Abstract
To identify relationships amongst tracheal and alveolar epithelial cells during lung development, we used conditional systems controlled by the rat CCSP and human SFTPC gene promoters to express Cre-recombinase in the developing mouse lung, thereby permanently labeling cells by expression of alkaline phosphatase or green fluorescent protein. When controlled by the rat CCSP promoter, continuous exposure of the fetus to doxycycline caused widespread recombination in conducting airway epithelial cells, including cells of the trachea, bronchi, and bronchioles before birth, and in both conducting and peripheral airways after birth. Neuroepithelial cells, identified by CGRP staining, were never labeled. Recombination and permanent labeling were observed in both ciliated and nonciliated respiratory epithelial cells, demonstrating their derivation from common progenitor cells during lung morphogenesis. Remarkable dorsal–ventral and cephalo–caudal labeling patterns, established before birth, were identified by recombination controlled by the rat CCSP gene promoter. In the trachea, subsets of epithelial cells labeled by the CCSP promoter were organized horizontally along the dorsal–ventral axis of the trachea, where selective labeling of cells juxtaposed to tracheal and bronchial cartilage was observed. In sharp contrast, recombination controlled by the human SFTPC gene promoter identified related cells that were organized in linear patterns along the cephalo–caudal axis of the conducting airways. Conditional expression of Cre-recombinase in the respiratory epithelium provides a useful model for the study of gene expression and function in the mouse respiratory tract and in the lung.
Keywords: CCSP, conditional gene targeting, CRE-recombinase, trachea, patterning
The lung forms by evagination of endoderm-derived cells from the foregut epithelium, which invade the surrounding splanchnic mesenchyme. The primary conducting airways are established early in lung morphogenesis (embryonic day [E] 9.5–E 11.5). Thereafter, peripheral lung tubules are formed by further budding of the intrapulmonary conducting airways, which leads to formation of the peripheral gas exchange regions, or alveoli, in the postnatal lung (1). Epithelial cells lining the respiratory tract undergo differentiation to produce the numerous, distinct, epithelial cell types characteristic of the mammalian lung. The numbers and types of cells lining conducting and peripheral airways vary with species, developmental stage, and along the cephalo–caudal axis. Factors controlling epithelial cell differentiation at various sites along the respiratory tract are not fully known, but are likely to be influenced by autocrine and paracrine interactions among epithelial cells, their precursors, and the underlying mesenchymally derived cells, including cartilage, stroma, smooth muscle, vascular, and marrow-derived cell types. Factors controlling the numbers and location of distinct epithelial cell types, including cells of the tracheal glands, ciliated, basal, intermediate, Clara, neuroepithelial, and goblet cells of the conducting airways, and squamous type I and cuboidal type II cells in the alveolar region, are poorly understood (2–4). Recent studies using gene addition and targeted deletion of genes in the respiratory epithelium of the mouse indicate that specification and differentiation of proximal and peripheral respiratory epithelial cells are influenced by multiple signaling pathways, including β-catenin (5), sonic hedgehog (6–8), BMP's (9, 10), Foxa2 (11), GATA-6 (12, 13), TTF-1 (14), Rb (15), and others.
In the present study, we used transgenic mice in which both rat CCSP and human SFTPC gene promoters were used to express the reverse tetracycline transactivator (rtTA) (16), thus placing the expression of Cre-recombinase (CRE) under conditional control of doxycycline during mouse lung morphogenesis. Expression of CRE was used to permanently activate alkaline phosphatase (AP) (17) or green fluorescent protein (GFP) (18) in subsets of respiratory epithelial cells in the conducting airways. Each promoter labeled respiratory epithelial cells in stereotypic patterns, either along the cephalo–caudal or dorsal–ventral axis or in relationship to tracheal-bronchial cartilage.
MATERIALS AND METHODS
Genotyping
Transgenic mice were identified using PCR primers specific for each transgene CCSP-rtTA: (5′ CCSP promoter: 5′-ACT GCC CAT TGC CCA AAC AC-3′ and the 3′ primer in rtTA coding sequence (5′-AAA ATC TTG CCA GCT TTC CCC-3′) forward CRE (5′-TGC CAC GAC CAA GTG ACA GCA ATG-3′) and reverse CRE (5′-AGA GAC GGA AAT CCA TCG CTC G-3′). Amplification of PCR products was performed as follows: denaturation at 94°C for 5 min; 30 cycles of denaturation at 94°C for 30 s, annealing at 58°C for 30 s, and extension at 72°C for 30 s, followed by a 5-min extension at 72°C. ZAP and ZEG mice were genotyped by positive β-gal staining on tissue.
Animal Use and Doxycycline Administration
Animals were housed in pathogen-free conditions in accordance with institutional guidelines. Animals were mated, and gestational age was determined by detection of the vaginal plug and then correlated with length and weight of each pup at the time of killing. Dams bearing double and triple transgenic pups were maintained on doxycycline containing food (625 mg/kg; Harlan Teklad, Madison, WI) or drinking water (Sigma Chemical Co., St. Louis, MO) at 1 mg/ml for various time spans. The mice were killed by either placing them in a CO2 chamber or by injection with 0.2–0.3 cc anesthetic (ketamine, xylazine, acepromazine). All experiments were performed with at least five triple transgenic mice from three independent litters.
Timing and Conditional Control of CRE Recombination
Doxycycline was administered to pregnant dams at E 0.5 and maintained until killing. E 0.5 was defined as 12 h after fertilization, as determined by the vaginal plug. To investigate recombination at different periods during embryonic development, doxycycline was administered for shorter time periods by providing the dam doxycycline food at the indicated time points and removing it after 48 h. For postnatal recombination, pregnant and nursing dams were placed on doxycycline food from E 18.5 until PN 9, and recombination was assessed in triple transgenic mice at 3 wk of age. AP staining or detection of fluorescent GFP expression was used to assess recombination. Each experiment represents a group of 3 to 4 pregnant females of a cross resulting in triple transgenic offspring (CCSP-rtTA/tetOCre/ZAP or CCSP-rtTA/tetOCre/ZEG).
Lung Histology and In Situ Hybridization
Timed matings were performed to produce litters with triple transgenic offspring. Pregnant dams were administered doxycycline food for times specified in each individual experiment. Before killing, mothers were injected with 0.2–0.3 cc anesthetic (ketamine, xylazine, acepromazine) to prevent pups from breathing. Genotyping was performed on tail DNA. The thorax of both triple transgenic mice and controls were immersion fixed overnight at 4°C with 4% paraformaldehyde in PBS. Lungs from PN5 to PN21 were inflation-fixed at 25 cm of pressure and fixed overnight at 4°C.
For detection of rtTA and CCSP mRNA by in situ hybridization, CCSP-rtTA transgenic pups were obtained from timed matings, lungs were isolated and fixed overnight in 4% paraformaldehyde at 4°C, washed with PBS, dehydrated through a graded series of ethanol solutions, and processed for paraffin embedding. Sections (5 μm) were loaded onto polysine slides. 35S-UTP labeled sense and antisense riboprobes were generated from a pGEM3z-rtTA and a pGEM4Z-CC10 DNA plasmid (Promega, Madison, WI) and transcribed in vitro with a riboprobe transcription kit (Promega). Conditions and solutions for hybridization were essentially as previously described (19). Hybridization was performed overnight at 55°C, and the sections were washed under highly stringent conditions. Slides were dipped in Kodak NTB2 emulsion (Fisher Scientific, Pittsburgh, PA), exposed for 7–10 d for detection of rtTA expression in the mouse lung and 10 wk for detection of CCSP expression in both mouse and rat lungs, and developed with Kodak D19.
For visualizing AP activity, samples were fixed in 0.2% glutaraldehyde in PBS, 0.02% Nonident P-40, and 0.01% SDS. Samples were washed with PBS, dehydrated through a graded series of ethanol solutions, and processed for paraffin embedding. Five-micron sections were loaded onto polysine slides. AP staining was performed on tissue sections as previously described (17). All sections were counterstained with nuclear fast red. Histology was documented with an Optronics digital camera (Optronics, Goleta, CA) attached to a Nikon Microphot FXA (Nikon, Tokyo, Japan). Images were processed with Optronics MagnaFIRE 1.1 and Adobe Photoshop software (Adobe Systems, San Jose, CA).
For studies of GFP expression and co-localization with other markers, samples were fixed in 4% paraformaldehyde in PBS. Images of whole mount trachea and lung parenchyma were acquired with an inverted Olympus microscope (Olympus, Lake Success, NY). For sections, samples were rinsed in PBS, cryoprotected in 30% sucrose in PBS, and infiltrated with a 2:1 mixture of 30% sucrose-OCT, before freezing in OCT. Cryosections were cut at 8 μm, loaded onto silanized slides, and dried at room temperature before storage at −80°C. Colocalization for GFP expression and specific cellular markers was performed by immunohistochemistry. GFP (green) and Alexa Fluor 568 (red) fluorescence were visualized with the appropriate filter sets, and images were acquired with a Zeiss Axioplan 2 Imaging Universal Microscope (Zeiss, Göttingen, Germany) and an Axiocam MRm black and white digital camera (Axiovision Release 4.3; Zeiss) and processed with an Apotome Slider (Zeiss) for pseudoconfocal imaging.
1. Ciliated cells were identified by labeling for β-tubulin IV (1:50, MU178-UC mouse monoclonal antibody OS1A6, Biogenex [San Ramon, CA]; 1:200, Alexa Fluor 568–conjugated donkey anti-mouse IgG1; Molecular Probes [Eugene, OR]).
2. Clara cells were identified by labeling for CCSP (1:500, affinity purified goat anti-rat polyclonal antibody, gift from Dr. Barry Stripp; 1:200, Alexa Fluor 568–conjugated donkey anti-goat IgG; Molecular Probes).
3. Type II cells were identified by labeling for pro–surfactant protein (SP)-C (1:1,000, R09337 mono-specific, rabbit anti-mouse polyclonal antibody, in house; 1:200, Alexa Fluor 568–conjugated goat anti-rabbit IgG; Molecular Probes).
4. Neuroendocrine cells were identified by labeling for CGRP (1:500, C8198 polyclonal rabbit anti-rat antibody; Sigma; 1:1,000; 1:200, Alexa Fluor 568–conjugated goat anti rabbit IgG; Molecular Probes).
RESULTS
Expression of rtTA under Control of the Rat CCSP Promoter
Distinct lines of transgenic mice were produced that express rtTA under control of the rat CCSP and human SFTPC gene promoters (16, 20). These mice were bred to tetO-CRE mice and either ZEG or ZAP mice (17, 18), creating triple transgenic mice (herein designated as CCSPrtTA/tetOCRE/ZEG, CCSPrtTA/tetOCRE/ZAP, SPCrtTA/tetOCRE/ZEG, SPCrtTA/tetOCRE/ZAP), in which respiratory epithelial cells permanently expressed either AP or GFP after doxycycline-induced recombination (Figure 1). These mice have been bred and maintained for more than 4 yr, with normal lung function and longevity in the vivarium. Sites of CRE expression and recombination in the peripheral lung under control of the SFTPC promoter have been described previously (21).
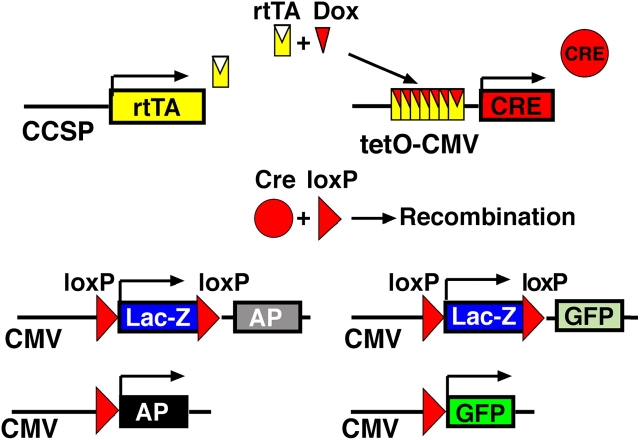
Figure 1.
Permanent cell labeling by controlled expression of Cre-recombinase. The rtTA gene is driven by a cell-type specific promoter (rat CCSP) and followed by a SV40 polyadenylation site. In the presence of doxycycline (Dox), the transactivator (rtTA) recognizes its specific DNA target sequence (tetO). Expression of CRE causes recombination at loxP sites. In Z/AP and Z/EG reporter mice β-gal expression changes to alkaline phosphatase (AP) or enhanced green fluorescent protein (GFP). Visualization of AP or GFP expression indicates doxycycline-induced CCSP-dependent recombination in triple transgenic CCSPrtTA/tetOCre/ZAP and CCSPrtTA/tetOCre/ZEG mice.
To identify the sites of rtTA expression in the CCSP-rtTA mouse, in situ hybridization for the reverse tetracycline activator mRNA was performed in mouse lung from E 13.5 to Postnatal Day (PN) 21 (Figure 2). No rtTA mRNA was found in lung epithelium before E 14.5. At E 14.5 and E 16.5, rtTA mRNA was confined to respiratory epithelial cells in the conducting airways (trachea and bronchi) (Figures 2A and 2B) and bronchioles. From E 18.5 to PN 21 rtTA mRNA was found in the conducting airways (Figures 2C–2H) and in a subset of peripheral epithelial cells, consistent with previous findings demonstrating the sites of expression of the rat CCSP promoter in type II epithelial cells of the adult mouse lung (22, 23). In the adult, rtTA mRNA was detected in epithelial cells adjacent to tracheal cartilage rings on the ventral side of both trachea and bronchi. In the dorsal epithelium of the trachea, rtTA mRNA appeared to be random and less frequently expressed (Figure 2I). This pattern of rtTA expression, and of subsequent recombination observed in the CCSPrtTA/tetOCRE mice, was distinct from that observed in SPCrtTA/tetOCRE mice, in which recombination was similar on the dorsal and ventral side of the trachea and increased along the proximal to distal axis (21). In situ hybridization for CCSP mRNA was performed on adult mouse and rat lung tissue to locate cellular expression of endogenous CCSP. In contrast to the mouse, where CCSP mRNA expression was restricted to the conducting airways (25), rat CCSP was expressed in a subset of alveolar type II cells, as well as in the conducting airways (Figure 3). This finding indicates that the rat CCSP promoter directs transgene expression to cells of both the conducting airways and the alveolar epithelium in transgenic mice (22). Therefore, rtTA mRNA expression in the mouse is consistent with endogenous rat CCSP gene expression.
Figure 2.

Localization of rtTA mRNA in CCSPrtTA transgenic mice. Radioactive in situ hybridization was performed on paraffin sections of lung from CCSP-rtTA transgenic mice killed at E 14.5 (A), E 16.5 (B), E 18.5 (C), PN 5 (D), PN 7 (E), PN 10 (F), PN 14 (G), and PN 21 (H, I). The CCSP promoter drives rtTA expression in the bronchus and bronchioles as early as E 14.5 and in the alveolar region as early as E 18.5. In the trachea of adult mice, rtTA expression was detected primarily in epithelial cells on the ventral side of the trachea. V, ventral; D, dorsal.
Figure 3.
Localization of endogenous CCSP mRNA in rat and mouse lung. Radioactive in situ hybridization was performed on paraffin sections of lung from adult rat (A, C) and mouse (B). Endogenous CCSP mRNA expression was detected in the bronchus, bronchioles, and a subset of alveolar type II cells in the rat (A). In the mouse, CCSP mRNA was detected in conducting airways but not in the alveoli (B). Hybridization with the sense RNA is shown on rat tissue (C). Size bars = 200 μm.
Mild but consistent enlargement of alveoli was noted in the lungs of all CCSPrtTA mice in the presence or absence of doxycycline or additional transgenes.
Conditional Recombination in the Postnatal Lung Controlled by CCSPrtTA
Exposure of the adult triple transgenic CCSPrtTA/tetOCRE/ZAP mice to doxycycline for 3 wk caused expression of AP in the conducting airways and in a subset of type II epithelial cells in the lung periphery (Figures 4A and 4B), demonstrating the sites and extent of CRE-mediated recombination. In the absence of doxycycline, very few cells in the bronchioles of adult and 3-wk-old mice were labeled (Figure 4A). Doxycycline-independent recombination was never found in the alveolar region (21) (Figures 4A, 4E, and 4F). AP staining was never found in double transgenic tetOCRE/ZAP mice (Figures 4C and 4D). When triple transgenic mice were exposed to doxycycline from E 18.5 to PN 9, clusters of AP-labeled epithelial cells were observed lining the bronchioles (Figure 4G). After this time of prolonged doxycycline exposure, a subset of type II cells was labeled (Figure 5H), consistent with the timing and extent of expression of the rtTA mRNA as detected by in situ hybridization (Figure 2). Labeling of alveolar type I cells was not observed in the CCSPrtTA/tetOCRE/ZAP mice. In contrast, both alveolar type I and type II cells were extensively labeled in SPCrtTA/tetOCRE/ZAP mice. This finding suggests that in the CCSPrtTA transgenic mice this subset AP labeled of type II cells does not differentiate into type I cells and, therefore, represents a distinct subset of type II cells from those targeted in SPCrtTA mice (21).
Figure 4.
CCSPrtTA mediated recombination in adult and E 18.5 to PN 9 mouse lung. Lung sections of adult (A, B) and PN21 (C–H) double and triple transgenic mice were analyzed for AP expression. In the absence of doxycycline, rare recombination in the conducting airways was observed in lung sections of adult (arrows in A) and PN 21 (arrowhead in E, F) triple transgenic CCSPrtTA/tetOCRE/ZAP mice. No AP-positive cells were found in the lung periphery (A, F). No AP-positive cells were detected in double transgenic control lungs (C, D). After 3 wk of doxycycline treatment, recombination was found in ciliated cells, Clara cells (arrows in B), and a subset of type II cells (arrowheads in B) in triple transgenic adult CCSPrtTA/tetOCRE/ZAP mouse lung. After doxycycline exposure from E 18.5 to PN 9, most nonciliated and ciliated cells in the bronchioles were positive for AP staining in triple transgenic CCSPrtTA/tetOCRE/ZAP PN 18 lungs. Cil, ciliated cells; Cla, Clara cells. Size bars: A, B = 50 μm; C–H = 10 μm.
Figure 5.

Recombination in embryonic lungs of CCSPrtTA/tetOCRE/ZAP mice after continuous exposure to doxycycline. Dams were treated with doxycycline from E 6.5 until killing. Mice were killed on E 14.5 (A), E 15.5 (B), E 16.5 (C), and at birth (D). Rare AP-positive cells were detected in conducting airways as early as E 14.5 (arrowhead), and in the lung parenchyma as early as E 16.5 (arrowhead). Numbers of labeled epithelial cells increased from E 14.5 until birth. Size bar = 50 μm.
Timing of Conditional Recombination in the Embryonic Respiratory Epithelium with the Rat CCSP Promoter
When the CCSPrtTA dams were exposed to doxycycline from E 6.5 and thereafter, labeling of conducting airway epithelial cells was first detected at E 14.5 (Figure 5). The numbers of labeled epithelial cells in the conducting airways increased between E 15.5–16.5 and thereafter. Most bronchiolar cells were labeled during this period of doxycycline exposure. Alveolar cells were first labeled at E 16.5 and thereafter (Figure 5), in contrast to findings in the SPCrtTA mice, where labeling of peripheral progenitor cells occurred very early in lung morphogenesis (21). Endogenous CCSP mRNA expression was detected in mouse bronchioles at E 16.5 (22), and protein expression was detected by E 17.5 (24). E 16.5 defines the end of the pseudoglandular stage, a time when specific cellular markers of the proximal and peripheral epithelium are increasingly expressed. Dams were exposed to doxycycline for 48-h periods to label progenitor cells at precise intervals (Figure 6). Lungs of triple transgenic CCSPrtTA/tetOCRE/ZAP offspring were analyzed at E18.5. When exposed to doxycycline from E 12.5–E 14.5 (Figures 6A, 6D, and 6G), infrequent tracheal and bronchial cells were labeled, but no cells in the bronchioles or peripheral saccules were labeled. Between E 14.5 and E 16.5 (Figures 6B, 6E, and 6H), extensive labeling was observed in trachea, bronchi and bronchioles, and frequently in the peripheral saccules. Between E 16.5 and E 18.5 (Figures 6C, 6F, and 6I), recombination was detected in virtually all cells lining the bronchioles, and in a subset of epithelial cells in the lung periphery (Figure 6). In contrast to SPCrtTA mice (21), thyroid and thymus were never labeled in the CCSPrtTA mice.
Figure 6.

Timing of recombination in the prenatal period. Dams were treated with doxycycline for 48-h periods. AP staining was assessed in triple transgenic CCSPrtTA/tetOCRE/ZAP lungs at E 18.5. When exposed to doxycycline from E 12.5–14.5, few cells were labeled in the bronchioles (A, D, G). After exposure from E 14.5–16.5, extensive labeling was observed in bronchioles and bronchiolar-alveolar portals, with a subset of cells labeled in peripheral saccules (B, E, H). After exposure from E 16.5–18.5, recombination was detected in most cells in the proximal and terminal bronchioles, but was rarely detected in peripheral lung saccules (C, F, I). Labeling was not detected in thymus or thyroid. Size bar = 100 μm.
Distinct Patterns of Recombination in Conducting Airways
ZEG mice, expressing GFP following recombination, were used to more precisely image the spatial organization of labeled epithelial cells in the lung. Dams were provided doxycycline from conception throughout pregnancy. Triple transgenic CCSPrtTA/tetOCRE/ZEG and SPCrtTA/tetOCRE/ZEG mice were analyzed on PN 7 (Figure 7). Continuous exposure of the dams to doxycycline caused a stereotypic pattern of recombination in subsets of conducting airway cells. CCSPrtTA/tetOCRE/ZEG, GFP expressing cells were observed in both cephalic and caudal regions of the trachea. GFP was expressed in a horizontal banding pattern along the ventral side of the trachea. Epithelial cells overlying the cartilage rings were labeled, while GFP labeling was rare in inter-cartilaginous regions (Figure 7A). This pattern of discontinuous labeling was observed from the larynx to the end of the cartilaginous region in the main stem and lobar bronchi. On the membranous, or dorsal, side of the trachea, GFP-labeled cells were distributed randomly (Figures 7C, 7E, 7G, 8A, and 8B). The same dorsal ventral pattern was found by in situ hybridization for rtTA mRNA in the trachea (Figure 2I). The pattern of labeling in the CCSPrtTA/tetOCRE/ZEG was distinct from that observed in SPCrtTA/tetOCRE/ZEG mice, in which epithelial cells of the trachea were labeled with increasing frequency in a proximal to distal gradient. Distal trachea and mainstem bronchi of SPCrtTA/tetOCRE/ZEG mice contained labeled cells forming longitudinal stripes that were similar on both dorsal and ventral surfaces (Figures 7D, 7F, and 7H).
Figure 7.
Pattern of recombination from the trachea to the alveolar epithelium. Dams were treated with doxycycline from E 6.5 to PN 7. Tracheas of triple transgenic CCSPrtTA/tetOCRE/ZEG (A, C, E, G, I, K) and SPCrtTA/tetOCRE/ZEG (B, D, F, H, J, L) mice were visualized using an inverted microscope with fluorescence optics. Whole mount of the proximal trachea is shown in A and B. The main stem bronchi are shown in C and D. Inserts in A and C demonstrate the ventral (A) and dorsal (C) aspects of the trachea at 4× higher magnification. In CCSPrtTA/tetOCRE/ZEG tracheas, labeled cells were present in a dorsal–ventral pattern with increased density of labeled cells overlaying the cartilage rings. In the SPCrtTA/tetOCRE/ZEG tracheas, labeled cells formed longitudinal stripes with increasing numbers of labeled cells observed from the proximal to distal region. In the mainstem bronchi (E, G), labeled cells where found in a random pattern in the lungs of CCSPrtTA/tetOCRE/ZEG mice. Longitudinal stripes were observed in the bronchi of SPCrtTA/tetOCRE/ZEG mice (F, H). Recombination was frequent in epithelial cells of the bronchioles, as well as in the alveoli of both transgenic lines (I–L). Size bars: A, B = 400 μm; E, F = 500 μm; G, H, I, J = 250 μm; K, L = 400 μm. Note: autofluorescence can be seen in all tissue. V, ventral; D, dorsal.
Figure 8.

Colocalization of GFP with β-tubulin and endogenous CCSP in the trachea. Dams were treated with doxycycline from E 6.5 to PN 7. Frozen sections of trachea (A, B) of triple transgenic CCSPrtTA/tetOCRE/ZEG mice were visualized with an upright microscope using fluorescence optics. Numerous GFP-positive cells were detected all along the ventral or cartilaginous side of the trachea (arrows in A) from the larynx to the mainstem bronchi (A, B). Tracheal glands lacked GFP staining (arrows in B). Frozen sections of CCSPrtTA/tetOCRE/ZEG trachea were stained for β-tubulin (C, D; red) or CCSP (E, F; red) and visualized for dual fluorescence with GFP (green). Ciliated cells on the dorsal side of the trachea were predominantly GFP-negative (C, arrows). The majority of ciliated cells (β-tubulin) on the ventral side of the trachea were labeled with GFP (D, arrow), although some ciliated cells were GFP-negative (C, D; arrowhead). CCSP did not colocalize with GFP anywhere in the trachea (E, F; arrowhead). Note: The yellow color on F results from close proximity of the red CCSP signal and the green GFP signal, but does not reflect colocalization. Arrows in all panels show GFP-negative cells that express differentiation markers; arrowheads demonstrate colocalization. Size bars: A, B = 500 μm; C, E = 200 μm; D, F = 50 μm.
Conditional Labeling in the Peripheral Lung
After continuous doxycycline treatment during embryonic development, GFP-positive cells lined small airways and bronchioles of both CCSPrtTA/tetOCRE/ZEG and SPCrtTA/tetOCRE/ZEG lungs, and GFP-positive cells were found in the alveolar regions (Figures 7I, 7J, 9A, and 9B). GFP-positive alveolar type I and type II cells were found in SPCrtTA/tetOCRE/ZEG lungs. GFP-positive alveolar type II cells, but not type I cells, were found in the lungs of CCSPrtTA/tetOCRE/ZEG mice.
Figure 9.

Colocalization of GFP with epithelial cell markers in the peripheral lung. Dams were treated with doxycycline from E 6.5 to PN 7. Most bronchiolar cells (A) and some alveolar type II cells (B) expressed GFP. At PN 7 frozen sections of CCSPrtTA/tetOCRE/ZEG lungs were stained for β-tubulin, CCSP, pro–SP-C, or CGRP (red) and visualized for dual fluorescence with GFP (green). Some ciliated (β-tubulin–positive) cells expressed GFP (arrowhead in C). In the bronchioles, some Clara cells (CCSP-positive) expressed GFP (arrowhead in D), although not all nonciliated, GFP-expressing cells expressed CCSP (arrows in D). A subset of alveolar type II cells (pro–SP-C) expressed GFP (arrowhead in E). GFP was not detected in squamous type I epithelial cells (E). GFP expression (green) was not colocalized with CGRP (arrows in F). Note: arrows in all panels show GFP-negative cells that do express differentiation markers; arrowheads show colocalization. Size bars: A = 500 μm; B = 125 μm; C–F (left) = 50 μm; C–F (right) = 10 μm.
Correlations between Airway Epithelial Differentiation and Recombination
GFP expression in the tracheal epithelium of CCSPrtTA/tetOCRE/ZEG mice was correlated with sites of immunohistochemical staining for β-tubulin (ciliated cells), CCSP (Clara cells), pro–SP-C (alveolar type II cells), and CGRP (neuroepithelial cells). Ciliated cells overlying tracheal cartilage on the ventral surfaces of the trachea and bronchi expressed GFP. In contrast, ciliated cells on the dorsal side of the trachea or in the bronchioles were GFP-negative (Figures 8C, 8D, and 9C). Since the CCSP promoter is not active in ciliated cells, these findings suggest that expression of the rat CCSPrtTA transgene occurred in progenitor cells that were labeled before ciliated cell differentiation. Exposure of the dams to doxycycline for 48-h periods, starting at E 12.5, E 14.5, or E 16.5, revealed that recombination in the tracheal epithelium occurred during doxycycline exposure from E 12.5 to E 14.5 and did not occur when the dam was exposed to doxycycline after E 16.5 (data not shown). Since targeting of tracheal epithelial cells occurred early in development, nontargeted ciliated cells are likely derived from progenitor cells that are distinct form those of GFP-positive ciliated cells on the ventral side of the trachea. Surprisingly, epithelial cells expressing endogenous mouse CCSP in the adult trachea did not express GFP. Thus, the rat CCSP promoter induced recombination in a subset of cells that have differentiated later and no longer express endogenous CCSP. Furthermore, differentiated CCSP-expressing cells in the trachea of postnatal mice are not susceptible to recombination.
Some, but not all bronchiolar epithelial cells co-expressed CCSP and GFP, indicating that the rat CCSP promoter targeted a subset of bronchiolar Clara cells in this transgenic mouse line (Figure 9D). In the peripheral lung, GFP was colocalized with pro–SP-C in a subset of alveolar type II cells (Figure 9E). This site of recombination is consistent with the expression of the rtTA mRNA under the control of the rat CCSPrtTA promoter and with expression of the endogenous CCSP gene in the rat. In both CCSPrtTA and SPCrtTA transgenic mice (21), GFP was never detected in CGRP-reactive cells, indicating that CCSP lineage did not overlap with that of the neuroepithelial cells identified by CGRP (Figure 9F). Cells of the tracheal glands (Figure 8B), which are located at the proximal end of the trachea, were not labeled by expression of doxycycline-induced CRE under the control of either the rat CCSP or human SPC promoter.
DISCUSSION
The CCSPrtTA and SPCrtTA transgenic lines have been used to conditionally express or delete expression of various genes from the developing and adult respiratory epithelium (5, 7, 11, 26, 27). By using either the SPCrtTA or CCSPrtTA transgenic mice, genes can be readily activated or inactivated at specific times during development. The sites and extent of gene activation or deletion can be influenced by the promoter, as well as timing of exposure to doxycycline. The pattern of rtTA expression controls time-dependent CRE activation and recombination that is useful for inducing gene addition or deletion in defined subsets of cells at specific times. The CCSP rtTA conditional system is particularly useful for alteration of gene expression in the conducting airways and for conditional regulation of genes that may be lethal if altered before birth. The present study compares the sites and timing of recombination, using the CCSPrtTA inducible system with previous studies using the SPCrtTA-inducible system (21). Because these mice are used by many investigators, knowledge of the utility and limitations of the models should be useful in the interpretation of experiments designed to discern gene function using this system.
Timing of Gene Expression and Recombination in CCSPrtTA versus SPCrtTA
CRE-mediated SPCrtTA-controlled recombination occurs before formation of definitive lung buds and mediates recombination throughout the intrapulmonary respiratory epithelium (21). In contrast, recombination with the CCSPrtTA transgene occurs after E 14.5 and targets subsets of epithelial cells, which are distinct from those targeted in the SPCrtTA mice. Since gene addition or deletion with the SPCrtTA line may occur throughout the epithelium, gene alterations may limit perinatal survival. The CCSPrtTA system targets subsets of epithelial cells later in lung morphogenesis and can be used to bypass potentially lethal gene effects. In the postnatal period, recombination with the CCSPrtTA line occurs more frequently than with the SPCrtTA mouse line (21), making the CCSPrtTA line more suitable for studies in which recombination is used to study the adult lung.
Cellular Localization of Recombination
Consistent and reproducible labeling patterns were found in the trachea and lung with both SPCrtTA/tetOCRE and CCSPrtTA/tetOCRE recombination systems. Analysis of both models indicated that extensive and widespread random migration or “shuffling” of cells does not occur during lung morphogenesis. In the SPCrtTA transgenic mice, exposure to doxycycline before E 9–10 targets only a few precursor cells that results in labeling of virtually all intrapulmonary epithelial cells (21). Thus, all intraparenchymal respiratory epithelial cells, except the neuroepithelial cells, are derived from a pool of common endodermal precursor cells identified by recombination with the SP-C promoter.
A rare subset of tracheal epithelial cells can be targeted with the SPCrtTA system in early lung development, providing a useful model for studying paracrine signaling between epithelial and mesenchymal cells of the trachea. For example, expression of Fgf18 (28) or deletion of Shh (7) in the tracheal epithelium from E 11.5–12.5 using the SPCrtTA system, resulted in aberrant cartilage ring formation in the trachea close to the laryngeal region. Later in development, SPCrtTA-induced gene expression or deletion in the respiratory epithelium adjacent to cartilaginous structures had no effect on cartilage ring formation, indicating that tracheal epithelial cells influence the sites and extent of tracheal-bronchial cartilage formation only during early developmental stages. Likewise, the labeling pattern of distinct subsets of related cells identified by CCSPrtTA-induced recombination supports the concept that cartilage-related cells provide paracrine signals that influence cell type patterning along the ventral side of the trachea and bronchi. Most GFP-positive cells were found ventrally, overlying the cartilage rings. Previous scanning electron microscopy studies of the rat trachea demonstrate that ciliated cells are concentrated in the tracheal ligament between cartilage rings, which may represent specialized regions of active mucous clearance (29). In the mouse, ciliated cells in the tracheal ligament were not extensively labeled using the CCSPrtTA mouse line, which suggests that the progenitor cells of these specialized ciliated cells were not targeted with the CCSPrtTA system. A subset of GFP-positive tracheal cells did not express the differentiation markers β-tubulin or CCSP, and may represent cells that have the potential for self renewal after injury, as described by Plopper and coworkers (30). In the bronchioles, a subset of CCSP-positive cells were also GFP-positive. Their random and scattered location, however, makes them distinct from the label retaining subset of Clara Cells found in association with neuroepithelial bodies in the airway (31). In addition, the rat CCSP promoter directed gene expression to a subset of Clara cells in contrast to the murine CCSP promoter, which labeled all of the Clara cells (31). Whether this subset of GFP-positive Clara Cells serves as a source of progenitor cells in the airway remains to be investigated.
Sites of rtTA Expression and Recombination Do Not Correlate in Adult Lung
In the rat, endogenous CCSP is expressed in a subset of alveolar type II cells, whereas in the mouse lung CCSP expression is restricted to Clara cells in the conducting airways. Transgenes driven by the rat CCSP promoter in the mouse were expressed in both conducting airways and in alveolar type II cells. In the postnatal period, rtTA expression directed by the rat CCSP promoter was detected at high frequency on the ventral side of the trachea. In the postnatal period recombination did not occur in Clara cells of the conducting airways, even though these cells do express endogenous CCSP. Thus, in the postnatal lung, rat CCSP promoter–driven rtTA expression and CRE-mediated recombination do not entirely overlap with sites and extent of endogenous mouse CCSP expression, indicating that either labeling occurs in precursor cells that differentiate into various cell types or that labeling occurs only in a subset of differentiated cells. Similarly in the alveolar region of the adult lung, only a subset of alveolar type II cells, which expresses CRE controlled by CCSPrtTA or SPCrtTA, undergoes recombination. In contrast to the paucity of doxycycline-induced recombination observed in type II cells in adult SPCrtTA mice, recombination occurred frequently in the adult CCSPrtTA mouse lung. Furthermore, labeling in alveolar type I cells was observed in SPCrtTA but not CCSPrtTA transgenic mice. Based on these results, we postulate that the CCSPrtTA and the SPCrtTA transgenes target distinct subsets of alveolar type II cells. The present SPCrtTA and CCSPrtTA systems are being widely used; therefore, characterization of the sites and timing of expression will be useful for design and interpretation of experiments. Because the sites and extent of gene expression can be controlled by the timing and duration of doxycycline treatment, the SPCrtTA and CCSPrtTA systems can be used to control differential gene-expression in subsets of lung epithelial cells. CCSPrtTA and SPCrtTA, when used with tetOCRE and other Tet operator–driven genes have been useful for conditional control of gene expression or for permanently altering gene expression in various subsets of respiratory epithelial cells in both fetal and postnatal mice. Moreover, SPCrtTA and CCSPrtTA transgenic mice can be used to manipulate gene expression during well-defined periods of development to target subsets of alveolar progenitor cells by the administration of doxycycline.
The present labeling experiments demonstrated an unexpected diversity of labeling and gene expression amongst morphologically indistinguishable cells. In the trachea, distinct dorsal–ventral and horizontal patterning of labeled cells was observed in the CCSPrtTA transgenic mouse line, while a proximal–distal gradient of longitudinal stripes was observed in the SPCrtTA transgenic mice (21). In the lung parenchyma, subpopulations of both ciliated and Clara cells were identified that were not directly lineage related. Likewise, sites and extent of cell types labeled with the CCSPrtTA or the SPCrtTA system were distinct. We conclude that the CCSPrtTA system is most useful for targeting gene expression or deletion later in development and in the adult lung. The SPCrtTA system is well suited for alterations of gene expression during embryonic lung development.
Acknowledgments
The authors thank Andreas Nagy, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Canada for providing the tetOCre transgenic mice. They also thank Corrinne G. Lobe, Sunnybrook and Women's College Health Science Centre, Toronto, Ontario, Canada for providing the ZAP and ZEG reporter mice.
This work was supported by the National Institutes of Health HL 56387 and the Research and Development Program of the Cystic Fibrosis Foundation.
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Ten Have-Opbroek AA. Lung development in the mouse embryo. Exp Lung Res 1991;17:111–130. [DOI] [PubMed] [Google Scholar]
- 2.Shannon JM. Induction of alveolar type II cell differentiation in fetal tracheal epithelium by grafted distal lung mesenchyme. Dev Biol 1994;166:600–614. [DOI] [PubMed] [Google Scholar]
- 3.Cardoso WV. Molecular regulation of lung development. Annu Rev Physiol 2001;63:471–494. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery PK. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis 1983;128:S14–S20. [DOI] [PubMed] [Google Scholar]
- 5.Mucenski ML, Wert SE, Nation JM, Loudy DE, Huelsken J, Birchmeier W, Morrisey EE, Whitsett JA. beta-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J Biol Chem 2003;278:40231–40238. [DOI] [PubMed] [Google Scholar]
- 6.Bellusci S, Furuta Y, Rush MG, Henderson R, Winnier G, Hogan BL. Involvement of Sonic hedgehog (Shh) in mouse embryonic lung growth and morphogenesis. Development 1997;124:53–63. [DOI] [PubMed] [Google Scholar]
- 7.Miller LA, Wert SE, Clark JC, Xu Y, Perl AK, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn 2004;231:57–71. [DOI] [PubMed] [Google Scholar]
- 8.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol 1998;8:1083–1086. [DOI] [PubMed] [Google Scholar]
- 9.Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 1996;122:1693–1702. [DOI] [PubMed] [Google Scholar]
- 10.Weaver M, Yingling JM, Dunn NR, Bellusci S, Hogan BL. Bmp signaling regulates proximal-distal differentiation of endoderm in mouse lung development. Development 1999;126:4005–4015. [DOI] [PubMed] [Google Scholar]
- 11.Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA. Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 2004;131:953–964. [DOI] [PubMed] [Google Scholar]
- 12.Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development 2002;129:2233–2246. [DOI] [PubMed] [Google Scholar]
- 13.Keijzer R, van Tuyl M, Meijers C, Post M, Tibboel D, Grosveld F, Koutsourakis M. The transcription factor GATA6 is essential for branching morphogenesis and epithelial cell differentiation during fetal pulmonary development. Development 2001;128:503–511. [DOI] [PubMed] [Google Scholar]
- 14.DeFelice M, Silberschmidt D, DiLauro R, Xu Y, Wert SE, Weaver TE, Bachurski CJ, Clark JC, Whitsett JA. TTF-1 phosphorylation is required for peripheral lung morphogenesis, perinatal survival, and tissue-specific gene expression. J Biol Chem 2003;278:35574–35583. [DOI] [PubMed] [Google Scholar]
- 15.Wikenheiser-Brokamp KA. Rb family proteins differentially regulate distinct cell lineages during epithelial development. Development 2004;131:4299–4310. [DOI] [PubMed] [Google Scholar]
- 16.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res 2002;11:21–29. [DOI] [PubMed] [Google Scholar]
- 17.Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy AZ. AP, a double reporter for cre-mediated recombination. Dev Biol 1999;208:281–292. [DOI] [PubMed] [Google Scholar]
- 18.Novak A, Guo C, Yang W, Nagy A, Lobe CGZ. EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 2000;28:147–155. [PubMed] [Google Scholar]
- 19.Wert SE, Glasser SW, Korfhagen TR, Whitsett JA. Transcriptional elements from the human SP-C gene direct expression in the primordial respiratory epithelium of transgenic mice. Dev Biol 1993;156:426–443. [DOI] [PubMed] [Google Scholar]
- 20.Tichelaar JW, Wert SE, Costa RH, Kimura S, Whitsett JA. HNF-3/forkhead homologue-4 (HFH-4) is expressed in ciliated epithelial cells in the developing mouse lung. J Histochem Cytochem 1999;47:823–832. [DOI] [PubMed] [Google Scholar]
- 21.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA 2002;99:10482–10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stripp BR, Sawaya PL, Luse DS, Wikenheiser KA, Wert SE, Huffman JA, Lattier DL, Singh G, Katyal SL, Whitsett JA. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem 1992;267:14703–14712. [PubMed] [Google Scholar]
- 23.Tichelaar JW, Lu W, Whitsett JA. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J Biol Chem 2000;275:11858–11864. [DOI] [PubMed] [Google Scholar]
- 24.Zhou L, Lim L, Costa RH, Whitsett JA. Thyroid transcription factor-1, hepatocyte nuclear factor-3beta, surfactant protein B, C, and Clara cell secretory protein in developing mouse lung. J Histochem Cytochem 1996;44:1183–1193. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Whitsett JA, Stripp BR. Regulation of Clara cell secretory protein gene transcription by thyroid transcription factor-1. Biochim Biophys Acta 1997;1350:359–367. [DOI] [PubMed] [Google Scholar]
- 26.Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. J Clin Invest 2004;113:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perl AK, Kist R, Shan Z, Scherer G, Whitsett JA. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis 2005;41:23–32. [DOI] [PubMed] [Google Scholar]
- 28.Whitsett JA, Clark JC, Picard L, Tichelaar JW, Wert SE, Itoh N, Perl AK, Stahlman MT. Fibroblast growth factor 18 influences proximal programming during lung morphogenesis. J Biol Chem 2002;277:22743–22749. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira MJ, Pereira AS, Guimaraes L, Grande NR. de Sa CM, Aguas AP. Zonation of ciliated cells on the epithelium of the rat trachea. Lung 2003;181:275–282. [DOI] [PubMed] [Google Scholar]
- 30.Plopper CG, Nishio SJ, Alley JL, Kass P, Hyde DM. The role of the nonciliated bronchiolar epithelial (Clara) cell as the progenitor cell during bronchiolar epithelial differentiation in the perinatal rabbit lung. Am J Respir Cell Mol Biol 1992;7:606–613. [DOI] [PubMed] [Google Scholar]
- 31.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Sripp BR. Clara Cell Secretory Protein–expressing cells of the airway neuroepithelial body environment include a label retaining subset and are critical for epithelial renewal after progenitor depletion. Am J Respir Cell Mol Biol 2001;24:671–681. [DOI] [PubMed] [Google Scholar]