Abstract
Mutations of bone morphogenetic protein receptor type II (BMPR-II) have been associated with familial and idiopathic pulmonary arterial hypertension (PAH). BMPR-II is a member of the transforming growth factor-β receptor superfamily. It consists of extracellular, transmembrane, and kinase domains, and a unique C-terminus with mostly unknown function. However, a number of PAH-causing mutations are predicted to truncate the C-terminus, suggesting that this domain plays an important role in the homeostasis of pulmonary vessels. In this study, we sought to elucidate the functional role of this C-terminus by seeking its interacting partners. Using yeast two-hybrid screening, we identified c-Src tyrosine kinase as a binding partner of this C-terminus. In vitro co-immunoprecipitation confirmed their interaction. Mutations truncating the C-terminus disrupted their interaction, while missense mutation within kinase domain reduced their interaction. In addition, BMPR-II and c-Src tyrosine kinase colocalized within intracellular aggregates when overexpressed in HEK293 cells. Moreover, mutations truncating the C-terminus disrupted their colocalization, whereas missense mutation within kinase domain had no effect on their colocalization. Furthermore, BMP ligand stimulation decreased c-Src–activating phosphorylation at Tyrosine 418 in pulmonary smooth muscle cells in both time- and concentration-dependent manners. Mutations that truncated the C-terminus abolished this response. Taken together, these results suggest a model in which proliferative effect of c-Src by vasoactive molecules is balanced by opposing effect of BMP signaling in basal state, and the loss of this balance due to BMPR2 mutations leads to increased c-Src activity and subsequently cell growth.
Keywords: bone morphogenetic protein receptor type II, c-Src tyrosine kinase, pulmonary arterial hypertension
Bone morphogenetic protein receptor type II (BMPR-II) is a member of the transforming growth factor-β (TGF-β) receptor superfamily. Members of the TGF-β receptor superfamily include type I and type II receptor proteins, and play a complex and multifunctional role in the regulation of cell proliferation, differentiation, and apoptosis during embryogenesis and throughout adult life (1). Homozygous BMPR-II knockout mice die very early in development during gastrulation (2), while mice that express a mutant BMPR-II, lacking half of the ligand-binding domain, undergo normal gastrulation, but die at midgestation with cardiovascular and skeletal defects (3).
The receptor ligands, bone morphogenetic proteins (BMPs), constitute a family of ∼ 20 growth factors. They initiate signaling pathway by binding to the heterotetramers of type I (BMPR-IA, BMPR-IB) and type II (BMPR-II) receptors, which are transmembrane serine/threonine protein kinases. The binding of ligands to the receptor complexes leads to phosphorylation of the receptor kinases and subsequently phosphorylation of signal transduction molecules, including Smads (1, 5, and 8) and MAPK kinases (ERK, JNK, and p38 MAPK). The activated signal transduction molecules then translocate to the nucleus and regulate the transcription of target genes (1).
The BMPR2 gene encoding this receptor protein consists of 13 exons and is located on the chromosome 2q33 (4–7). The exons 1–3 encode an extracellular domain, exon 4 the transmembrane domain, exons 5–11 a serine/threonine kinase domain, and exons 12 and 13 a large intracellular C-terminal domain. However, the function of C-terminal domain is largely unknown. It comprises almost half of the BMPR-II protein and is a unique feature within the TGF-β receptor superfamily (6). This region shares low sequence conservation with the Drosophila homolog wit (8), suggesting that it may have evolved as a unique function to the protein. Both a long form of BMPR-II that contains the large C-terminal domain, and a short form that has only 30 amino acids after the kinase domain, are expressed. Both forms of BMPR-II can activate BMP type I receptors to phosphorylate Smad proteins (4–7). However, mutants with truncated C-terminal domain showed decreased activity as compared with the long form of the receptor (9).
Both our group and the International PPH Consortium have previously shown that heterozygous mutations of the BMPR2 gene are associated with familial and idiopathic pulmonary arterial hypertension (PAH) (previously known as primary pulmonary hypertension [PPH], MIM 178600) (10–12). To date, BMPR2 gene mutations have been identified in ∼ 40% of familial PAH and ∼ 15% of idiopathic PAH (13). PAH is characterized by progressive narrowing and obstruction of small pulmonary arteries, leading to sustained elevation of pulmonary arterial pressure, progressive right heart hypertrophy, and eventually heart failure. It is typically associated with abnormal proliferation of endothelial and smooth muscle cells within the small pulmonary arteries (14). Most of the PAH mutations within the C-terminal domain of BMPR-II are nonsense and frameshift mutations, and are predicted to truncate this domain, suggesting that this domain may play an important role in the homeostasis of pulmonary arterial wall. However, the signaling mechanism involving the C-terminal domain is still largely unknown.
In this study, we sought to elucidate the function of this C-terminal domain by identifying molecules it interacts with. Using a yeast two-hybrid screening system, we identified c-Src tyrosine kinase as an interacting partner of the C-terminal domain of the BMPR-II. The gene encoding c-Src tyrosine kinase is located on chromosome 20q12–13. It is a nonreceptor tyrosine kinase and the cellular homolog to the potent transforming v-Src viral oncogene (for review see Ref. 15). It functions at the hub of a large array of signal transduction cascades that influence cellular proliferation, differentiation, motility, and survival. Aberrant activation of c-Src tyrosine kinase has been documented in over 50% of tumors derived from the colon, liver, lung, breast, and pancreas (16). We demonstrated that BMPR-II and c-Src tyrosine kinase co-immunoprecipitated in vitro, and furthermore, that PAH-causing mutations (which truncated the C-terminal domain of BMPR-II) disrupted this interaction. In addition, we showed that BMPR-II and c-Src tyrosine kinase colocalized together within intracellular aggregates, when both were overexpressed in HEK293 cells. Moreover, truncation of the C-terminal domain of BMPR-II disrupted this colocalization between BMPR-II and c-Src tyrosine kinase. In contrast, missense mutation within the kinase domain of BMPR-II had minimal effect on their colocalization. One of the tyrosine phosphorylation sites of c-Src is located at amino acid Tyrosine 418 (Tyr418) in the catalytic SH2 domain. Phosphorylation of Tyr418 upregulates the c-Src activity (17). We showed that stimulation by ligands BMP-2, -4, and -7 decreased c-Src phosphorylation at the Tyr418 in human pulmonary arterial smooth muscle cells. Furthermore, mutations that truncated BMPR-II C-terminal domain abolished the decrease of c-Src phosphorylation in response to BMP-2. These findings identify a novel function of the C-terminal domain of BMPR-II and raise the possibility that dysregulation of c-Src tyrosine kinase may involve in the pathogenesis of PAH.
MATERIALS AND METHODS
Yeast Two-Hybrid Screens
The BD Matchmaker Yeast Two-hybrid System 3 (Clontech, Mountain View, CA) was used for the yeast two-hybrid screening with the C-terminal domain of BMPR-II as a bait. The BstXI-BamHI fragment of human BMPR-II cDNA (nucleotides 1725–3114, GenBank NM_001204), consisting of partial exon 10 and exons 11–13, and encoding the C-terminal domain of BMPR-II (amino acids 575–1038), was cloned in frame into the BstXI and BamHI sites of pGBK-T7 vector (Clontech) to generate a bait fusion construct of Gal4 DNA-binding domain and BMPR-II. The fusion construct pGSK-T7-BMPR-II was confirmed with direct DNA sequencing bidirectionally using the Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) and an automated capillary sequencer (ABI 3100). The plasmid was transformed into Saccharomyces cerevisiae strain AH109 (Clontech) by lithium acetate method and grown on tryptophan-deficient selective media. Colonies able to grow on tryptophan-deficient media were selected and mated with a human heart cDNA library, which expressed fusions with the GAL4-Activation domain pretransformed in yeast strain Y187 (Clontech). If the fusion proteins interact, the reporter genes would be activated, permitting growth on selective medium lacking adenine and histidine (nutritional markers) and activation of the lacZ gene (enzymatic reporter gene). The cDNA clones from positive colonies were isolated, transformed into XL-1 Blue bacterial competent cells (Stratagene, La Jolla, CA), and identified by direct sequencing.
DNA Constructs and Site-Directed Mutagenesis
The pcDNA3–c-Src tyrosine kinase expression plasmid encoding the HA-tagged c-Src tyrosine kinase was provided by Dr. Robert Lefkowitz (Duke University). Untagged BMPR2 cDNA cloned in pBSSK vector was provided by Dr. Jan Rosenbaum (Procter and Gamble Pharmaceuticals, Mason, OH). The 6xHIS-tagged pcDNA4-BMPR2 plasmid was made by PCR cloning the full-length BMPR2 cDNA (using pBSSK-BMPR2 as a template) and ligated into the pcDNA4 vector using TOPO TA cloning kit (Invitrogen, Carlsbad, CA). All of BMPR2 mutant constructs were made using the wild-type pcDNA4-BMPR2 as a template and the QuikChange Site-Directed Mutagenesis Kit (Stratagene) following the manufacturer's instructions. The primers used for construct cloning and mutageneses are listed in Table 1. All constructs were verified by direct sequencing as described above. The BMPR-II (K230fsX21) mutant construct carried a deletion/insertion mutation (nucleotides 690–691 delAGinsT) (12) and was predicted to truncate the kinase and the C-terminal domain. The R491W mutation has been identified in several families with PAH (12). This mutation was resulted from missense mutation at nucleotide 1471 C>T and was predicted to alter an evolutionally highly conserved arginine residue in the kinase domain. The N861fsX10 mutation (a deletion at nucleotide 2579 delT) would cause a frameshift and truncate part of BMPR-II C-terminal domain (11, 12).
TABLE 1.
Primers used in sequencing, cloning, and mutagenesis
| Primer Name | Sequence |
|---|---|
| Matchmaker 5′AD Sequencing | CTATTCGATGATGAAGATACCCCACCAAACC |
| Matchmaker 3′AD Sequencing | GTGAACTTGCGGGGTTTTTCAGTATCTACGATT |
| Matchmaker 5′DBD Sequencing | TCATCGGAAGAGAGTAG |
| Matchmaker 3′DBD Sequencing | GTCACTTTAAAATTTGTAT |
| BMPR2 wild-type (1–18) | ATGACTTCCTCGCTGCAG |
| BMPR2 wild-type (3114–3096) | CAGACAGTTCATTCCTATA |
| BMPR2 (K230fsX21) forward | GTCCAGTTGCTGTAAATTGTTTTCCTTTGC |
| BMPR2 (K230fsX21) reverse | GCAAAGGAAAACAATTTACAGCAACTGGAC |
| BMPR2 (R491W) forward | CAGGATGCAGAGGCTTGGCTTACTGCACAG |
| BMPR2 (R491W) reverse | CTGTGCAGTAAGCCAAGCCTCTGCATCCTG |
| BMPR2 (N861fsX10) forward | GACACCCGGCTGAATATAATTCCAGTCCTG |
| BMPR2 (N861fsX10) reverse | CAGGACTGGAATTATATTCAGCCGGGTGTC |
Cell Culture and Transfection
HEK-293 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cells were maintained in Minimum Essential Medium supplemented with 2 mM L-glutamine and Earle's BSS adjusted to contain 1.5 g/liter sodium bicarbonate, 0.1 mM nonessential amino acids, 1.0 mM sodium pyruvate, 10% heat-inactivated horse serum, and 1% penicillin/streptomycin at 37°C in a humidified 5% CO2 atmosphere. The human pulmonary arterial smooth muscle cells (hPASMC) from normal subjects were purchased from Cambrex. The cells were cultured in smooth muscle growth medium (SMGM) (Cambrex, East Rutherford, NJ) at 37°C in a humidified 5% CO2 atmosphere and used at the fourth to sixth passage. Transient transfection of HEK-293 cells and hPASMC was performed by using SuperFect (Qiagen, Valencia, CA) and Lipofectamine (Invitrogen), respectively, according to the manufacturer's instructions.
Scanning Laser Confocal Microscopy
Transiently transfected HEK-293 cells in collagen-coated 35-mm glass bottom dishes were washed with Dulbecco's PBS, fixed with 10% paraformaldehyde for 30 min at room temperature, permeabilized with absolute methanol for 10 min at −20°C, and blocked with 1% BSA/5% FBS for 3 h at room temperature. For visualization of HA–c-Src tyrosine kinase and HIS–BMPR-II, cells were stained with 1:200 dilution of rhodamine-conjugated monoclonal anti-HA antiserum and 1:2,000 dilution of FITC-conjugate anti-HIS antiserum for 1 h at 37°C and washed with PBS before examination. Confocal microscopy was performed on a Zeiss LSM510 laser scanning microscope (Zeiss, Thornwood, NY) using a Zeiss 63 × 1.4 numerical aperture water immersion lens with dual line switching excitation (488 nm for FITC, 568 nm for rhodamine) and emission (515–540 nm for FITC, 590–610 rhodamine) filter sets.
Co-Immunoprecipitation and Immunoblotting
Immunoprecipitation experiments were performed by using transiently transfected HEK-293 cells in 100-mm dishes. Cells were lysed in 500 μl lysis buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 10% glycerol, 1% Triton X-100, and 1 mM EDTA) and the lysates were incubated overnight at 4°C with 2 μl monoclonal antibody against HIS epitope (Roche, Indianapolis, IN) and 50 μL 20% protein G-Sepharose beads (Sigma, St. Louis, MO). After washing the beads 5 times in IP washing buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 100 μM ZnCl2, 2 mM EDTA, 1% Nonidet P-40 and 10% glycerol), they were suspended and boiled in Laemmli sample buffer. Samples were resolved by SDS-PAGE and probed with monoclonal antibody against HA epitope (Roche) using UnBlot In-Gel Chemiluminescent Detection Kit for Mouse (Pierce Biotechnology, Rockford, IL).
c-Src Phosphorylation Assay
The phosphorylation of c-Src at Tyr418 was determined using FACE c-Src chemiluminescent assay (Active Motif, Carlsbad, CA), according to the manufacturer's instructions. The amount of c-Src phosphorylation was normalized by the number of cells using crystal violet cell staining with absorbance at 595 nm using TECAN ULTRA plate reader (Tecan Inc., San Jose, CA). The ligands BMP-2, -4, and -7 were obtained from R&D Systems, Inc. (Minneapolis, MN).
RESULTS
Isolation of c-Src Tyrosine Kinase as an Interacting Protein of the C-Terminal Domain of BMPR-II in Yeast Two-Hybrid System
To identify proteins that interact with the C-terminal domain of human BMPR-II, we screened a human heart cDNA library using a GAL4 yeast two-hybrid system. We chose to use a human heart cDNA library, as there was no commercially available lung cDNA library and we had observed that BMPR-II was highly expressed in the heart (data not shown). The C-terminal domain of BMPR-II was expressed as a fusion protein with the GAL4–DNA-binding domain (DBD) of the yeast transcription factor GAL4. The GAL4 DBD was located in the 5′ end to maintain a free C-terminal domain of BMPR-II in the expressed fusion protein.
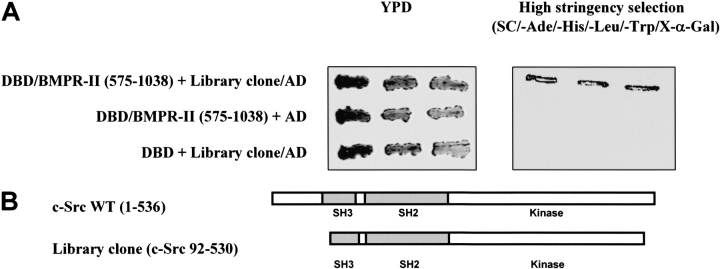
From ∼ 1 × 106 clones screened, we obtained a positive cDNA clone based on growth on selective media and activation of the lacZ reporter gene. The library-derived plasmid was purified and retransformed together with the bait plasmid to verify the phenotype and confirm the interaction of the two proteins. The interaction between the C-terminal domain of BMPR-II and the library clone was specific because the growth of colonies on high-stringency selective plates, which lacked adenine and histidine (nutritional markers) and contained X-α-Gal (enzymatic reporter gene), was apparent only in the presence of both DBD/BMPR-II-C-terminal domain and the library clone (Figure 1A, right panel). Yeast transformed with DBD/BMPR-II-C-terminal domain and activation domain (AD) alone, or yeast transformed with the library clone with DBD only, were not able to grow on the high-stringency selective plates (Figure 1A, right panel). Transformation per se did not affect the growth of the yeast host because all three types of transformants were capable of growing on rich, nonselective yeast peptone dextrose (YPD) medium (Figure 1A, left panel), as well as on plates selecting for the presence of bait or library clone (data not shown). DNA sequencing of this positive library clone showed that it encoded the c-Src tyrosine kinase (amino acids 92–530), but lacked parts of the N-terminal and C-terminal domains. It contained most of the SH3 motif, the SH2 motif, and most of the kinase domain (Figure 1B).
Figure 1.
Isolation of protein interacting with C-terminal domain of BMPR-II. (A) Growth of yeast transformants on YPD and high-stringency selection plates. One colony of each of the indicated yeast transformants was resuspended in 0.5 ml of water and streaked with three repeats onto rich yeast peptone dextrose (YPD) medium (left panel) or high-stringency selection plates containing X-α-Gal (right panel). Yeast cells were transformed with the following plasmids: (1) the C-terminal domain of BMPR-II (pGBKT7-DBD-BMPR-II [575–1038]) and the isolated positive library clone (pGADT7-library clone-AD) from the yeast two-hybrid screening; (2) C-terminal domain of BMPR-II and pGADT7 empty vector; and (3) pGBKT7-DBD empty vector and the isolated library clone. (B) Schematic representation of the library clone isolated from the yeast two-hybrid screening. The isolated library clone was identified by direct sequencing and consisted of partial sequence of the full-length c-Src tyrosine kinase. The numbers of the amino acid residues were indicated. The isolated library clone consisted of the Src Homology (SH) domains 2 and 3, and the catalytic tyrosine kinase.
Co-Immunoprecipitation of BMPR-II with c-Src Tyrosine Kinase
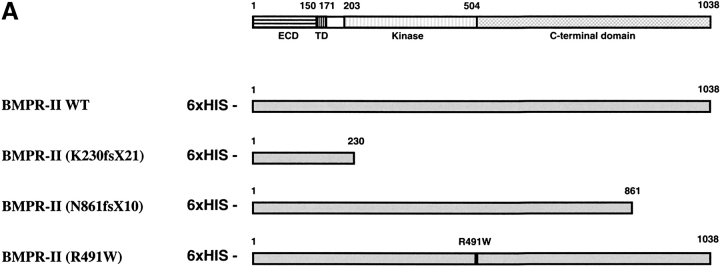
In vitro co-immunoprecipitation was performed to confirm the interaction between BMPR-II and c-Src tyrosine kinase. Wild-type and various mutant BMPR-IIs were tagged in the N-terminal with 6xHIS epitope (Figure 2A) and c-Src tyrosine kinase was tagged in the N-terminal with HA epitope. The expression constructs encoding these proteins were transfected into HEK293 cells. The lysates of the transfected cells were immunoprecipitated with anti-HIS antibody, and the presence of c-Src tyrosine kinase was detected with anti-HA antibody. When the c-Src tyrosine kinase was overexpressed alone and immunoprecipitated with anti-HIS antibody, no c-Src tyrosine kinase was detected with anti-HA antibody in the immunoblot (Figure 2B, lane 1, negative control). However, when BMPR-II was expressed together with the c-Src tyrosine kinase, both proteins were immunoprecipitated together with an anti-HIS antibody and the c-Src tyrosine kinase was detected with anti-HA antibody (Figure 2B, lane 2). This finding was in agreement with the results from the yeast two-hybrid experiment and demonstrated the specific interaction between BMPR-II and c-Src tyrosine kinase when overexpressed in mammalian cells.
Figure 2.
Co-immunoprecipitation of BMPR-II with c-Src tyrosine kinase. (A) Schematic representation of expression constructs of wild-type, truncation, and point mutants of BMPR-II. The number of amino acids of BMPR-II and its various domains were indicated: ECD (extracellular domain), TD (transmembrane domain), Kinase, and C-terminal domain. All of the expression constructs were N-terminal tagged with 6xHIS. (B) Co-immunoprecipitation of BMPR-II with c-Src tyrosine kinase. The HEK293 cells were transfected with the plasmid encoding HA-tagged c-Src tyrosine kinase and without (lane 1) or with the plasmid encoding 6xHIS-tagged wild-type BMPR-II (lane 2) or with various mutant BMPR-IIs (lanes 3–5). The cell lysates were immunoprecipitated with anti-HIS antibody and immunoblotted with anti-HA antibody to detect for the presence of c-Src tyrosine kinase.
To further determine whether PAH mutations of BMPR2 affect its interaction with c-Src tyrosine kinase, each of the mutant BMPR2 expression constructs was cotransfected with c-Src tyrosine kinase expression construct into HEK293 cells. The frameshift mutations (K230fsX21) of BMPR-II (12), which truncated the majority of kinase and the C-terminal domain, abolished its interaction with c-Src tyrosine kinase (Figure 2B, lane 3). In contrast, missense mutation in the kinase domain (R491W) (12) only slightly reduced the interaction between BMPR-II and c-Src tyrosine kinase (Figure 2B, lane 4). Similar to the K230fsX21, partial truncation of C-terminal domain (N861fsX10) (11, 12) also abolished its interaction with c-Src tyrosine kinase (Figure 2B, lane 5). These findings suggested that the C-terminal domain of BMPR-II was critical for its interaction with c-Src tyrosine kinase.
Colocalization of Full-Length BMPR-II and c-Src Tyrosine Kinase in HEK293 Cells
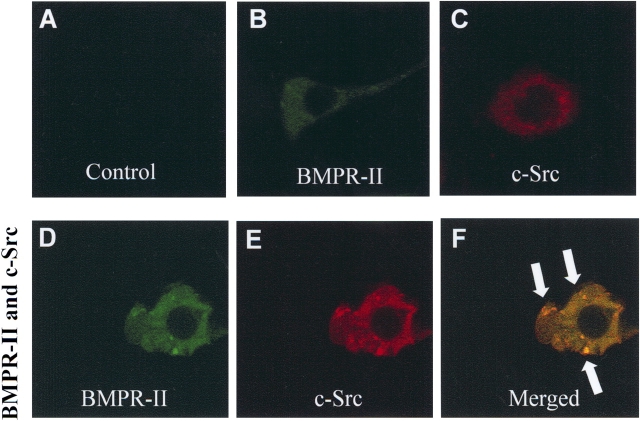
To investigate whether BMPR-II and c-Src tyrosine kinase colocalized in vivo, HEK293 cells were transiently transfected with plasmids expressing wild-type HIS-tagged BMPR-II and HA-tagged c-Src tyrosine kinase. We chose the HEK293 cells for this study because of their well-defined cellular morphology and the mRNA expression for all BMP receptors (BMPR-IA, BMPR-IB, and BMPR-II) and c-Src tyrosine kinase in these cells (data not shown). In addition, we found that BMPR-II protein was highly expressed in human kidney epithelial cells (data not shown). The proteins BMPR-II and c-Src tyrosine kinase were localized by indirect immunofluorescence using HIS- and HA-epitope specific antibodies, respectively. The BMPR-II and c-Src tyrosine kinase proteins, when expressed alone, were diffusely distributed throughout the cytoplasm (Figures 3B and 3C). However, when they were expressed together, BMPR-II and c-Src tyrosine kinase colocalized together within intracellular vesicle-like aggregates (Figures 3D–3F).
Figure 3.
Recruitment and co-localization of c-Src tyrosine kinase with BMPR-II. (A) Confocal images of negative control (without primary antibody). (B) 6xHIS-BMPR-II expressed alone. HEK293 cells were transfected with wild-type BMPR-II, permeablilized, and stained with antibody against 6xHIS and FITC-conjugate anti-HIS antibody to visualize BMPR-II (green). (C) HA–c-Src tyrosine kinase expressed alone. Cells were detected by using antibody against HA and rhodamine-conjugated monoclonal anti-HA antibody to visualize c-Src tyrosine kinase (red). (D–F) Confocal images of (D) 6xHIS-BMPR-II and (E) HA-c-Src tyrosine kinase in the same HEK293 cell overexpressing both BMPR-II and c-Src tyrosine kinase. (F) 6xHIS-BMPR-II colocalized with HA-c-Src tyrosine kinase in intracellular vesicle-like aggregates (indicated by arrows) in the merged image. Data were representative of three independent experiments.
Effects of PAH-Causing Mutations within C-Terminal Domain of BMPR-II on its Interaction with c-Src Tyrosine Kinase
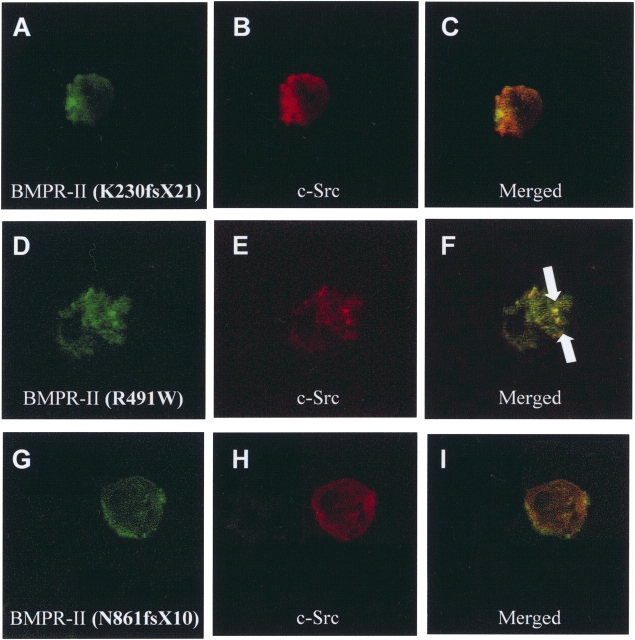
A number of BMPR2 mutations from patients with PAH are predicted to truncate the C-terminal domain of the protein. To determine whether these mutations affect the colocalization with c-Src tyrosine kinase, HEK293 cells were cotransfected with each of the mutant BMPR2s and the c-Src tyrosine kinase expression plasmids. When the BMPR-II mutant K230fsX21 and wild-type c-Src tyrosine kinase were overexpressed in HEK293 cells, both proteins were diffusely distributed throughout the cytoplasm (Figures 4A–4C). This mutation disrupted the colocalization of BMPR-II and c-Src tyrosine kinase in aggregates compared with the wild-type BMPR-II (Figures 3D–3F). In contrast, missense mutation within the BMPR-II kinase domain (R491W) had no effects on the colocalization with the c-Src tyrosine kinase (Figures 4D–4F). Similar to the K230fsX21 mutation, the frameshift mutation N861fsX10, which caused truncation of the amino acids 872–1038 of C-terminal domain, also abolished the aggregation with the c-Src tyrosine kinase (Figures 4G–4I). These findings were consistent with the results from the co-immunoprecipitation and suggested that the C-terminal domain of BMPR-II, particularly the region between amino acids 872 and 1038, was essential for its interaction with c-Src tyrosine kinase.
Figure 4.
Effects of PAH mutations on the colocalization of BMPR-II and c-Src tyrosine kinase in HEK293 cells. (A–C) HEK293 cells were transiently transfected with HA–c-Src tyrosine kinase and truncation mutant BMPR-II (K230fsX21), permeablilized, and stained with antibody against 6xHIS and FITC-conjugate anti-HIS antibody to visualize BMPR-II (K230fsX21) (green) and with antibody against HA and rhodamine-conjugated monoclonal anti-HA antibody to visualize c-Src tyrosine kinase (red). Merged image depicted lack of colocalization of BMPR-II (K230fsX21) with c-Src tyrosine kinase. (D–F) HEK293 cells expressing BMPR-II (R491W) point mutant and c-Src tyrosine kinase. Colocalization of BMPR-II point mutant and c-Src tyrosine kinase was found in the merged image (aggregates indicated by arrows). (G–I) Similar to the K230fsX21 BMPR-II mutant, lack of colocalization between BMPR-II and c-Src tyrosine kinase was also found with N861fsX10 BMPR-II mutant. Data were representative of three independent experiments.
BMP-2, -4, and -7 Stimulation Decrease c-Src Phosphorylation
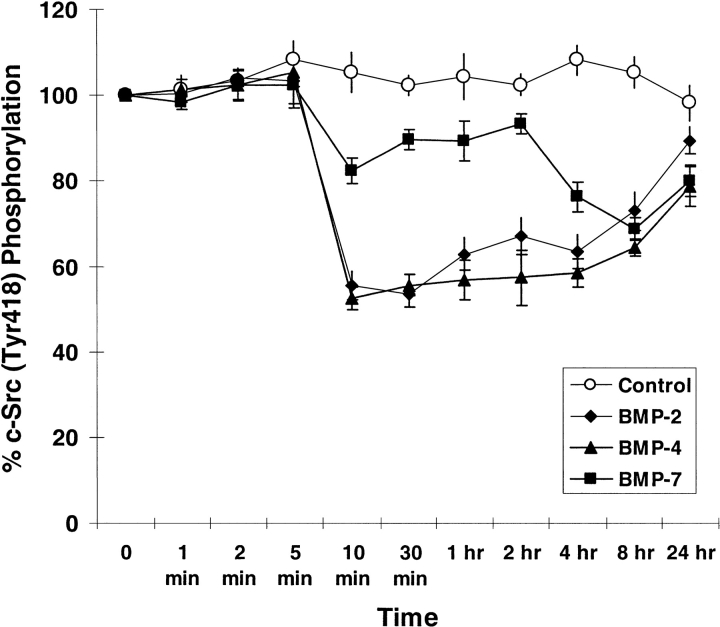
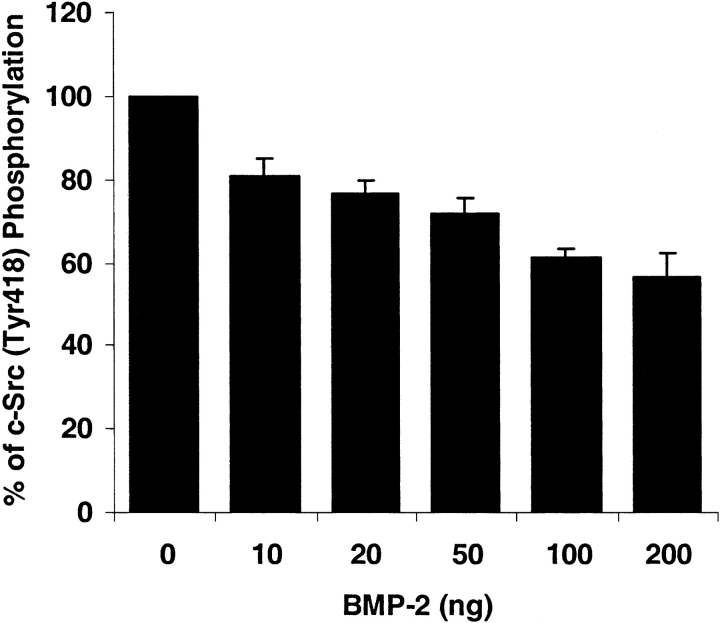
One of the tyrosine phosphorylation sites of c-Src is located at amino acid Tyr418 in the catalytic SH2 domain. Phosphorylation of Tyr418 upregulates the c-Src activity. As shown in Figure 5, after incubation with 200 ng/ml of either BMP-2, -4, or -7, hPASMC experienced a rapid decrease in c-Src phosphorylation at Tyr418 as compared with control. At as little as 10 min after stimulation, c-Src (Tyr418) phosphorylation was decreased by ∼ 50% in response to BMP-2 and BMP-4, and by ∼ 20% in response to BMP-7. This decrease lasted for several hours and gradually returned to the endogenous c-Src phosphorylation level. Interestingly, the decrease in response to BMP-7 was slower than BMP-2 and BMP-4. At 8 h after stimulation, BMP-7 decreased c-Src phosphorylation by ∼ 30%, which was similar to the response to BMP-2 and BMP-4. Moreover, the c-Src phosphorylation response to BMP-2 was concentration-dependent. At 10 min after stimulation, the c-Src phosphorylation level started to decrease when exposed to 10 ng/ml of BMP-2, and continued to decrease with increasing concentration of BMP-2 (Figure 6).
Figure 5.
BMP-2, -4, and -7 stimulations inhibit c-Src phosphorylation (Tyr418). The human pulmonary smooth muscle cells were stimulated with 200 ng/ml of BMP-2, BMP-4, BMP-7 or without ligand as control for the indicated time periods. After ligand stimulation, cells were immediately fixed with 4% formaldehyde in PBS and assayed for the phosphorylation of c-Src (Tyr418). Results were normalized with the number of cells as described in MATERIALS AND METHODS. Data represented the mean ± SEM from two independent experiments with duplicates.
Figure 6.
BMP-2 exposure results in concentration-dependent decrease of c-Src phosphorylation (Tyr418). The human pulmonary smooth muscle cells were plated and treated with various concentrations of BMP-2. Cells were then fixed and assayed for the phosphorylation of c-Src (Tyr418). Results were normalized with the number of cells. Data represented the mean ± SEM from two independent experiments with duplicates.
Effects of PAH-Causing Mutations of BMPR-II on c-Src (Tyr418) Phosphorylation
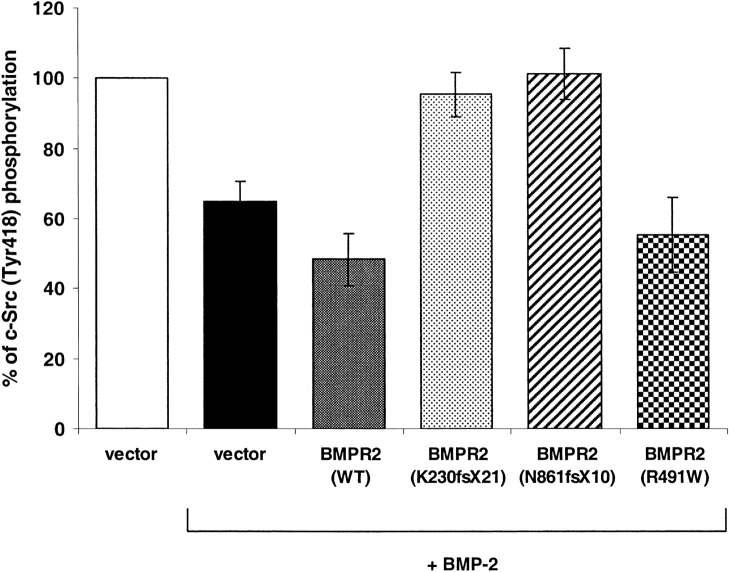
To determine the functional role of the interaction between BMPR-II mutations and c-Src on c-Src phosphorylation, hPASMC were overexpressed with wild-type or mutant BMPR-II, treated with 200 ng/ml of BMP-2, and assayed for c-Src phosphorylation. As shown in Figure 7, with the stimulation of BMP-2, overexpression of wild-type BMPR-II further decreased c-Src phosphorylation compared with the vector control. In contrast, overexpression of BMPR-II mutant K230fsX21 (truncated the majority of kinase and the C-terminal domain) or mutant N861fsX10 (truncated part of C-terminal domain) abolished the c-Src (Tyr418) phosphorylation. Similar to the wild-type BMPR-II, missense mutation in the kinase domain (R491W) reduced on c-Src phosphorylation (Tyr418) by ∼ 50%. These results suggested that the C-terminal domain of BMPR-II is essential for the inhibition of c-Src phosphorylation at Tyr418.
Figure 7.
Effects of BMPR-II mutations on c-Src phosphorylation (Tyr418). The human pulmonary arterial smooth muscle cells were transfected with BMPR-II wild-type or mutant (K230fsX21, N861fsX10, or R491W) expression constructs or empty vector (as control). Forty-eight hours after transfection, cells were treated with or without BMP-2 for 10 min. The cells were then fixed and assayed for c-Src phosphorylation (Tyr418). Results were normalized with the number of cells. Data represented the mean ± SEM from two independent experiments with duplicates.
DISCUSSION
In this study, we have identified c-Src tyrosine kinase as an interacting partner of C-terminal domain of BMPR-II in a yeast two-hybrid screening. This interaction was observed in vivo in yeast and confirmed by in vitro co-immunoprecipitation. In addition, BMPR-II and c-Src tyrosine kinase were found to colocalize in cytoplasmic aggregates when both proteins were overexpressed in HEK293 cells. Morever, PAH-causing mutations within the C-terminal domain disrupted its interaction with c-Src tyrosine kinase and abolished the colocalization of these proteins in cytoplasmic aggregates. Furthermore, BMP ligand stimulation decreased c-Src–activating phosphorylation at Tyr418, and mutations of BMPR-II C-terminal domain abolished this response in hPASMC. These findings provide a novel function of the C-terminal domain of BMPR-II, which is a unique among the members of TGF-β receptor superfamily.
Similar to other TGF-β type II receptors, BMPR-II transduces signals by forming heteromeric complexes with its type I receptors (BMPR-IA and BMPR-IB). Binding of BMP ligand to the receptor complexes initiates a signal transduction cascade, leading to the phosphorylation of signaling proteins, such as Smads (1, 5, and 8) and MAPK kinases (ERK, JNK, and p38 MAPK) (1). However, unlike other TGF-β receptors, BMPR-II has a large C-terminal domain that encompasses more than one-half of the protein and is unique in the genome. The single large exon 12 of BMPR2 gene encodes most of this C-terminal domain and is absent in an alternatively spliced isoform (4–7). Comparative analysis of BMPR-II showed that the C-terminal domain is the least conserved region of the protein among various vertebrate and invertebrate homologs, suggesting that this region may have conferred new and evolutionarily advantageous function to the protein (unpublished data from our laboratory).
Although the C-terminal domain is not required for signaling through Smads and p38MAPK pathways (7, 9, 18) a number of nonsense mutations from patients with PAH are predicted to truncate the C-terminal domain, strongly suggesting that it has crucial biochemical function and may participate in other signaling pathways that regulate cellular proliferation (10–12). Analyses of the PAH mutations revealed a functional heterogeneity of the BMPR-II mutants. Mutations within the extracellular and kinase domains abolished the BMP signaling and altered BMPR-II subcellular localization, whereas truncation of C-terminal domain retained the ability to transducer BMP signals via Smads (9, 19). This functional heterogeneity suggested that the effects of BMPR-II mutations are mediated by different molecular mechanisms. A recent study has shown that the C-terminal domain interacted with LIM kinase 1 (LIMK1) and altered the dynamics of the actin cytoskeleton (20). Another study has demonstrated that this C-terminal domain interacted with Tctex-1, a light chain of the motor complex dynein (21). Using two-dimensional gel electrophoresis and mass spectrometry, it was shown that the C-terminal domain may also serve as a docking site for several different proteins in mouse myoblast C2C12 cells, including MAPKKK8, Rab geranylgeranyl transferase α subunit, and Forkhead box L1 (22). These findings suggest that the C-terminal domain of BMPR-II may have multiple functional roles in cellular functions.
Using the yeast two-hybrid system, we identified the c-Src tyrosine kinase as a novel interacting partner of the C-terminal domain of BMPR-II. This finding provides a new dimension to the complexity of BMP signaling. The c-Src tyrosine kinase is the cellular counterpart of the viral oncogene v-Src and is implicated in a range of human cancers (16). It mediates diverse biological effects, including cell growth, migration, and apoptosis. Overexpression or overactivation of c-Src tyrosine kinase promotes the development of cancer and is found in a large percentage of colon and breast tumors. In addition to cell proliferation, c-Src tyrosine kinase regulates cell adhesion, invasion, and motility, which may contribute to tumor progression and metastasis (15).
The c-Src tyrosine kinase is composed of an amino-terminal myristylation sequence, SH3 and SH2 protein interaction domains, and a tyrosine kinase domain. Its activity is regulated by several mechanisms, including phosphorylation, intramolecular protein–protein interactions involving its SH2 and SH3 domains, and degradation by ubiquitin-proteasome pathway. Phosphorylation at Tyr418 residue upregulates the enzyme activity (17). In contrast, phosphorylation at the carboxy-proximal tyrosine-527 residue leads to the binding of this region to the SH2 domain and results in a “closed” or inactive conformation (23). Intramolecular interaction between the SH3 and the kinase domains can also inactivate the protein (23, 24). Conversely, displacement of the SH3- and SH2-mediated intramolecular interactions by higher affinity binding partners may cause conformational activation of c-Src tyrosine kinase. A number of c-Src tyrosine kinase–interacting partners have been identified that could disrupt the intramolecular interactions and result in the open and active configuration of the protein. These include the platelet-derived growth factor receptor (PDGFR), protein phosphatase-α, focal adhesion kinase (FAK), and FAK binding partner, p130CAS (25, 26). It is not yet clear whether the binding of BMPR-II via its C-terminal domain to c-Src tyrosine kinase will cause conformational change.
However, results from this study demonstrated that PAH-causing mutations within the C-terminal domain of BMPR-II disrupted its interaction with c-Src tyrosine kinase. In addition, these mutations abolished the decrease in c-Src phosphorylation (Tyr418) in response to BMP ligand stimulation. These findings indicated a potential role of this interaction in the pathogenesis of PAH. Indeed, accumulating evidence has suggested a crucial role of c-Src tyrosine kinase in vascular homeostasis. c-Src tyrosine kinase is abundant in vascular smooth muscle cells (27). However, activation of c-Src tyrosine kinase can lead to either vasorelaxation or vasoconstriction, depending on the types of cell and signaling pathways of activation. In bovine pulmonary artery endothelial cells, angiotensin-II stimulates c-Src tyrosine kinase, which in turn activates the MAPK pathway, resulting in an increase in protein expression and activity of endothelial nitric oxide synthase (eNOS) (28). This stimulation of eNOS by angiotensin-II leads to an increased production of the potent vasodilator nitric oxide (NO), resulting in vasorelaxation. Conversely, activation of c-Src tyrosine kinase by serotonin (5-hydroxytryptamine) leads to inhibition of voltage-dependent and Ca2+-activated K+ channel (MaxiK, BK), and consequently to vasoconstriction (29). In contrast, pharmacologic inhibition of c-Src tyrosine kinase decreases phosphorylation of MaxiK, leading to vasorelaxation.
On the other hand, activation of c-Src tyrosine kinase by serotonin 2B receptor (5-HT2B-R) induces phosphorylation of PDGFR and ERK1/2 MAPKs, and activates cell cycle regulators such as cyclins D and E, leading to cell proliferation (30). The inhibiting effects of BMP signaling on c-Src phosphorylation may oppose the growth signals of such mediators as 5-HT and PDGF, by downregulation of the cell cycle regulators and therefore subsequently prevent cell proliferation. Similar to BMPR-2, the activin-like kinase 1 (ALK-1) and endoglin (ENG) are members of the TGF-β superfamily. Mutations in the ALK-1 and endoglin have been implicated in Hereditary Hemorrhagic Telangiectasia (HHT)-associated pulmonary hypertension (31–34). Interestingly, it has recently been shown that TGF-β1 activated epidermal growth factor receptor (EGFR) and c-Src, and both contribute to Akt oncogene phosphorylation and cell survival in hepatocytes (35). Other studies had indicated that the sensitivity to TGF-β1–induced apoptosis is regulated by crosstalk between the Akt and Smad pathways through a mechanism that involves a direct interaction between Akt and Smad3 (36, 37). Future studies will be needed to determine whether BMP signaling is also regulated by crosstalk between the Smad and c-Src in pulmonary arterial smooth muscle cells and how defects in these two pathways may cause PAH.
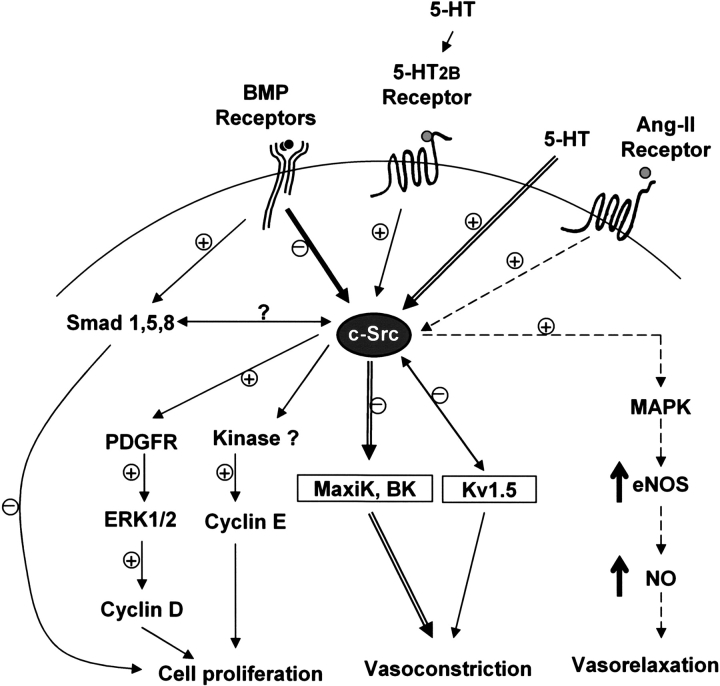
Of note, the 5-HT2B receptor has also been implicated in the pathologic effects of norfenfluramine—a metabolite of the anorectic agent fenfluramine that has been associated with PAH (38, 39). The anorectic agents fenfluramine and dexfenfluramine, which have been associated with PAH, also inhibit K+ current, principally via the voltage-gated K+ channel (Kv1.5) (40). Interestingly, c-Src tyrosine kinase has a direct protein–protein interaction with Kv1.5 in human myocardium that suppresses outward potassium current (41). This is the same direction of effect that is seen in smooth muscle cells of patients with PAH, which are observed to have downregulated the Kv1.5 channel (42). This leads to a decrease in the outward K+ current, causing membrane depolarization and subsequent calcium entry through L-type calcium channels, ultimately leading to vasoconstriction and cell proliferation. Thus, the c-Src tyrosine kinase may not only provide a central hub for the various susceptibility factors of PAH including the BMPR-II, serotonin, and Kv1.5, but also a potential therapeutic target for this disease (Figure 8).
Figure 8.
Schematic representation of c-Src tyrosine kinase as a potential hub for signaling pathways involved in PAH. The interaction between BMPR-II and c-Src tyrosine kinase may inhibit c-Src tyrosine kinase activity in the presence of BMP ligand by reducing its phosphorylation at tyrosine-418 residue. The inhibition of c-Src activity by BMP signaling may inhibit downstream cell cycle regulators such as cyclins D and E and subsequently prevent smooth muscle cell proliferation. Moreover, BMP ligand stimulation may also inhibit cell proliferation through the Smad pathway. In contrast, activation of c-Src tyrosine kinase by 5-HT2B receptor causes the induction of cell cycle regulators (cyclins D and E) via ERK/MAPK pathway and subsequently triggers cell proliferation. In addition, activation of c-Src tyrosine kinase by serotonin inhibits voltage-dependent and Ca2+-activated K+ channel (MaxiK, BK), leading to vasoconstriction. Protein–protein interaction between c-Src tyrosine kinase and voltage-gated K+ channel (Kv1.5) suppresses outward potassium current and ultimately causes vasoconstriction. In contrast, activation of c-Src tyrosine kinase by angiotensin-II (Ang-II) receptor leads to increased eNOS gene expression and activities via MAPK pathway and subsequently causes vasorelaxation. The “+” and “−” signs indicated possible activation and inhibition, respectively, while “?” indicated potential cross-talk of the smad and c-Src signaling pathway.
In summary, our results provide a novel signaling function of BMPR-II and demonstrate a discrete function of its C-terminal domain. Given the crucial role of c-Src tyrosine kinase in cell proliferation and vascular remodeling, future studies will be needed to determine how the disruption of the interaction between BMPR-II and c-Src tyrosine kinase may contribute to PAH. Better understanding of the role of c-Src tyrosine kinase in BMP signaling and their interplay with the other susceptibility factors of PAH will help elucidate the pathogenesis of PAH and may lead to potential therapeutic treatment for this disease.
The study was supported by the National Heart, Lung and Blood Institute grant NIH-HL 60056 (J.H.M.). This work was done during the tenure of fellowships from the Pulmonary Hypertension Association and the Stony-Wold Herbert Fund, Inc. (W.K.P.W.).
Conflict of Interest Statement: W.K.P.W. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.A.K. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.H.M. has received no money but has a patent on BMPR2 mutations in pulmonary hypertension, as required by Columbia University.
References
- 1.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell 2003;113:685–700. [DOI] [PubMed] [Google Scholar]
- 2.Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 2000;221:249–258. [DOI] [PubMed] [Google Scholar]
- 3.Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development 2003;130:209–220. [DOI] [PubMed] [Google Scholar]
- 4.Kawabata M, Chytil A, Moses HL. Cloning of a novel type II serine/threonine kinase receptor through interaction with the type I transforming growth factor-beta receptor. J Biol Chem 1995;270:5625–5630. [DOI] [PubMed] [Google Scholar]
- 5.Rosenzweig BL, Imamura T, Okadome T, Cox GN, Yamashita H, ten Dijke P, Heldin CH, Miyazono K. Cloning and characterization of a human type II receptor for bone morphogenetic proteins. Proc Natl Acad Sci USA 1995;92:7632–7636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu F, Ventura F, Doody J, Massague J. Human type II receptor for bone morphogenic proteins (BMPs): extension of the two-kinase receptor model to the BMPs. Mol Cell Biol 1995;15:3479–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nohno T, Ishikawa T, Saito T, Hosokawa K, Noji S, Wolsing DH, Rosenbaum JS. Identification of a human type II receptor for bone morphogenetic protein-4 that forms differential heteromeric complexes with bone morphogenetic protein type I receptors. J Biol Chem 1995;270:22522–22526. [DOI] [PubMed] [Google Scholar]
- 8.Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhaes TR, Goodman CS. Wishful thinking encodes a BMP type II receptor that regulates synaptic growth in Drosophila. Neuron 2002;33:545–558. [DOI] [PubMed] [Google Scholar]
- 9.Nishihara A, Watabe T, Imamura T, Miyazono K. Functional heterogeneity of bone morphogenetic protein receptor-II mutants found in patients with primary pulmonary hypertension. Mol Biol Cell 2002;13:3055–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, Ward K, Yacoub M, Mikhail G, Rogers P, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet 2000;37:741–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3, Loyd JE, Nichols WC, Trembath RC. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 2000;26:81–84. [DOI] [PubMed] [Google Scholar]
- 12.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000;67:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation 2005;111:534–538. [DOI] [PubMed] [Google Scholar]
- 14.Rubin LJ. Primary pulmonary hypertension. N Engl J Med 1997;336:111–117. [DOI] [PubMed] [Google Scholar]
- 15.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer 2004;4:470–480. [DOI] [PubMed] [Google Scholar]
- 16.Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta 2002;1602:114–130. [DOI] [PubMed] [Google Scholar]
- 17.Hunter T. A tail of two src's: mutatis mutandis. Cell 1987;49:1–4. [DOI] [PubMed] [Google Scholar]
- 18.Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem 2002;277:5330–5338. [DOI] [PubMed] [Google Scholar]
- 19.Rudarakanchana N, Flanagan JA, Chen H, Upton PD, Machado R, Patel D, Trembath RC, Morrell NW. Functional analysis of bone morphogenetic protein type II receptor mutations underlying primary pulmonary hypertension. Hum Mol Genet 2002;11:1517–1525. [DOI] [PubMed] [Google Scholar]
- 20.Foletta VC, Lim MA, Soosairaiah J, Kelly AP, Stanley EG, Shannon M, He W, Das S, Massague J, Bernard O. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol 2003;162:1089–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado RD, Rudarakanchana N, Atkinson C, Flanagan JA, Harrison R, Morrell NW, Trembath RC. Functional interaction between BMPR-II and Tctex-1, a light chain of Dynein, is isoform-specific and disrupted by mutations underlying primary pulmonary hypertension. Hum Mol Genet 2003;12:3277–3286. [DOI] [PubMed] [Google Scholar]
- 22.Hassel S, Eichner A, Yakymovych M, Hellman U, Knaus P, Souchelnytskyi S. Proteins associated with type II bone morphogenetic protein receptor (BMPR-II) and identified by two-dimensional gel electrophoresis and mass spectrometry. Proteomics 2004;4:1346–1358. [DOI] [PubMed] [Google Scholar]
- 23.Hubbard SR. Src autoinhibition: let us count the ways. Nat Struct Biol 1999;6:711–714. [DOI] [PubMed] [Google Scholar]
- 24.Williams JC, Weijland A, Gonfloni S, Thompson A, Courtneidge SA, Superti-Furga G, Wierenga RK. The 2.35 A crystal structure of the inactivated form of chicken Src: a dynamic molecule with multiple regulatory interactions. J Mol Biol 1997;274:757–775. [DOI] [PubMed] [Google Scholar]
- 25.Kanner SB, Reynolds AB, Vines RR, Parsons JT. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci USA 1990;87:3328–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kypta RM, Goldberg Y, Ulug ET, Courtneidge SA. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell 1990;62:481–492. [DOI] [PubMed] [Google Scholar]
- 27.Oda Y, Renaux B, Bjorge J, Saifeddine M, Fujita DJ, Hollenberg MD. cSrc is a major cytosolic tyrosine kinase in vascular tissue. Can J Physiol Pharmacol 1999;77:606–617. [PubMed] [Google Scholar]
- 28.Li X, Lerea KM, Li J, Olson SC. Src kinase mediates angiotensin II-dependent increase in pulmonary endothelial nitric oxide synthase. Am J Respir Cell Mol Biol 2004;31:365–372. [DOI] [PubMed] [Google Scholar]
- 29.Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci USA 2002;99:14560–14565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nebigil CG, Launay JM, Hickel P, Tournois C, Maroteaux L. 5-hydroxytryptamine 2B receptor regulates cell-cycle progression: cross-talk with tyrosine kinase pathways. Proc Natl Acad Sci USA 2000;97:2591–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdalla SA, Gallione CJ, Barst RJ, Horn EM, Knowles JA, Marchuk DA, Letarte M, Morse JH. Primary pulmonary hypertension in families with hereditary haemorrhagic telangiectasia. Eur Respir J 2004;23:373–377. [DOI] [PubMed] [Google Scholar]
- 32.Chaouat A, Coulet F, Favre C, Simonneau G, Weitzenblum E, Soubrier F, Humbert M. Endoglin germline mutation in a patient with hereditary haemorrhagic telangiectasia and dexfenfluramine associated pulmonary arterial hypertension. Thorax 2004;59:446–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison RE, Berger R, Haworth SG, Tulloh R, Mache CJ, Morrell NW, Aldred MA, Trembath RC. Transforming growth factor-beta receptor mutations and pulmonary arterial hypertension in childhood. Circulation 2005;111:435–441. [DOI] [PubMed] [Google Scholar]
- 34.Trembath RC, Thomson JR, Machado RD, Morgan NV, Atkinson C, Winship I, Simonneau G, Galie N, Loyd JE, Humbert M, et al. Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 2001;345:325–334. [DOI] [PubMed] [Google Scholar]
- 35.Murillo MM, del Castillo G, Sanchez A, Fernandez M, Fabregat I. Involvement of EGF receptor and c-Src in the survival signals induced by TGF-beta1 in hepatocytes. Oncogene 2005;24:4580–4587. [DOI] [PubMed] [Google Scholar]
- 36.Conery AR, Cao Y, Thompson EA, Townsend CM Jr, Ko TC, Luo K. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat Cell Biol 2004;6:366–372. [DOI] [PubMed] [Google Scholar]
- 37.Remy I, Montmarquette A, Michnick SW. PKB/Akt modulates TGF-beta signalling through a direct interaction with Smad3. Nat Cell Biol 2004;6:358–365. [DOI] [PubMed] [Google Scholar]
- 38.Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, et al. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol 2000;57:75–81. [PubMed] [Google Scholar]
- 39.Humbert M, Deng Z, Simonneau G, Barst RJ, Sitbon O, Wolf M, Cuervo N, Moore KJ, Hodge SE, Knowles JA, et al. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J 2002;20:518–523. [DOI] [PubMed] [Google Scholar]
- 40.Weir EK, Reeve HL, Huang JM, Michelakis E, Nelson DP, Hampl V, Archer SL. Anorexic agents aminorex, fenfluramine, and dexfenfluramine inhibit potassium current in rat pulmonary vascular smooth muscle and cause pulmonary vasoconstriction. Circulation 1996;94:2216–2220. [DOI] [PubMed] [Google Scholar]
- 41.Holmes TC, Fadool DA, Ren R, Levitan IB. Association of Src tyrosine kinase with a human potassium channel mediated by SH3 domain. Science 1996;274:2089–2091. [DOI] [PubMed] [Google Scholar]
- 42.Yuan XJ, Wang J, Juhaszova M, Gaine SP, Rubin LJ. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet 1998;351:726–727. [DOI] [PubMed] [Google Scholar]