones AC, Barst RJ, Hodge SE, Wilhelmsen KC, Nygaard TG. Mapping of familial primary pulmonary hypertension locus (PPH1) to chromosome 2q31-q32. Circulation 1997;95:2603–2606. [DOI] [PubMed] [Google Scholar]
- 59.Nichols WC, Koller DL, Slovis B, Foroud T, Terry VH, Arnold ND, Siemieniak DR, Wheeler L, Phillips JA, Newman JH, et al. Localization of the gene for familial primary pulmonary hypertension to chromosome 2q31–32. Nat Genet 1997;15:277–280. [DOI] [PubMed] [Google Scholar]
- 60.The International PPH Consortium, Lane KB, Machado RD, Pauciulo MW, Thompson JR, Philips III JA, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2 encoding a TGF-B receptor cause familiar pulmonary hypertension. Nat Genet 2000;26:81–84. [DOI] [PubMed] [Google Scholar]
- 61.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. Familial primary pulmonary hypertension (Gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000;67:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, Simonneau G, Galie N, Loyd JE, Humbert M, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001;345:325–334. [DOI] [PubMed] [Google Scholar]
- 63.Cool CD, Rai MD, Yeager ME, Hernandez-Saavedra D, Serls AE, Bull TM, Geraci MW, Brown KK, Routes JM, Tuder RM, et al. Expression of human herpesvirus 8 in primary pulmonary hypertension. N Engl J Med 2003;349:1113–1122. [DOI] [PubMed] [Google Scholar]
- 64.Bull TM, Coldren CD, Moore M, Sotto-Santiago SM, Pham DV, Nana-Sinkam SP, Voelkel NF, Geraci MW. Gene microarray analysis of peripheral blood cells in pulmonary arterial hypertension. Am J Respir Crit Care Med 2004;170:911–919. [DOI] [PubMed] [Google Scholar]
- 65.Zhao YDD, Courtman DW, Deng YP, Kugathasan L, Zhang QW, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res 2005;96:442–450. [DOI] [PubMed] [Google Scholar]