Abstract
Interplay between electronic effects imparted by phosphinothiol substituents and steric effects imposed by amino-acid reactants affect the rate of the traceless Staudinger ligation of peptides in a predictable manner.
Chemical synthesis enables the incorporation of nonnatural functionality into proteins, harboring the potential to elucidate function and enhance performance.1 The most common peptide ligation strategy is “native chemical ligation”, but this method is limited to ligations at junctions containing a cysteine residue.2 Efforts in our laboratory have been aimed at developing another strategy for peptide ligation called the “traceless Staudinger ligation”, which alleviates this limitation.
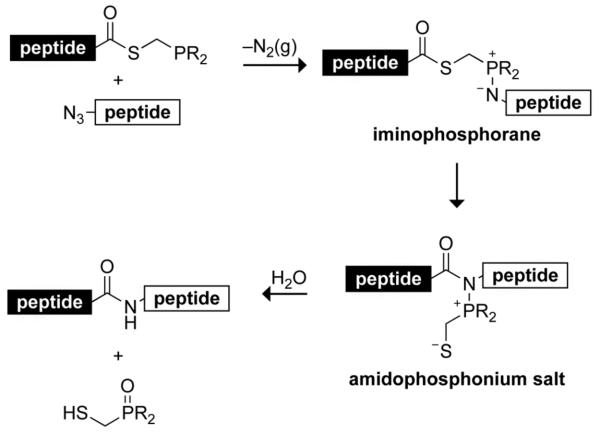
The Staudinger ligation joins a peptide having a C-terminal phosphinothioester with a peptide having an N-terminal azide through an intermediate iminophosphorane (Scheme 1). Notable attributes of this ligation strategy include its ability to generate an amide product without either residual atoms,3 or racemization,4 and a high reaction rate.5 (Diphenylphosphino)methanethiol (1) is the most often used of known reagents for effecting the traceless Staudinger ligation, and has mediated the orthogonal assembly of a protein,6 site-specific immobilization of peptides and proteins,7-9 and synthesis of glycopeptides.10, 11
Scheme 1.
Putative mechanism of the traceless Staudinger ligation.
Recently, we reported a new reagent, di(4-methoxyphenyl)phosphinomethanethiol (2), that is effective at mediating ligations between encumbered residues.12 Results indicated that electron-donating substituents on the aryl rings disfavor the formation of a side product, thereby leading to an increased yield (>80%) of the desired amide. Here, we report on electronic and steric effects on the rate of different steps in the traceless Staudinger ligation.
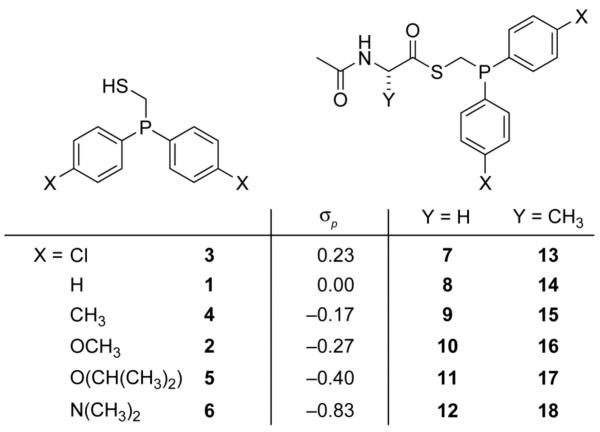
We surveyed the ligation rates mediated by a range of phosphinothiol reagents (1–6) with varying degrees of electron-donating character imparted by para substituents on their aryl rings (Fig. 1). The phosphinothiols were synthesized via a route analogous to that reported for similar phosphinothiols.13 Their respective thioesters (7–18) were prepared by coupling the phosphinothiols to AcGlyOH or AcAlaOH to provide model reactants of more or less sterically encumbered ligations, respectively.
Fig. 1.
Phosphinothiols, phosphinothioesters, and the σp values of their aryl substituents.14
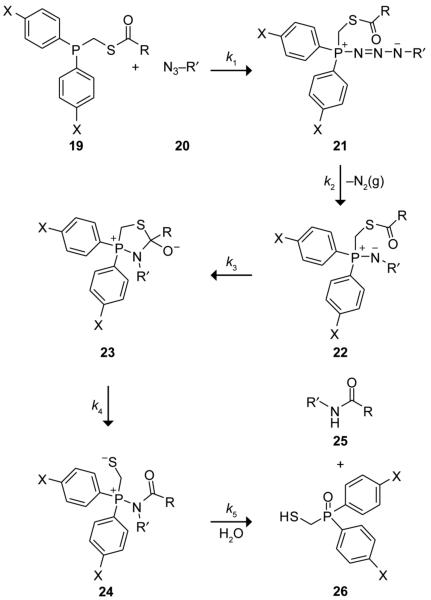
A detailed mechanism of the traceless Staudinger ligation is depicted in Scheme 2. In this mechanism, phosphinothioester 19 and azide 20 react to form phosphazide 21 (Step 1), which gives iminophosphorane 22 with concomitant release of N2(g) (Step 2). Formation of the tetrahedral intermediate 23 follows (Step 3), which collapses to amidophosphonium salt 24 (Step 4). Hydrolysis of 24 yields the desired amide 25 and phosphine oxide 26 (Step 5).
scheme 2.
Putative mechanism of the traceless Staudinger ligation.
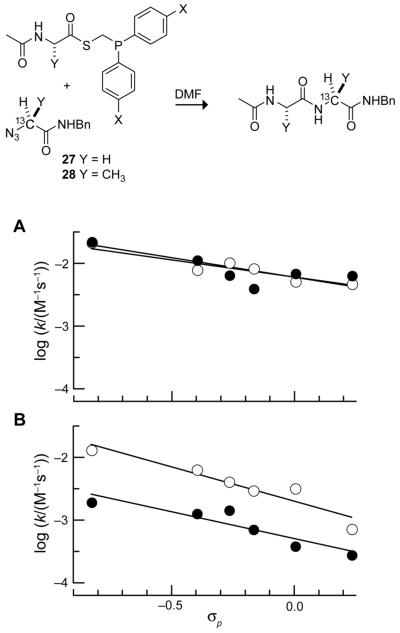
A 13C NMR-based assay developed previously in our laboratory was used to monitor the fate of azides enriched with 13C on their α-carbon during the course of a traceless Staudinger ligation.5 This assay enables the determination of the rate constants for both the disappearance of the azide reactant and the appearance of the amide product. Phosphinothioesters with various aryl substituents (7–12) were reacted with 13C-labeled glycyl azide 27, and it was found that both of these rate constants correlate well with the Hammett σp values of the substituents (Fig. 2, ○), which is indicative of an electronic effect.15 In general, substituents with greater electron-donating ability provide higher rates of reaction.
Fig. 2.
Hammett plots for the reaction of glycyl phosphinothioesters 7–12 with 13C-labeled glycyl azide 27 (○) and for the reaction of alanyl phosphinothioesters 13–18 with 13C-labeled alanyl azide 28 (●) in DMF at room temperature. Reactant concentrations: 0.146 M. Lines depict a linear least-squares fit. (A) Second-order rate constants for azide consumption (○: ρ = −0.62; ●: ρ = −0.55). (B) Second-order rate constants for amide formation (○: ρ = −1.09; ●: ρ = −0.86).
The trend was also evident in the more sterically encumbered reaction between alanyl phosphinothioesters 13–18 and 13C-labeled alanyl azide 28 (Fig. 2, ●). Interestingly, the rate constants for azide consumption were similar for the Gly+Gly and Ala+Ala ligations (Fig. 2A). This similarity indicates that the intermolecular reaction rate of the phosphinothioester and azide is influenced by electronic but not steric effects. On the other hand, the rate constants for amide formation were markedly lower for the ligation of the bulkier alanyl reactants (Fig. 2B). Apparently, the rate of an intramolecular step is diminished by steric effects. The values of ρ for amide formation are, however, highly negative, indicating that this rate can benefit substantially from electron-donating substituents.
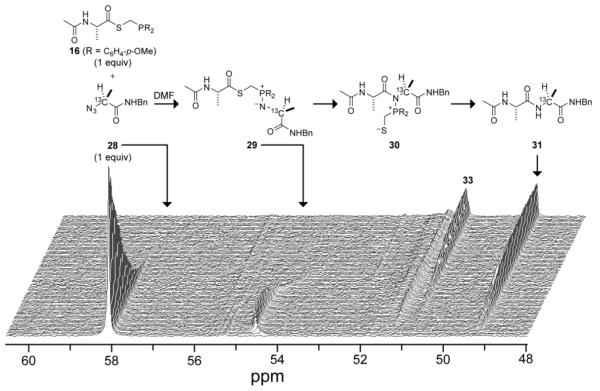
Previous reports have assigned the rate-determining step in the Staudinger ligation to be the association of starting phosphinothioester and azide to form phosphazide intermediate 21.16, 5 This assignment appears to be true for Gly+Gly ligations, as no intermediates accumulate during the course of that reaction. In an Ala+Ala ligation, however, an intermediate does accumulate (Fig. 3), indicating that the rate of amide formation is indeed influenced by the rate of an intramolecular step.
Fig. 3.
13C NMR-based assay of an Ala+Ala traceless Staudinger ligation in DMF at room temperature. Reactant concentrations: 0.146 M. Ninety spectra were acquired over a 12-h time course.
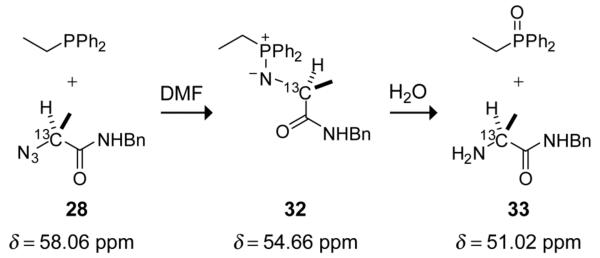
To correlate the observed chemical shifts with discrete intermediates along the reaction pathway for an Ala+Ala ligation, mimics of the intermediate were synthesized and characterized by 13C NMR spectroscopy.5 A mimic of iminophosphorane 29 in an Ala+Ala ligation was synthesized by the reaction of diphenylethylphosphine and 13C-labeled alanyl azide 28, both in the absence of water (to generate iminophosphorane 32) and in the presence of water (to generate amine 33; Scheme 3). The chemical shifts of iminophosphorane 32 and amine 33 were obtained by 13C NMR spectroscopy.
Scheme 3.
13C Chemical shifts of reaction-intermediate mimics.
Iminophosphorane 32 was found to have a chemical shift of 54.66 ppm, which supports the assignment of the signal at 54.64 ppm in Fig. 3 to iminophosphorane 29. Thus, the intermediate that accumulates in ligations between bulkier reactants is the iminophosphorane, and Step 3 partially limits the rate of amide formation. Presumably, the steric effects hinder S→N acyl transfer. Analogous steric effects have been observed in non-traceless Staudinger ligations.16 In the traceless Staudinger ligation, these steric effects can be mitigated by electron-donating substituents (Fig. 2B).
Electron-donating substituents are also known to benefit Step 4 of the reaction pathway (Scheme 2). Previously, we demonstrated that increasing electron density of the phosphorus increases yields at bulkier ligation sites by discouraging tetrahedral intermediate 23 from forming a P–O bond and hence an oxazaphosphetane.12 Thus, electron-rich phosphinothiols aid in both Step 3 and Step 4 of the traceless Staudinger ligation.
Electron-donating substitutuents do, however, have a detrimental effect. Hydrolysis of 32 gave a signal at 51.02 ppm for amine 33, which is similar to the 51.27 ppm signal in Fig. 3. Thus, an amine is the major byproduct of more sterically encumbered ligations. Furthermore, amine was formed to a greater extent when azide 28 was reacted with the more electron-donating phosphinothioesters (Table 1). We suspect that increased electron density renders the iminophosphorane nitrogen more susceptible to protonation by trace amounts of water. That protonation would impair the desired S→N acyl transfer, as well as make the adjacent phosphorus more electrophilic and thereby expedite hydrolysis.
Table 1.
Yields of amide and amine produced during Ala+Ala traceless Staudinger ligations of 0.146 M reactants in DMF at room temperature for 12 h.
| phosphinothioester | σp | azide | Amide Yield |
Amine Yield |
|---|---|---|---|---|
| 16 | −0.27 | 28 | 51% | 49% |
| 17 | −0.40 | 28 | 45% | 55% |
| 18 | −0.83 | 28 | 36% | 59% |
In conclusion, electronic and steric effects play multiple roles in determining the rate and yield of traceless Staudinger ligations. Most notably, electron-donating phosphinothiol substituents have dichotomous consequences. They are advantageous in accelerating the intermolecular reaction, enhancing S→N acyl transfer in the iminophosphorane, and discouraging undesirable P–O bond formation in the ensuing tetrahedral intermediate. Electron-donating substituents are deleterious in promoting the protonation of the iminophosphorane nitrogen, which impairs S→N acyl transfer in the iminophosphorane and leads to amine formation. Overall, electron-donating substituents increase the ligation rate of sterically encumbered reactants (Fig. 2), but decrease the yield of amide product (Table 1). The optimal choice of phosphinothiol for a particular ligation (especially between non-glycyl reactants) requires consideration of these electronic and steric effects.17
Supplementary Material
Acknowledgments
We thank Dr. E. L. Myers for contributive discussions. This work was supported by grant GM044783 (NIH). M.B.S. was supported by an ACS Division of Organic Chemistry Fellowship, sponsored by Abbott Laboratories.
Footnotes
Electronic Supplementary Information (ESI) available: Synthesis and characterization of all new compounds. See DOI: 10.1039/b000000x/
Notes and references
- 1.Nilsson BL, Soellner MB, Raines RT. Annu. Rev. Biophys. Biomol. Struct. 2005;34:91–118. doi: 10.1146/annurev.biophys.34.040204.144700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawson PE, Kent SBH. Annu. Rev. Biochem. 2000;69:923–960. doi: 10.1146/annurev.biochem.69.1.923. [DOI] [PubMed] [Google Scholar]
- 3.Nilsson BL, Kiessling LL, Raines RT. Org. Lett. 2001;3:9–12. doi: 10.1021/ol006739v. [DOI] [PubMed] [Google Scholar]
- 4.Soellner MB, Nilsson BL, Raines RT. J. Org. Chem. 2002;67:4993–4996. doi: 10.1021/jo025631l. [DOI] [PubMed] [Google Scholar]
- 5.Soellner MB, Nilsson BL, Raines RT. J. Am. Chem. Soc. 2006;128:8820–8828. doi: 10.1021/ja060484k. [DOI] [PubMed] [Google Scholar]
- 6.Nilsson BL, Hondal RJ, Soellner MB, Raines RT. J. Am. Chem. Soc. 2003;125:5268–5269. doi: 10.1021/ja029752e. [DOI] [PubMed] [Google Scholar]
- 7.Soellner MB, Dickson KA, Nilsson BL, Raines RT. J. Am. Chem. Soc. 2003;125:11790–11791. doi: 10.1021/ja036712h. [DOI] [PubMed] [Google Scholar]
- 8.Watzke A, Kohn M, Gutierrez-Rodriguez M, Wacker R, Schroder H, Breinbauer R, Kuhlmann J, Alexandrov K, Niemeyer CM, Goody RS, Waldmann H. Angew. Chem. Int. Ed. 2006;45:1408–1412. doi: 10.1002/anie.200502057. [DOI] [PubMed] [Google Scholar]
- 9.Gauchet C, Labadie GR, Poulter CD. J. Am. Chem.Soc. 2006;128:9274–9275. doi: 10.1021/ja061131o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y, Hinklin RJ, Chang JY, Kiessling LL. Org. Lett. 2004;6:4479–4482. doi: 10.1021/ol048271s. [DOI] [PubMed] [Google Scholar]
- 11.Liu L, Hong ZY, Wong CH. ChemBioChem. 2006;7:429–432. doi: 10.1002/cbic.200500437. [DOI] [PubMed] [Google Scholar]
- 12.Soellner MB, Tam A, Raines RT. J. Org. Chem. 2006;71:9824–9830. doi: 10.1021/jo0620056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tam A, Soellner MB, Raines RT. J. Am. Chem. Soc. 2007;129:11421–11430. doi: 10.1021/ja073204p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansch C, Leo A, Taft RW. Chem. Rev. 1991;91:165–195. [Google Scholar]
- 15.Hammett LP. Chem. Rev. 1935;17:125–136. [Google Scholar]
- 16.Lin FL, Hoyt HM, Van Halbeek H, Bergman RG, Bertozzi CR. J. Am. Chem. Soc. 2005;127:2686–2695. doi: 10.1021/ja044461m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.