Abstract
To study the change in intrapulmonary bacterial growth rate over time during Gram-negative pneumonia, a two-hit model of recurrent bacterial aspiration was developed in mice. A mutant of Klebsiella pneumoniae was isolated that could be distinguished from the wild type when cultured on appropriate media. These strains were intranasally administered, 4 h apart, to mice whose lungs were quantitatively cultured 24 h later. The relative burden of each aspirated inoculum was determined, and, using the administered dose and the number of bacteria from each inoculum present at the end of the experiment, first-order growth constants for each inoculum were calculated. Results indicate that after an initial aspiration of this organism, subsequently aspirated bacteria proliferate more slowly. When two aspirations occurred 4 h apart, the bacteria aspirated first represented 96% of total lung burden at 24 h. The growth constant of the second inoculum was related to the magnitude of the first inoculum in an inverse, nonlinear fashion. When parallel experiments were performed in complement C3-deficient mice, no suppression of the second inoculum was noted, suggesting that early upregulation of antibacterial activity in the lung is a C3-mediated event.
Keywords: pneumonia, mathematical models, complement, Klebsiella pneumoniae
Acute bacterial pneumonia, whether community or hospital acquired, is usually the result of recurrent low-volume aspirations of pharyngeal secretions harboring pathogens that have colonized the pharynx (1). In such a system, an initial aspiration event takes place in a quiescent lung in which only a few sentinel immune detectors and effectors are present. In these early hours, the respiratory pathogen may have the advantage and may enjoy relatively unfettered proliferation. If host immunity is intact, subsequent bacterial aspirations encounter a more lethal lung environment. This occurs within hours due to the upregulation and recruitment of innate humoral and cellular responses and within days as a consequence of adaptive responses. The “repeated hit” mechanism of lung infection is often overlooked in animal models of bacterial pneumonia. Experimentally framing lung infection as a series of bacterial inocula entering a progressively inflamed lung raises questions not easily addressed in single inoculation models, such as: In terms of accumulated bacterial burden, what is the relative contribution of the initial inoculum versus later aspirations? Is the intensity of upregulation of intrapulmonary antibacterial activity an “all or none” phenomenon, or is it related to the magnitude of recent exposures? What host factors drive bactericidal upregulation in the early hours of pneumonia when repeated exposures are occurring?
The answers to each of these questions require a quantitative assessment of bacterial growth (or clearance) in the lung over time. Although it is not possible to measure in vivo the rate of bacterial division or the rate of killing by host effectors, the rate of change in bacterial content in the lung over time is the sum of these effects. If one knows the size of the initial inoculum and the number of bacteria present at the end of some period of time, then an apparent growth constant based on first-order kinetics can be determined to describe net intrapulmonary proliferation. Although such an estimate does not describe some aspects of intrapulmonary bacterial behavior, it does allow easier handling of the exponential nature of bacterial proliferation and host-mediated killing. First-order growth terms have positive values in the setting of net bacterial accumulation and negative values when host defenses successfully render intrapulmonary bacteria nonviable.
A more difficult technical problem is simultaneously measuring the apparent growth constants of multiple bacterial populations that have been aspirated into the lung at different times. In the present, work we developed a quantitative means of assessing the changes in the rate of bacterial proliferation over time in the first 24 h of bacterial pneumonia in mice. We established a two-hit system with Klebsiella pneumoniae using a wild-type bacterial strain and a closely related galactokinase mutant strain; these were similarly virulent but could be phenotypically differentiated by their ability or inability to ferment galactose. The two-hit model, in addition to modeling the clinically relevant problem of sequential injuries to the lung, used the second aspirated inoculum as a means of directly measuring changes in the host environment during the delay between the first and second inocula. When the two strains were delivered 4 h apart, the difference in their observed growth constants was a reflection of changes in host–pathogen interactions during the interinoculum period.
With this strategy, we addressed three issues related to early host defense in the infected lung. First, we examined the relative contribution of two sequential aspirations to total lung bacterial burden 24 h after the onset of disease. Second, we characterized the dose–response relationship between the magnitude of the initial exposure and the growth constants of subsequent bacterial inocula. We hypothesized that larger inocula would recruit host defenses more vigorously and, therefore, would enable the host to suppress subsequent bacterial proliferation more efficiently. Third, to evaluate the utility of this model in studying underlying mechanisms of the dynamics of intrapulmonary bacterial growth, we used it to quantify the roles of two early innate immune proteins, complement C3 and C5, in the upregulation of host defenses in the first few hours of pneumonia. These proteins manifest direct effects against the bacterial surface (C3 by opsonization for subsequent phagocytosis and C5 by initiating the assembly of the membrane attack complex) and produce proinflammatory signals (C5 by producing C5a and C3 by producing C3a and by engaging complement receptors on several host immune cells). The absence of either is known to greatly impair host defense against bacterial pneumonia (2–5). We hypothesized that changes in observed bacterial proliferation rates over time would differ between mice sufficient and deficient in each complement protein.
MATERIALS AND METHODS
K. pneumoniae 43816 was obtained from ATCC. Serratia marcescens strain IGX2 was obtained from the Caenorhabditis Genetics Center, University of Minnesota. Mouse strains included outbred ICR (Harlan-Sprague-Dawley, Indianapolis, IN), C3−/− animals (B6.129S4-C3tm1Crr/J) and their C57BL/6J control strain, and the spontaneous C5-deficient mutant B10.D2-Hc0 H2d H2-T18c/oSnJ and its control strain, B10.D2-Hc1 H2d H2-T18c/nSnJ (Jackson Laboratories, Bar Harbor, ME). BODIPY FL-labeled Escherichia coli bioparticles were purchased from Molecular Probes (Eugene, OR). Phycoerythrin-labeled anti-Ly-6G antibodies were obtained from R&D Systems (Minneapolis, MN). Luria Bertani (LB) broth, MacConkey, MacConkey base, and minimal agars were obtained from Difco (Detroit, MI). All other reagents were purchased from Sigma Chemical Co. (St. Louis, MO) unless otherwise stated. Animal protocols were approved by the institution's animal use committee.
Generation and Characterization of a Galactose-Nonfermenting Phenotype Strain of K. pneumoniae
To differentiate bacterial inocula aspirated at different time points, we exploited the capacity of wild-type K. pneumoniae to use galactose as a carbon source. Spontaneous galactokinase-deficient mutants were identified by plating bacteria on minimal agar containing 1% glycerol and 0.05% 2-deoxygalactose, the latter being metabolized into the toxin 2-deoxygalactose-1-phosphate in the presence of functional galactokinase (6). Colonies growing under these strongly selective conditions were confirmed to possess functional galactokinase mutations by their phenotype on MacConkey galactose agar. One colony was selected for all further study. To characterize the mutation producing the galactose nonfermenting phenotype (Gal−) phenotype, the gene-encoding galactokinase (galK) gene was cloned from wild-type and Gal− K. pneumoniae as follows. The galactokinase sequence was identified by a BLAST search of the published E. coli K12 gene sequence against a published K. pneumoniae genome sequence (7, 8). From this identified sequence, polymerase chain reaction (PCR) primers spanning 50 bp upstream and downstream from the identified gene were constructed (5′-gaattccgcgccgtcagcgacgtc, 3′-aagcttttaaattgctcaagggtgacatcgcg and 5′-gaattccagcctcgctggagtcgcc, 3′-aagcttgctgccatggccggccgt). The resulting PCR products provided 2× coverage for most of the gene. After standard amplification, the PCR products from wild-type and Gal− strains were cloned into pGem-3Z vectors (Promega, Madison, WI), sequenced, and submitted to GenBank under accession number AY902196.
Murine Pneumonia Model
Midlog growth bacteria were washed and resuspended to the desired CFU content using a turbidimetric method (5). Mice anesthetized with 2% inhaled isoflurane (Abbott Laboratories, North Chicago, IL) were inoculated with a 30-μl droplet of bacterial suspension placed in the nares. Upon full recovery, animals were returned to standard housing. Animals in the two-hit protocol received a second aspirated inoculum 4 h after the first. To control for any unrecognized differences between the wild-type and Gal− strains, experiments were stratified such that half of the animals in any experimental group received the wild-type followed by the Gal− strain, and the other half received the strains in reverse order. Unless stated otherwise, mice were killed 24 h after the first inoculation.
Estimation of Aspirated Dose
To determine what fraction of the intranasally administered inoculum entered the lower airway, wild-type K. pneumoniae were grown in LB broth containing 500 μCi [111In] oxime. These were administered as described previously (n = 10). The animals were killed 1 h later, and their lungs, tracheae, and heads above the level of the glottis (constituting the entire pharynx) were assayed for activity. In this assay, 18.5 ± 5.2% of the nasally administered dose was recovered from the lower airway, and 11.5 ± 4.3% remained in the naso-, oro-, or hypopharynx. Although it is likely that retained pharyngeal bacteria were available for later aspiration, all subsequent calculations assumed that only 18.5% of any intranasal dose participated in lung infection.
Confirmation of Comparable Growth and Virulence of Wild-Type and Gal− Strains
Three strategies confirmed that the galK mutation did not significantly affect the pathogenic characteristics of K. pneumoniae. First, their growth rates were compared during batch growth in LB broth. Second, animals were inoculated with just wild-type or Gal− bacteria, and their lungs were cultured at 24 or 48 h (n = 5 per strain per time point) to confirm similar intrapulmonary bacterial burden. Third, the 7-d lethality of a 1,000-CFU intranasal inoculum was compared between strains (n = 10 mice per strain). Survival differences between mice infected with the strains were compared using proportional hazards modeling as previously described (5).
Measurement of Lung Bacterial Burden and Estimation of Apparent Growth Constants
Lungs from infected animals were homogenized in 3 ml of sterile phosphate-buffered saline. Quantitative cultures were performed using serial dilutions on MacConkey galactose agar. We assumed first-order exponential growth, that the burden of intrapulmonary bacteria in a living animal was much smaller than the bacterial carrying capacity of that animal (i.e., that any limitation in growth was a result of host defense and not a result of limited substrate), and that loss of bacteria into the blood or lymphatics or from mucociliary clearance was negligible compared with total lung burden. Accordingly, the bacterial content B at any time t for the ith microaspiration could be determined as follows:
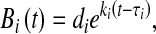 |
where di represents the inhaled dose in CFU, ki denotes that dose's growth rate in hr−1, and τi represents the delay, in hours, between the beginning of the experiment and the ith aspiration.
For each animal, growth constants k1 and k2, describing the growth of the inoculum initially delivered and that delivered 4 h later could be determined as follows:
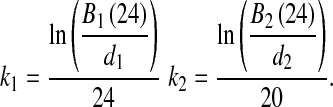 |
The serial dilutions used to measure B in our system had a lower limit of detection of 200 CFU/animal. Accordingly, when an inoculum was undetectable in a quantitative culture, the value assigned to that experiment was 200, rather than 0, CFU.
The relative contributions of both inocula to the total lung burden were described for each animal as the ratio of CFU of the second inoculum to those of the first inoculum at 24 h. In addition to this measured ratio, we generated an expected ratio of second-to-first inoculum burden by assuming that both doses had grown at rate k1 (i.e., under the null hypothesis that bacterial growth rate is unchanged over time during lung infection). The frequency distributions of the measured and the expected ratios were displayed as histograms, and two were compared using Wilcoxon nonparametric methods.
Dose–Response Relationship Between Initial Inoculum and Bacterial Growth Rates
We hypothesized that lung antibacterial activity would increase (and hence that apparent bacterial growth rate would decrease) in proportion to the magnitude of the initial bacterial challenge. To test this, a series of animals were studied in which the size of the first inoculum (d1) was varied over a tenfold range. The dose-response effect between initial inoculum magnitude and intrapulmonary growth was considered in two ways. First, the magnitude of the first inoculum d1 was plotted against k1 and k2. The results suggested a general trend that as d1 increased, ki asymptotically decreased. To validate this hypothesis statistically, we proposed a model where ki was a function of d1:
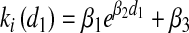 |
and performed a nonlinear regression on the βs for i = 1 and 2. Estimation of the span β1, decay constant β2, and plateau β3 was performed using the Gauss-Newton method used by the NLIN procedure in SAS 8.12 (SAS Institute, Cary, NC). This statistical model provided 95% confidence intervals (CI) and overall F-tests for the regressions (9).
The second strategy used to examine the dose–response relationship explicitly acknowledged that, at higher doses of initial inoculation, the second inoculum was frequently undetectable at 24 h. We therefore treated the 24-h lung content of the first and the second hits as binary response variables (i.e., detectable or no detectable bacteria) rather than as continuous variables. A logistic model of the probability P, over the range of first inocula, of an inoculum being detectable was constructed as follows:
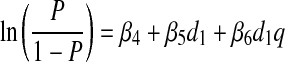 |
where q was a dummy variable with a value of 0 for k1 and 1 for k2, and β4, β5, and β6 were the fitted model parameters.
Confirmation of Key Findings with S. marcescens in a Two-Pathogen System
For some experiments, S. marcescens was used as the first or second inoculum in conjunction with K. pneumoniae. S. marcescens IGX2 has been demonstrated to be pathogenic in murine models of pneumonia (10). Standard MacConkey agar (containing lactose as its carbon source) was used to distinguish S. marcescens (lactose nonfermenting phenotype) and K. pneumoniae (lactose-fermenting phenotype) in lung homogenates. In pilot studies, Serratia strain was cleared more extensively from the lung than Klebsiella. Therefore, an intranasal dose of 108 CFU was used when Serratia was administered as the first or second inoculum.
Many members of Enterobacteriaceae secrete colicins that inhibit the growth of other Gram-negative rods. We confirmed that no significant interpathogen interactions via colicins existed between K. pneumonia 43816 and S. marcescens IGX2 using a mitomycin C-based assay (11). No interaction between the two species was found.
Role of Complement C3 and C5 in Early Changes in Bacterial Proliferation
Complement C3 knockout mice and congenitally C5-deficient mice were used to study the role of these two innate immune proteins in modulating observed bacterial proliferation rates over time. In these experiments, 3,000 CFU were used for each inoculum. Control mouse strains were chosen according to recommendations from the vendor. Differences in observed bacterial growth rates between C3- and C5-sufficient and -deficient mice were compared using ANOVA with Tukey post-hoc comparisons.
Measurement of Intrapulmonary Neutrophil Recruitment and Phagocytosis
In C3+/+ and C3−/− mice, 103 CFU wild-type K. pneumoniae was administered intranasally in saline spiked with BODIPY FL-labeled E. coli bioparticles. Animals were killed 6 h later and underwent bronchoalveolar lavage (BAL) with ice-cold phosphate-buffered saline with 5 mmol ethylenediaminetetraacetic acid. Cells were washed twice and resuspended in sheath fluid. Neutrophil number and extent of phagocytosis was measured using a Coulter Epics XL cytometer. Gates for alveolar macrophages and neutrophils were established preliminarily using forward- and side-scatter from infected and uninfected animals. The contents of the neutrophil gate were confirmed to be stainable with PE-anti-Ly-6G. The phagocytosis negative control condition consisted of lung neutrophils from mice infected with K. pneumoniae in the absence of fluorescent particles. Phagocytosis in animals inoculated with K. pneumoniae and labeled particles was quantified as the percentage of cells staining more brightly than 99% of the negative control cells.
RESULTS
Characterization of a Gal− Mutant of K. pneumoniae
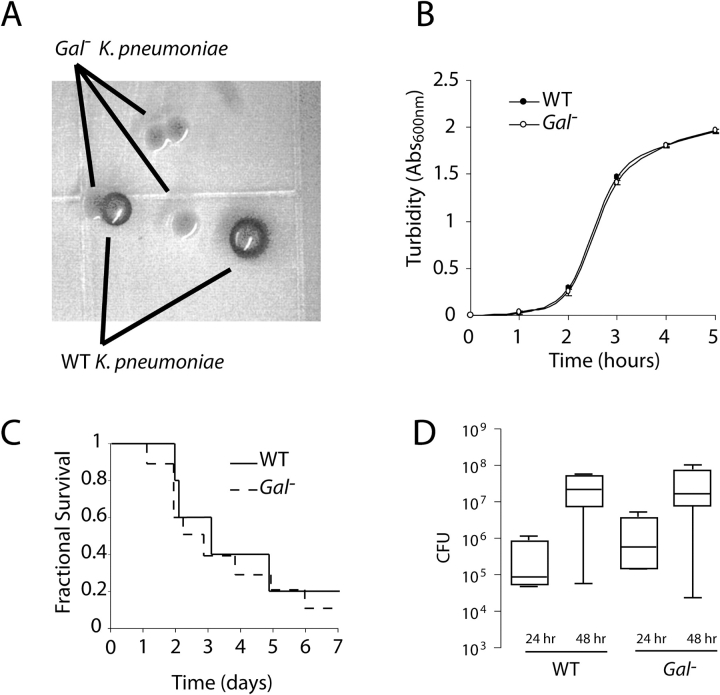
Comparison of the wild-type and mutant galK gene sequences revealed the nucleotide substitution C476T, corresponding to the replacement of a glutamine with a stop codon. This was confirmed using high-fidelity polymerase amplification of the gene from genomic DNA followed by direct sequencing of the PCR product. This premature stop codon presumably produces the Gal− phenotype by terminating galK translation before the C-terminal portion of the catalytic site, including the critical residue Asp-183 (12). Culture of lung homogenates containing wild-type and Gal− strains on MacConkey base agar supplemented with 1% galactose revealed clear phenotypic differences between the two strains, allowing the strains to be tracked individually over the course of our 24-h model (Figure 1A). The galK mutation was not associated with a change in bacterial growth in LB broth (n = 3 per strain, P = nonsignificant [ns]) (Figure 1B), wherein the growth constants at midlog growth were 1.24 h−1 (95% CI, 1.106–1.386) and 1.24 h−1 (95% CI, 1.086–1.386) for the wild-type and mutant strains, respectively. The 7-d survival curves of the wild-type and Gal− strains were indistinguishable (n = 10 animals per group, P = ns) (Figure 1C). Lung burden at 24 and 48 h after infection with either stain alone was similar (n = 5 animals per group per time point, P = ns) (Figure 1D). Thus, the Gal− did not seem to significantly affect the growth or pathogenicity of K. pneumoniae 43816.
Figure 1.
In vitro and in vivo characterization of a Gal− mutant of K. pneumoniae. (A) Appearance of wild-type and Gal− strains on MacConkey base agar containing 1% galactose 24 h after culture from a dually infected mouse lung. (B) Growth of the wild-type and Gal− strains in LB broth, where rates of proliferation were indistinguishable (results shown as means ± SD of three experiments, P = ns). (C) Seven-day survival curves for both strains after a 1,000-CFU intranasal inoculation (n = 10 animals per group, P = ns). (D) Comparable intrapulmonary burden of wild-type and Gal− strains at 24 and 48 h after inoculation when delivered as single-hit inocula (n = 5 per strain per time point, P = ns).
Relative Contributions of the First and Second Inocula to Total Lung Bacterial Burden at 24 h
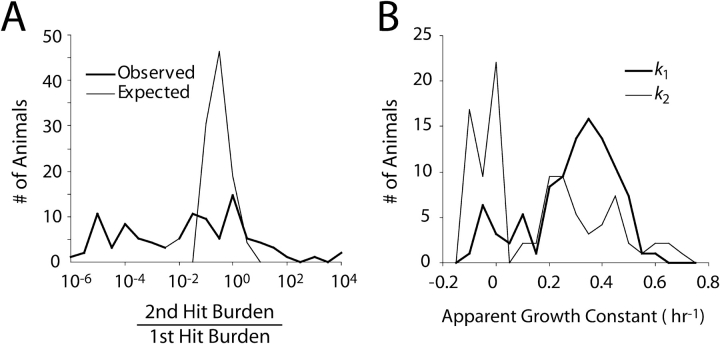
Ninety-five ICR-strain mice were studied to evaluate the relative contribution of the first and second inocula and in subsequent analyses of the relationship between k1, k2, and the dose of the initial inoculum. The median ratio of the second inoculum to the first inoculum was 0.04, indicating that in most animals the progeny of the first inoculum were the predominant organisms present at the end of the experiment (Figure 2A). The median value for the expected ratio had k remained fixed during pneumonia was 0.30 (P = 0.0001), demonstrating that k for sequential inocula was changing during the early hours of infection.
Figure 2.
Bacterial growth during 24 h of lung infection. (A) Relative contribution of the two aspirated inocula expressed as the ratio of second-hit bacterial content divided by first-hit content at 1 d. The “Observed” histogram depicts actual experimental results. The median value for this ratio was 0.04, and in 87% of cases the ratio was < 1, indicating that in most animals the bulk of the bacteria present at 24 h were the progeny of the initial inoculation. The “Expected” curve shows how this ratio would appear had the bacteria given in the second hit grown at the same growth constant as those administered in the first hit. The difference between the two histograms is a result of the greater antibacterial conditions encountered by, and thus the smaller apparent growth constant displayed by, the second inoculum (P < 0.0001). (B) Frequency distribution of growth constants for the first (k1) and second (k2) inocula. The median growth constant for k2 was 0.22 h−1 slower than for k1 (P < 0.0001), another reflection of the more adverse conditions encountered by bacteria entering a lung primed by an inoculation occurring 4 h earlier. Experiments were conducted in 95 ICR strain mice, and histograms were compared in both panels using Wilcoxon nonparametric methods.
Change in Apparent Growth Constant Between First and Second Inocula
Of the 95 animals studied, the median apparent growth constant for the initial inoculum was 0.34 h−1, a value that fell to 0.11 h−1 for the later inoculum, indicating a significant slowing of apparent intrapulmonary growth and corresponding to a prolongation of doubling time from 2 to 6.3 h (Figure 2B).
Effect of Initial Inoculum Magnitude on Subsequent Bacterial Proliferation
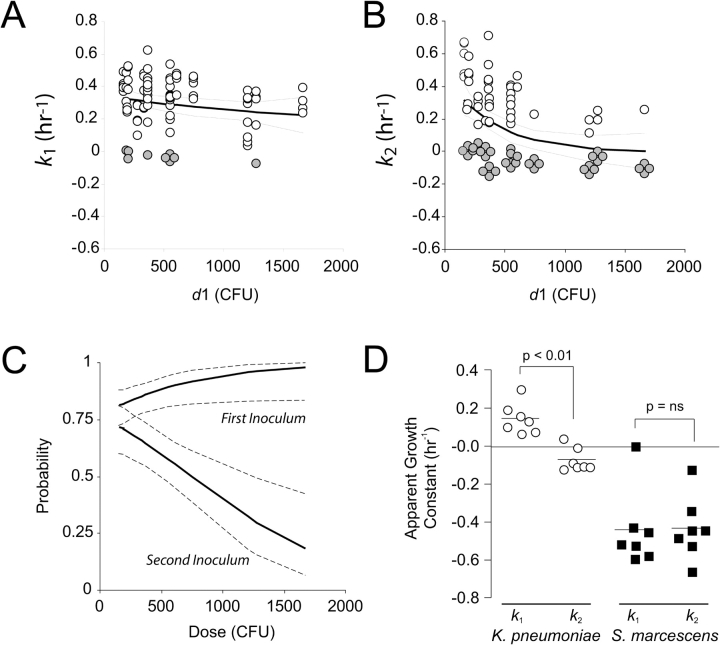
Measurement of bacterial growth constants for initial and subsequent aspirated inocula revealed a complex relationship that varied with the size of the initial inoculum. The relationships between d1 and k1 and k2 are shown in Figure 3, and the corresponding regression fits are shown in Table 1. Across a 10-fold dose range, there was no statistically detectable relationship between the size of the initial inoculum and that inoculum's growth rate over the next 24 h (n = 95, P = 0.1742) (Figure 3A). Distinctly different was the relationship between the initial inoculum and the dose administered 4 h later. There was a decrease in apparent growth constant of the second inoculum as the initial inoculum increased, supporting the hypothesis that larger initial bacterial exposures produce greater early antibacterial responses (P < 0.0001) (Figure 3B).
Figure 3.
Dose–response of intrapulmonary growth constants versus the magnitude of the initial inoculum. (A) Values for the growth over 24 h of the initial inoculum expressed as a first-order growth constant, k1 (h−1), and the best-fit exponential decay function and its 95% CI. No statistical relationship between the size of the initial inoculum and k1 was detected (n = 95 animals, P = 0.1742). In A and B, filled circles represent instances where an inoculum was undetectable by quantitative culture at the end of the 24-h experiment. (B) Similar analyses for the growth constant of the second hit (k2). A dose response was statistically supported (P < 0.0001). (C) Dose response of the probability of detecting the first hit (upper band) or the second hit (lower band) in lung culture at 24 h. Solid lines represent the estimated probability, by logistic regression, of detecting an inoculum. Dotted lines span the 95% CI for the estimate (P < 0.0001 for the two regions being statistically distinct). (D) Interaction between K. pneumoniae and S. marcescens in a two-hit model. The apparent growth of K. pneumoniae was significantly faster when it was introduced as an initial inoculum (k1) rather than 4 h after S. marcescens (k2) (n = 10 mice per condition, P < 0.05). S. marcescens was cleared so effectively that no difference between its growth as a primary inoculum and as a subsequent inoculum was detected.
TABLE 1.
Nonlinear regression estimates for dose–response curves of k1 and k2
| Parameter | Estimate | 95% CI | Overall F test P value |
|---|---|---|---|
| First inoculum (k1) | 0.1742 | ||
| Span (β1, h−1) | 0.17 | −0.36–0.70 | |
| Decay (β2, CFU−1) | 8.04 × 10−4 | −4.73 × 10−3–6.34 × 10−3 | |
| Plateau (β3, h−1) | 0.18 | −0.45–0.81 | |
| Second inoculum (k2) | < 0.0001 | ||
| Span (β1, h−1) | 0.48 | 0.24–0.72 | |
| Decay (β2, CFU−1) | 2.46 × 10−3 | −3.90 × 10−4, 5.1 × 10−3 | |
| Plateau (β3, h−1) | −5.42 × 10−3 | −0.14–0.13 |
At higher initial doses, the second inoculum was frequently not detectable by culture at 24 h. In the nonlinear regression analysis, values for these animals were assigned to the lower limit of detection to allow their inclusion in the model, violating an assumption (normal distribution of the dependent variable) made in this type of analysis. We therefore further examined the dose–response relationship by modeling the probability, for any given initial inoculum, that the first and second inocula would be reduced to the level of undetectability during their intrapulmonary course. This analysis (Figure 3C, Table 2) also revealed a strong dose effect on the second inoculum (P < 0.0001).
TABLE 2.
Logistic regression analysis of the magnitude of the initial inoculum and the probability of inocula persisting at 24 h after infection
| Parameter | Estimate | P value* |
|---|---|---|
| β4 | 1.20 | 0.0002 |
| β5 | 4.89 × 10−3 | 0.0007 |
| β6 | −3.25 × 10−3 | < 0.0001 |
See text for model details.
Overall P value < 0.0001.
Growth Kinetics in a Polymicrobial Model
We considered our findings with K. pneumoniae in a broader context by studying in some animals a two-hit injury using two different organisms: wild-type K. pneumoniae 43816 and S. marcescens IGX2. Pilot studies revealed that IGX2 was more susceptible to intrapulmonary killing; therefore, intranasal doses of 108 CFU of this organism were used in these experiments. When introduced as a first hit, S. marcescens was able to reduce the growth rate of subsequently aspirated K. pneumoniae (n = 10 per strain, P < 0.05) (Figure 3D). However, Serratia's growth constants, which when compared with K. pneumoniae were much lower when Serratia was used in the first or the second inoculum, were similar regardless of the order of inoculation.
The Role of Two Complement Proteins on Early Changes in Lung Bactericidal Activity
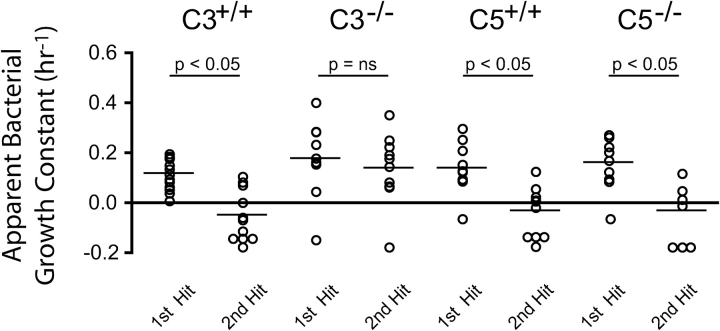
An important strength of our two-hit strategy is its ability to explore specific host and pathogen factors that affect intrapulmonary bacterial proliferation. We used our experimental model to examine the independent contribution of two complement proteins, C3 and C5, to the upregulation of host antibacterial function in the lung early in the course of pneumonia. At an initial intranasal dose of 3,000 CFU, C3-sufficient and C5-sufficient and -deficient mice mounted an immune response that significantly reduced k2 (n = 10, P < 0.05 for each) (Figure 4). This was not the case with C3-deficient mice, which enjoyed no diminution of bacterial proliferation after the initial inoculation. These data pointed to a more important role for C3 than C5 in the development of a bactericidal intrapulmonary environment early in the course of illness.
Figure 4.
Contribution of complement C3 and C5 on intrapulmonary bacterial growth rates. To evaluate the role of these complement pathway proteins in directed bacterial clearance early in the course of pneumonia, apparent growth constants of the first and second hits were determined in C3- and C5-sufficient and -deficient mice. In each instance (except among C3-deficient mice), the mean value for k2 was < 0 and statistically less than k1, indicating that on average these animals mounted a antibacterial response in the first 4 h of infection. C3-deficiency was the exception, suggesting a critical role for this complement protein in upregulating the antibacterial defenses of the lung early in the course of pneumonia (n = 5 animals per group) and acting as evidence-in-proof of the two-hit strategy to evaluate specific mechanisms of host–pathogen interaction.
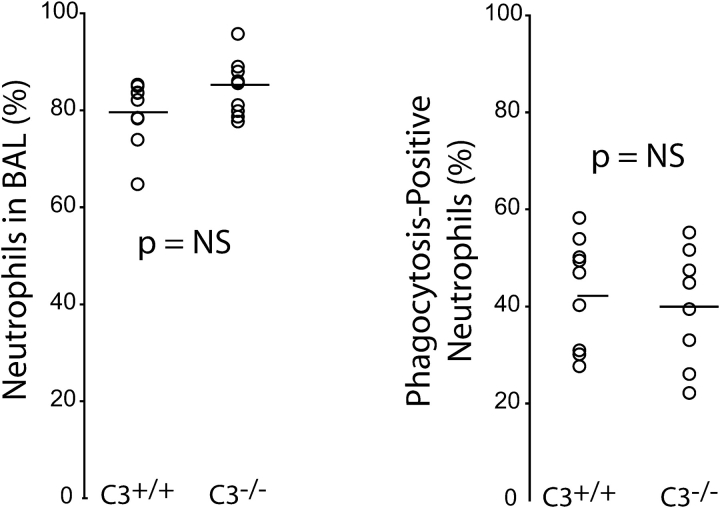
To further characterize the role of C3 in this system, experiments were performed to examine the in vivo ability of C3-sufficient and -deficient mice to recruit neutrophils into their lungs early in the course of infection and for those neutrophils to phagocytose intrapulmonary bacteria. We used a flow cytometry-based assay of BAL-recoverable neutrophils in mice coinoculated with Klebsiella and BODIPY FL-labeled E. coli bioparticles. At 6-h after infection, C3-deficient and wild-type mice had similar recruitment of neutrophils into the lung (Figure 5). Furthermore, among neutrophils recovered from the lung, there was no difference between C3+/+ and C3−/− animals in the extent of ingested particles. The mechanism by which C3 contributes to early host defense is therefore not solely a result of its ability to promote neutrophil recruitment or to promote the phagocytosis of foreign material.
Figure 5.
Effect of C3 on intrapulmonary neutrophil recruitment and phagocytosis during pneumonia. C3+/+ and C3−/− mice were inoculated with 103 CFU of K. pneumoniae mixed with fluorescently labeled E. coli bioparticles. BAL was performed 6 h later, and the intrapulmonary content of neutrophils (as a percentage of all BAL-recovered cells) was determined using a flow cytometer (see text for details). The effectiveness of phagocytosis by recruited neutrophils was assessed by measuring the fraction of neutrophils containing fluorescently labeled particles. No difference between C3-sufficient and C3-deficient mice was seen in either measurement (n = 9 per group, P = ns).
DISCUSSION
We used a novel murine model of repeated bacterial aspiration to characterize early host–pathogen dynamics in the lung. By treating proliferation as a first-order phenomenon and by independently tracking distinct bacterial aspirates over the first 24 h of illness, we have begun to explore the kinetics of bacterial proliferation and host-mediated killing that occur in pneumonia. Because our focus was intrapulmonary bacterial proliferation, our results address the central functional outcome of pneumonia (i.e., the clearance of the pathogen) rather than the inflammatory events mounted to achieve that outcome. Our data indicate that the host response to aspirated bacteria early in the course of illness may have a profound effect on the net growth of subsequently inhaled organisms and that these interactions, rather than being limited to multiple aspirations of a single organism, can occur between multiple species aspirated into the lung at different times.
Penetration into the lower airway by pathogenic bacteria is a defining event in the transition from a colonized to an infected respiratory tract. Our data shed some light on the nature of this transition. Early microaspiration events, if sufficiently large, can activate host responses that limit the growth of subsequently aspirated organisms. Our model suggests that in the setting of recurrent aspiration leading to pneumonia, the bacterial burden found in the lung may not be a result of the accumulation of bacteria repeatedly entrained from the upper airway. Rather, early inocula may be of prime importance as they enjoy the most favorable growth conditions; subsequently inhaled bacteria encounter a progressively hostile environment that, assuming sufficient effectiveness of host defenses, renders latecomers less relevant to the overall course of infection.
We feel there is significant value in considering intrapulmonary bacterial behavior as a kinetic process. The use of growth constants facilitates the comparison of host defense and bacterial pathogenic factors (and, more globally, host and bacterial strains) in a rigorous fashion that is easily portable between laboratories and published reports. On a more fundamental level, the application of kinetic parameters describing host–pathogen interactions and the empiric arrival at estimates for those parameters is a key step in establishing a useful mathematical simulation of disease. Our two-hit model suggests that parameters describing bacterial growth and host-mediated bacterial killing are not constant values; rather, they modulate during the course of illness in relationship to the timing and magnitude of earlier events. This is an important consideration for future attempts at modeling lung infection.
There are several challenges involved in quantifying intrapulmonary bacterial dynamics during pneumonia. Chief among these is the exponential behavior of bacterial growth and host-mediated bacterial killing and the exponential experimental error associated with these behaviors. Although first-order kinetic growth is known to be an imperfect approximation of bacterial growth and killing, transforming our raw culture results using such a model was a convenient means of handling bacterial burdens typically spanning several orders of magnitude within the experimental sample (13–15). Another technical challenge is the study of multiple bacterial species within a single infected animal. Differences in overall pathogenicity may necessitate considerable fine tuning of the inoculum size of each strain to allow reliable measurement of intrapulmonary growth. For example, in the current study, a 10,000-fold excess dose of S. marcescens was required to study interactions with a roughly LD50 dose of K. pneumoniae. Under what circumstances a less pathogenic strain such as Serratia would, in a colonized host, so outnumber a more virulent organism remains to be established.
A noteworthy finding in our work was the substantial interanimal variability in observed values for k in outbred mice. The experimental results from ICR mice and from the C57Bl/6J and B10.D2 strains used in the complement-related experiments reveal that significantly greater variability exists in the former mouse strain. Although not surprising and potentially problematic from the standpoint of sample sizes needed in future studies, there may be useful information contained in the observed variability. In particular, an examination of the frequency distributions for k1 and k2 in Figure 2 suggests bimodal behavior and raises the possibility of two phenotypically distinct subpopulations within the ICR strain. This deserves confirmation and, if true, further investigation.
In conclusion, in the current study we demonstrate in vivo changes in intrapulmonary bacterial growth rate over time using a model of repeated microaspiration. With this model, which allows for direct quantification of functional antibacterial host responses in the early hours of pneumonia, we show that after two sequential aspiration events, the bacteria entering the lung first are responsible for the majority of bacterial burden 1 d later. The slowing of apparent proliferation rates of subsequent aspirates was, in the current model, dependent on complement C3 but not detectably dependent on C5. C3's contribution to limiting the growth of sequential bacterial inocula did not seem to be solely related to its ability to promote neutrophil accumulation or to facilitate osponophagocytosis.
Acknowledgments
The authors thank Dr. Robert Bender for his advice in developing, and Dr. Robert Lyons for his assistance in sequencing, the Gal− strain of K. pneumoniae used in this work.
This project was supported by grants from the National Institute of General Medical Sciences (GM69438, J.G.Y.), the American Lung Association of Michigan (J.G.Y.), and the Alfred P. Sloan Foundation (T.L.J.).
References
- 1.Johanson W, Pierce A, Sanford J. Changing pharyngeal bacterial flora of hospitalized patients: emergence of Gram-negative bacilli. N Engl J Med 1969;281:1137–1140. [DOI] [PubMed] [Google Scholar]
- 2.Gross G, Rehm S, Pierce A. The effect of complement depletion on lung clearance of bacteria. J Clin Invest 1978;57:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watford W, Ghio A, Wright J. Complement-mediated host defense in the lung. Am J Physiol Lung Cell Mol Physiol 2000;279:L790–L798. [DOI] [PubMed] [Google Scholar]
- 4.Wessels M, Butko P, Ma M, Warren H, Lage A, Carroll M. Studies of group B streptococcal infection in mice deficient in complement component C3 or C4 demonstrate an essential role for complement in both innate and acquired immunity. Proc Natl Acad Sci USA 1995;92:11490–11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Younger J, Shankar-Sinha S, Mickiewicz M, Brinkman A, Younkin E, Sarma J, Standiford T, Zetoune F, Ward P. Murine complement interactions with Pseudomonas aeruginosa and their effects during acute pneumonia. Am J Respir Cell Mol Biol 2003;29:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alper M, Ames B. Positive selection of mutants with deletions of the gal-chl region of the Salmonella chromosome as a screening procedure for mutagens that cause deletions. J Bacteriol 1975;121:259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debouck C, Riccio A, Schumperli D, McKenney K, Jeffers J, Hughes C, Rosenberg M, Heusterspeute M, Brunel F, Davison J. Structure of the galactokinase gene of Escherichia coli, the last (?) gene of the gal operon. Nucleic Acids Res 1985;13:1841–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Available from: http://www.genomeold.wustl.edu/projects/bacterial/kpneumoniae/index.php (accessed Sept. 28, 2005).
- 9.The NLIN procedure. In: SAS/STAT user's guide. Cary, NC: SAS Institute, Inc.; 1994.
- 10.Kurz C, Chauvet S, Andres E, Aurouze M, Vallet I, Michel G, Uh M, Celli L, Filloux A, De Bentzmann S, et al. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J 2003;22:1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pugsley A, Oudega B. Methods for studying colicins and their plasmids. In: Hardy K, editor. Plasmids: a practical approach. Washington, D.C.: IRL Press; 1987. p. 105.
- 12.Holden H, Rayment I, Thoden J. Structure and function of enzymes of the LeLoir pathway for galactose metabolism. J Biol Chem 2003;45:43885–43888. [DOI] [PubMed] [Google Scholar]
- 13.Baranyi J, Pin C. A parallel study on bacterial growth and inactivation. J Theor Biol 2001;210:327–336. [DOI] [PubMed] [Google Scholar]
- 14.Casolari M. 1988. Microbial death. In: Bazin M, Prosser J, editors. Physiological models in microbiology, CRC Series in Mathematical Models in Microbiology. Boca Raton, FL: CRC Press, Inc.; 1988. pp. 1–44.
- 15.Swinnen I, Bernaerts K, Dens E, Geeraerd A, Van Impe F. Predictive modeling of the microbial lag phase. Int J Food Microbiol 2004;94:137–159. [DOI] [PubMed] [Google Scholar]