Abstract
Eosinophils, leukocytes involved in allergic, inflammatory and immunoregulatory responses, have a distinct capacity to rapidly secrete preformed granule-stored proteins through piecemeal degranulation (PMD), a secretion process based on vesicular transport of proteins from within granules for extracellular release. Eosinophil-specific granules contain cytokines and cationic proteins, such as major basic protein (MBP). We evaluated structural mechanisms responsible for mobilizing proteins from within eosinophil granules. Human eosinophils stimulated for 30–60 min with eotaxin, regulated on activation, normal, T-cell expressed and secreted (RANTES) or platelet activating factor exhibited ultrastructural features of PMD (e.g. losses of granule contents) and extensive vesiculotubular networks within emptying granules. Brefeldin A inhibited granule emptying and collapsed intragranular vesiculotubular networks. By immunonanogold ultrastructural labelings, CD63, a tetraspanin membrane protein, was localized within granules and on vesicles outside of granules, and mobilization of MBP into vesicles within and extending from granules was demonstrated. Electron tomography with three dimension reconstructions revealed granule internal membranes to constitute an elaborate tubular network able to sequester and relocate granule products upon stimulation. We provide new insights into PMD and identify eosinophil specific granules as organelles whose internal tubulovesicular networks are important for the capacity of eosinophils to secrete, by vesicular transport, their content of preformed and granule-stored cytokines and cationic proteins.
Keywords: CD63, electron tomography, eosinophil secretion, major basic protein, chemokines, PAF, piecemeal degranulation, secretory pathway, vesicular transport
Eosinophils, cells of the innate immune system, have functions in health and in the pathogeneses of asthma, allergies and other diseases (1–4). Eosinophils are terminally differentiated, end-stage leukocytes that when mature contain a dominant population of cytoplasmic granules, termed specific or secretory granules. These granules are also referred to as crystalline granules, because they exhibit an ultrastructurally unique morphology that includes a crystalline core. The core contains major basic protein (MBP), one of the eosinophils' four distinctive granule-derived cationic proteins (5). Classical roles of eosinophils are based on their acute, effector responses involving secretory processes to secrete these distinct cationic proteins. Other more recently indicated functions of eosinophils are based on their capacity to release cytokines (1,4). Since the first report of human eosinophils as a cytokine source by our group (6), these cells are now known to synthesize numerous cytokines with multiple biologic activities including transforming growth factor-α (TGF-α) (6), granulocyte macrophage colony-stimulating factor (GM-CSF) (7), tumor necrosis factor-α (TNF-α) (8), interleukin (IL)-3 (9), IL-5 (10), IL-4 (11), IL-16 (12), regulated on activation, normal, T-cell expressed and secreted (RANTES) (13), vascular endothelial growth factor (VEGF) (14), stem cell factor (SCF) (15) and IL-13 (16). Both cytokines and distinct eosinophil cationic proteins (ECPs) are stored in preformed pools within eosinophil specific granules and can be released upon stimulation (3,5,17). Several stimuli, including cross-linking of different subclasses of immunoglobulin receptors, interferon-γ and the chemokines, eotaxin and RANTES, are known to induce selective protein release from eosinophils (18,19).
The release of granule-derived proteins from human eosinophils occurs mainly by progressive emptying of specific granule contents in the absence of granule-to-granule or granule-to-plasma membrane fusions. This process of cell secretion, defined ultrastructurally and termed piecemeal degranulation (PMD), was described nearly 30 years ago (20). Since the early description in basophils, PMD has been documented in diverse inflammatory cells and in a variety of experimental models and diseases (21,22). In eosinophils, PMD is the most frequently encountered secretory process in cells from subjects with a range of inflammatory and allergic disorders (23–26). Observations of PMD in chief cells of the parathyroid gland (27), enteroendocrine cells of the gastrointestinal tract and chromaffin cells of the adrenal medulla (28) indicate that PMD may also be a distinct and more common degranulation mechanism for endocrine secretion (28). Piecemeal degranulation is based on vesicular transport of small packets of materials from the cytoplasmic granules to the cell surface (8,29,30).
In spite of being a well-documented mode of mediator release in human eosinophils, the structural mechanisms underlying PMD remain to be delineated. To date, most studies have documented the consequences of PMD based on the ultrastructural identification of emptying secretory granules. However, the intragranular organization and events responsible for the selective mobilization and release of eosinophil proteins remain to be defined. The relevance of this issue is underlined by the fact that functional and structural responses within eosinophil granules are crucial for the regulated release of cytokines and other proteins during eosinophil responses to allergic and inflammatory diseases, ranging from transplant rejection to wound healing (23–26). Thus, it is clear that intragranular events must be elucidated to more fully understand PMD.
Here, we have studied PMD in human eosinophils stimulated with physiologic agonists. Using transmission electron microscopy (TEM) and tomography and immunonanogold localization of CD63, a tetraspanin membrane protein, and granule-derived MBP, we show that eosinophil secretory granules are elaborate and compartmentalized organelles with internal membranous vesiculotubular domains able to sequester and relocate granule products upon stimulation. These events are, for the first time, associated with PMD-mediated secretion of proteins in human eosinophils.
Results
Specific granules in physiologically stimulated human eosinophils undergo brefeldin A-inhibitable PMD
To characterize ultrastructural events within secretory granules that underlie agonist-elicited PMD, freshly isolated human eosinophils were stimulated with physiologic stimuli [two chemokines, eotaxin and RANTES and the lipid, platelet activating factor (PAF)] or medium alone for 1 h, immediately fixed while still in suspension in a mixture of glutaraldehyde and paraformaldehyde, embedded in agar and prepared for TEM.
Specific granules in stimulated eosinophils showed dramatic changes in ultrastructure compared with those in unstimulated cells. Remarkable alterations were seen both inside and at the surface of granules with each of the different stimuli used. In unstimulated eosinophils (Figure 1A), granules were seen as round or elliptical structures, full of electron-dense contents, with a well-defined core and an outer granule matrix surrounded by a delimiting trilaminar membrane. However, after 1 h of stimulation, granule contents exhibited clear losses classically associated with PMD (Figures 1B and 2). Both chemokines and PAF induced the same morphological changes within granules. To quantify the number of granules undergoing PMD, eosinophil sections showing the entire cell profile and nucleus were evaluated, and a total of 3945 granules were analyzed. Eosinophil activation induced significant increases in the numbers of emptying granules (Figure 1C). These emptying granules were always intermingled, in the same cell section, with resting, non-mobilized granules (Figure 2B, asterisk), revealing a diversity of granule morphologies within the same cell. Whereas only 8% of granules in unactivated eosinophils had granules undergoing PMD, 43% (eotaxin), 25% (RANTES) and 34% (PAF) showed emptying granules (Figure 1C). Moreover, the responses of eosinophils were not uniformly distributed. For example, in scoring the numbers of granules that exhibited loss of granule contents indicative of PMD, in unstimulated cells, 70% of eosinophils had <10% of granules with losses, whereas eotaxin elicited a marked heterogeneity of granule-emptying responses within eosinophils such that >15% of eosinophils had >90% of their granules exhibiting content losses (Figure 3A). Fusion of granules with each other or with plasma membranes was only rarely seen and did not increase significantly with activation [0.6 ± 0.2 fused granules/eosinophil in unactivated cells compared with 1.2 ± 0.9 in eotaxin, 0.8 ± 0.2 in RANTES and 1.0 ± 0.2 in PAF-activated eosinophils (mean ± SEM), n = 95 cells].
Figure 1. Morphologic effect of three physiologic stimuli on human eosinophil specific granules.
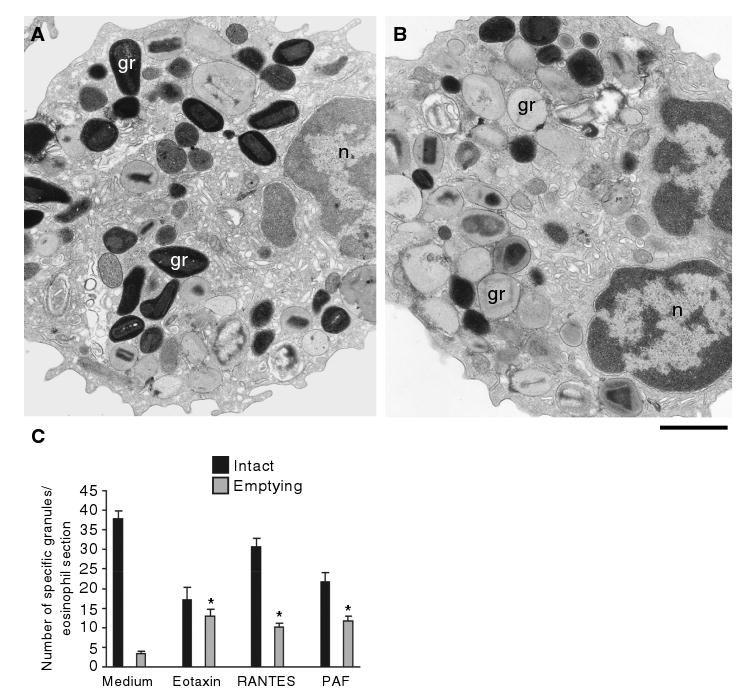
Cells were incubated with control buffer (A, C), 100 ng/mL eotaxin (B, C), 1 μM platelet-activating factor (PAF) (C) or 100 ng/mL regulated on activation, normal, T-cell expressed and secreted (RANTES) (C), immediately fixed and prepared for transmission electron microscopy. After 1 h of stimulation, granules exhibited progressive emptying of their contents and showed morphological diversity. Not all granules exhibited content losses. (C) Significant increases in numbers of emptying granules occurred after stimulation with the three stimuli (*p < 0.05). Eosinophils were isolated by negative selection from healthy donors. Counts were derived from three experiments with a total of 3945 granules counted in 95 electron micrographs randomly taken and showing the entire cell profile and nucleus. gr, granule; n, nucleus. Scale bar, 1.9 μm (A, B).
Figure 2. Morphology of emptying specific granules from stimulated human eosinophils.

Granules exhibited lucent areas in their cores, matrices or both (A–D); reduced electron density (A, *); disassembled matrices and cores (A–D); residual cores (A, arrowheads) or membrane empty chambers (A, **). Intact, non-emptying granules (typical morphology B, *) were seen close to emptying granules. In (B and D), the arrows point to sites of vesicle budding profiles. A round small vesicle and large, vesiculotubular structures termed Eosinophil Sombrero Vesicles (EoSVs) are indicated by arrowheads, respectively, in (C and D). In (C), round profiles (circles) are within, and tubules surround (thin arrows) an emptying granule. Over 450 electron micrographs, ranging from ×10 000 to ×75 000 and taken from three different experiments, were evaluated. Eosinophils were isolated and stimulated for 1 h with eotaxin (A), platelet activating factor (B, D) or regulated on activation, normal, T-cell expressed and secreted (RANTES) (C) as in Figure 1, fixed and processed for transmission electron microscopy. n, nucleus. Scale bar, 630 nm (A) and 300 nm (B–D).
Figure 3. Brefeldin A (BFA) effects on eosinophil specific granules.
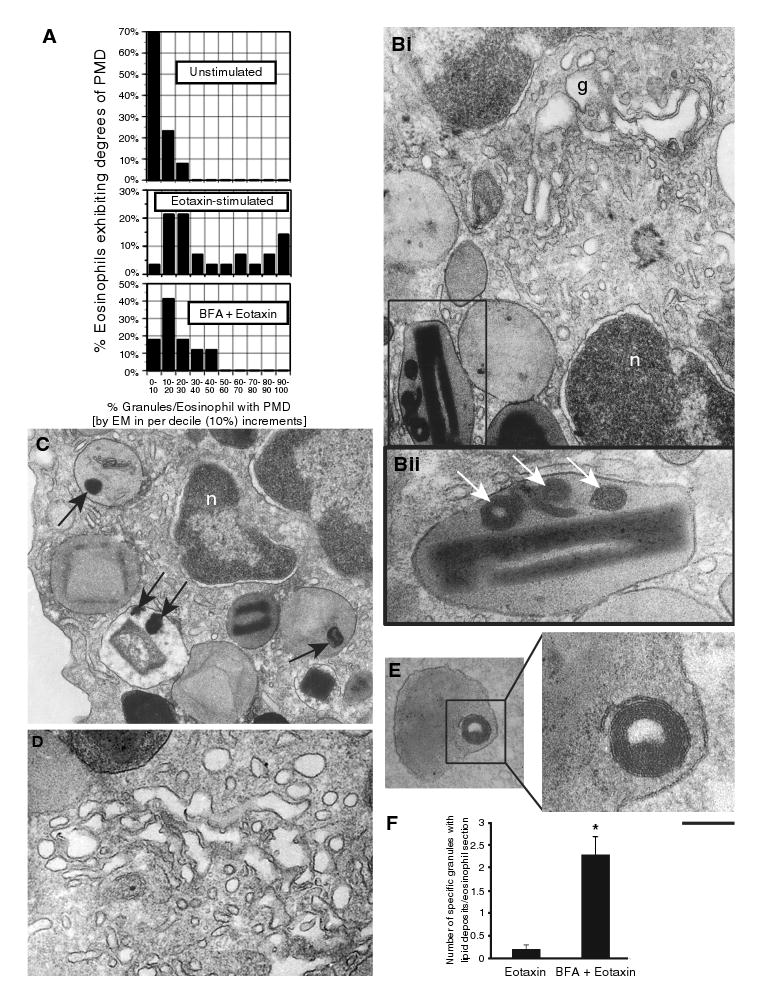
(A) Heterogeneity of granule responses in unstimulated, eotaxin-stimulated and eotaxin plus BFA-pretreated (1 μg/mL, 30 min prior to eotaxin) eosinophils. Brefeldin A elicited the formation of membrano-lipid deposits within secretory granules (Bii and C, arrows. Bii corresponds to the boxed area in Bi), imaged as collapsed membranous structures (E, boxed area) and, as expected, disruption of the Golgi complex (Bi, D). (F) BFA induced significant increases in granule numbers showing internal lipid deposits (*p < 0.05). Granules with lipid deposits were numerated in a total of 50 electron micrographs randomly taken and showing the entire cell profile and nucleus. g, Golgi complex; n, nucleus; PMD, piecemeal degranulation. Scale bar, 650 nm (Bi), 208 nm (Bii), 630 nm (C), 500 nm (D), 340 nm (E) and 140 nm (E, higher magnification).
In previous work, using a sensitive, dual antibody capture and detection immunofluorescent microscopic assay (the EliCell assay), we have demonstrated brefeldin A (BFA)-inhibitable IL-4 cytokine release from eotaxin-stimulated human eosinophils (19). Here, we have used BFA, a potential inhibitor of vesicular transport (31), to investigate its effect on specific granules. Pretreatment with BFA prior to eotaxin stimulation significantly inhibited eotaxin-induced granule emptying [13.2 ± 1.8/section in eotaxin vs. 6.2 ± 0.8/section in BFA + eotaxin (mean ± SEM), n = 50 cells] (Figure 3A). Moreover, BFA acted within granules to elicit the formation of distinct electron-dense lipid deposits (Figure 3Bi,Bii,C) composed of collapsed membranous structures (Figure 3E) in addition to affecting the Golgi complex (Figure 3Bi,D), its classical organellar target (31). Electron microscopy (EM) quantitative analyses showed that the numbers of granules exhibiting internal lipid deposits with BFA increased significantly compared with stimulation with eotaxin alone (Figure 3F).
Vesicles as well as tubules were frequently observed attached to or surrounding specific granules (Figure 2C,D). These vesicles were electron-lucent or showed light or very dense contents. These structures represent a vesiculotubular system comprised of both small spherical and large tubular vesicles [termed by us as Eosinophil Sombrero Vesicles (EoSVs)], involved in the trafficking of products from granules to the cell surface (unpublished data). Morphological evidence for this secretory pathway included vesicles profiles budding from emptying granule containers (Figure 2B,D, arrows). These findings show that the activation of eosinophils induces dramatic morphological changes in specific granules with significant losses of their contents associated with vesicular traffic.
Early stimulus-induced granule changes in human eosinophils
To investigate early mechanisms associated with stimulus-induced granule emptying, we stimulated eosinophils for 30 min with physiologic stimuli and analyzed them by TEM. After 30 min, granules developed into irregular structures with progressive protrusions from their surfaces (Figure 4A, arrows). In addition, these protrusions were preferentially present on intact granules that had an ill-defined core and matrix (Figure 4A). Clear ultrastructural alterations were observed at perigranular sites. Small spherical vesicles, EoSVs and smooth membrane-bound tubules were prominently associated with specific granules at sites where granule membranes exhibited alterations in electron density. These vesicles (Figure 4B, arrows) and tubules (Figure 4B, arrowheads) frequently formed a row closely associated with a large area of granule membranes. These morphological alterations, detected after 30 min of agonist stimulation, represent early aspects of eosinophil PMD, when most granules did not yet show signs indicative of content losses.
Figure 4. Early stimulus-induced granule events in human eosinophils.
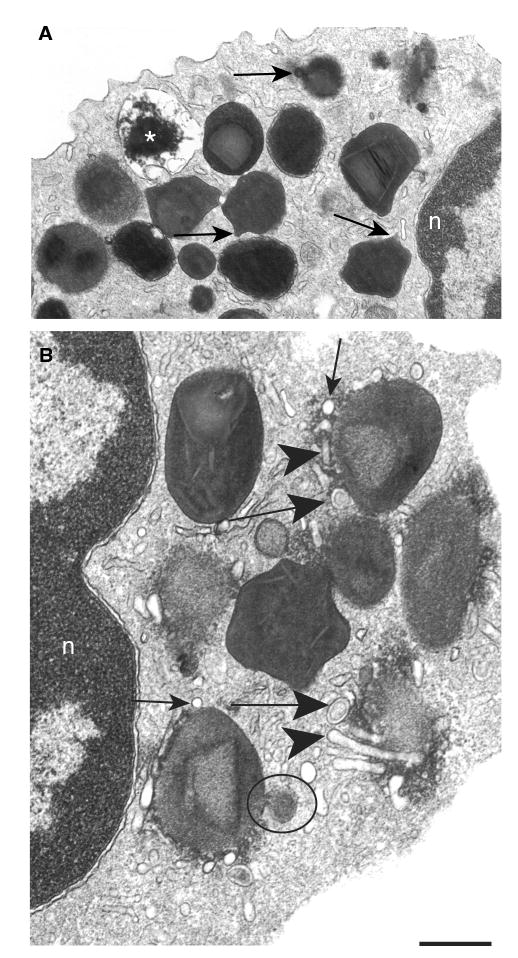
(A) After 30 min of stimulation, specific granules showed ill-defined cores and matrices and became irregular with elongation of their surfaces (arrows). An emptying granule is indicated (*). (B) A prominent system of vesicular compartments composed of small round vesicles (thin arrows), Eosinophil Sombrero Vesicles (EoSVs) (large arrows) and tubules (arrowheads) was seen at the granule surface. A large vesicle profile budding from the granule surface shows the same granule density (circle). Eosinophils were isolated and stimulated with eotaxin as in Figure 1. Transmission electron microscopic evaluations were derived from three experiments, and specimens were studied at magnifications ranging from ×5000 to ×75 000. n, nucleus. Scale bar, 720 nm (A) and 400 nm (B).
Ultrastructural activation-induced changes within eosinophil specific granules are associated with vesicular trafficking of MBP
Because a prominent vesicular system composed of round vesicles, tubules and vesiculotubular structures was seen surrounding (Figures 2C and 4B) and apparently budding from specific granules in stimulated cells (Figure 2B,D), we investigated vesicular secretion of MBP, one of the most abundant cationic proteins stored within and recognized as a marker of eosinophil specific granules (5). We performed MBP immunolabeling using pre-embedding immunonanogold EM for precise epitope preservation and subcellular localization. The use of a secondary antibody (Fab fragment) conjugated to 1.4 nm gold particles (Nanogold) enabled us to detect a prominent system of MBP-containing vesicles around mobilized granules in eotaxin-stimulated eosinophils (Figure 5A,C). Control cells in which the primary antibody was replaced by an irrelevant antibody were negative (Figure 5B). Notably, MBP was localized within disarranged cores (Figure 5D,Ei), matrices (Figure 5D,Ei,Eii) and within vesicles surrounding or attached to the surface of emptying granules (Figure 5C, Di, Dii, Eii). MBP-containing vesicles often lacked the density of granule cores and included both spherical small vesicles (Figure 5C,Di,Dii) and large tubular carriers (Figure 5Eii, arrowheads). This vesicular system was associated with a secretory pathway from eosinophil-specific granules and not with a biosynthetic route from the trans-Golgi network, which was rarely labeled for MBP (Figure 5Ei).
Figure 5. Vesicular transport of major basic protein (MBP) from within eotaxin-stimulated human eosinophils.

Immunonanonogold electron microscopy revealed MBP-positive vesicles surrounding (A, C, E) and apparently arising (C, D) from mobilized specific granules. Control cells were negative for MBP labeling (B). In (Di), MBP labeling is seen on the disarranged granule core and matrix and within a small, spherical vesicle attached to the granule surface (box). Intragranular vesicles were also labeled for MBP. (Dii) corresponds to the boxed area of (Di). In (Ei), an eosinophil section shows the Golgi (G) region negative for MBP and MBP labeling within mobilized granules. MBP-positive, granule-associated large tubular carriers are indicated in (Eii) (arrowheads). (Eii) corresponds to the boxed area of (Ei). Cells were processed for pre-embedding immunonanogold labeling with mouse anti-MBP monoclonal antibody followed by 1.4 nm gold-conjugated goat-anti-mouse Fab fragments. In control cells, the primary antibody was replaced by an irrelevant antibody. In all experiments, eosinophils were stimulated as described in Figure 1. n, nucleus; gr, granule. Scale bar, 600 nm (A, B), 270 nm (C), 250 nm (Di), 150 nm (Dii), 500 nm (Ei) and 220 nm (Eii).
Eosinophil-specific granules contain internal membranous vesiculotubular domains
Novel insights into the structural mechanisms underlying PMD were identified. Notably, as specific granule content was mobilized in activated eosinophils, as seen after 1 h of stimulation, an extensive network of tubules and vesicles was revealed within emptying granules, mainly in the matrix area (Figure 6A). The internal tubular network was imaged as an elaborate system of convoluted tubules or individual tubular profiles (Figure 6B) at times associated with small, round or sac-like vesicle profiles. The fixation and staining procedures employed in the present study have enabled us to distinguish membrane bilayers delimiting this intragranular system (Figure 6B,C). Intragranular vesicles also contained immunolabeled MBP (Figure 5Di).
Figure 6. Emptying granules exhibit internal membranous domains in agonist-stimulated eosinophils.
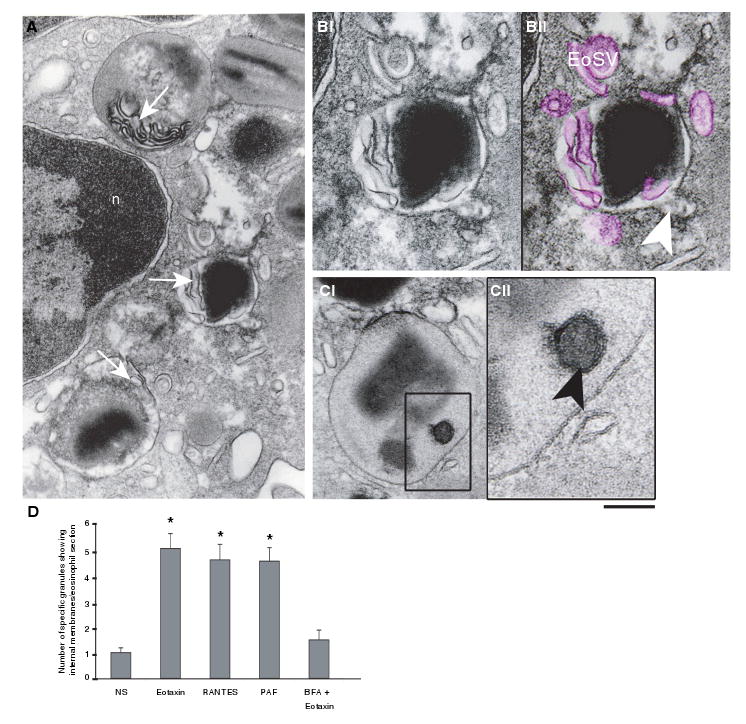
(A) Transmission electron microscopy (TEM) revealed intragranular membranous subcompartments organized as a tubular system in the matrix area (arrows). (B) Higher magnification of an emptying granule with internal membranous tubules (highlighted in pink in Bii) seen in conjunction with mobilized core. Eosinophil Sombrero Vesicles (EoSVs) around the granule are also colored pink. A budding vesicle is indicated (arrowhead). (Ci) A mobilized granule shows part of its content inside a subcompartment (box). (Cii) Higher magnification of the boxed area shows that a true internal membrane (arrowhead), with the same trilaminar appearance as exhibited by the outer granule membrane, encircles part of the granule content. (D) The numbers of granules showing internal membrane domains significantly increased after stimulation (*p < 0.05), but in brefeldin A (BFA)-pretreated eosinophils, these numbers did not differ from unstimulated cells. Cells were stimulated with eotaxin (A, B), regulated on activation, normal, T-cell expressed and secreted (RANTES) (C, D), platelet-activating factor (PAF) (D) or BFA prior to eotaxin (D), processed for TEM and analyzed as in Figure 1. n, nucleus. Scale bar, 480 nm (A), 300 nm (B), 350 nm (Ci) and 138 nm (Cii).
Quantitative EM analyses demonstrated that, in parallel with a significant increase of emptying granule numbers (Figure 1C), there were significant increases in numbers of granules showing internal membrane domains in response to the three stimuli (Figure 6D). Moreover, membranous subcompartments, frequently seen delimiting electron-dense granule products (Figure 6C), were prominent in granules with mobilized contents (Figure 6B,C) and/or were in contact with the inner surface of the granule membrane (Figure 6A). In BFA-pretreated and eotaxin-stimulated eosinophils, numbers of granules exhibiting internal membranes were suppressed (Figure 6D). Instead, it is this intragranular membranous system that was collapsed after BFA treatment, being imaged as electron-dense lipid deposits in the granule matrix area (Figure 3B,C,E).
To provide additional evidence that membrane domains are present within eosinophil granules, we next performed labeling for CD63, a transmembrane tetraspanin protein previously implicated in eosinophil granule secretion (32). By immunofluorescence microscopy, both stimulated (Figure 7A) and unstimulated (Figure 7B) eosinophils, embedded in an agarose matrix, exhibited CD63-positive granules. Images from deconvolution microscopy showed a clear labeling for CD63 at the granule periphery (Figure 7C). Control cells assayed with an irrelevant antibody were negative.
Figure 7. CD63 is localized to membranes of eosinophil specific granules.

Phase-contrast and fluorescence microscopy of identical fields of a representative eotaxin-stimulated (A) and unstimulated (B) eosinophil. Both stimulated and unstimulated eosinophils exhibited fluorescent immunoreactive staining for CD63 on specific granules. In (C), deconvolution microscopy revealed peripheral CD63 labeling on specific granules from an unstimulated eosinophil. (D, E). Immunonanogold electron microscopy revealed CD63 labeling on the granule outer membranes (D) and within granule matrices (D, E, circles). (F) CD63-positive cytoplasmic vesicles showing membrane-bound labeling are indicated (arrows). (G) A control eosinophil showing negative labeling for CD63. Cells were stimulated and processed for immunofluorescence or pre-embedding immunogold labeling with anti-CD63 monoclonal antibody followed by, respectively, Alexa-488 (A), Alexa-594 (B) or 1.4 nm gold-conjugated (D–G) antibodies. In control cells, the primary antibody was replaced by an irrelevant antibody. Eosinophils were isolated and stimulated as Figure 1. n, nucleus. gr, granule. Scale bar, 6 μm (A, B), 5 μm (C), 350 nm (D), 400 nm (E, F) and 600 nm (G).
We next used pre-embedding immunonanogold for subcellular localization of CD63 by EM in eotaxin-stimulated eosinophils. CD63 was localized to the granule limiting membranes (Figure 7D) as well as to the granule matrices (Figure 7D,E). The intragranular pool of CD63 was mainly associated with emptying granules (Figure 7D,E). Cytoplasmic vesicles around specific granules were also positive for CD63 (Figure 7F). In all immunonanogold experiments, controls treated with an irrelevant (Figure 7G) or secondary antibody alone were negative.
We used subcellular fractionation to quantify the levels of CD63 in isolated granules (before and after permeabilization) from unstimulated eosinophils. We wondered whether the intragranular pool of CD63 could be detected and quantified in resting granules with their normal content of compacted proteins. Isolated specific granules, with and without permeabilization with saponin, were analyzed by flow cytometry. Permeabilized granules showed 28% greater anti-CD63 mean fluorescence intensity (MFI) compared with non-permeabilized granules (data not shown). Our findings complement a prior report of CD63 within isolated eosinophil granules after permeabilization (32) and localize CD63 for the first time to intragranular membranous domains.
Our data demonstrate a pool of CD63 within secretory granules in both unstimulated and stimulated eosinophils. The presence of an intragranular membranous vesiculotubular network indicates that eosinophil specific granules are complex and compartmentalized organelles which undergo dynamic changes in their structure and contents in response to stimuli.
Intragranular subcompartments are organized as a tubular network and can sequester and relocate granule products
To obtain greater insights into the structural mechanisms related to mobilization of intragranular products, eosinophils were studied by automated electron tomography (ET). For this purpose, we analyzed, by dual-axis ET, the same sample of eotaxin-activated eosinophils evaluated by conventional TEM after 1 h of stimulation. While conventional TEM studies are usually performed on approximately 80 nm-thick sections, the tomographic slices used in this work were only 4 nm thick and offer a significant advantage over typical serial ‘thin sections’ for tracking cell structures in three dimensions (3D). Tomographic reconstructions revealed that mobilized eosinophil granule contents are rearranged within intragranular vesiculotubular compartments. Figure 8(A–F) [see also Movie 1 available on-line at http://www.traffic.dk/videos/6_10.asp] shows a granule subcompartment that is partially contained within the 3D volume and from which electron-dense content was being translocated to the granule outer membrane. Intragranular membranous subcompartments were imaged in 3D models as an aggregate of flattened tubular networks and tubules, with interconnections in some planes (Figure 8H–K and Movie 2 available on-line at http://www.traffic.dk/videos/6_10.asp). Remarkably, structural connections were revealed between the intragranular membranous network and the granule limiting membrane [Figure 8J and Movie 2 available on-line at http://www.traffic.dk/videos/6_10.asp]. This may represent membrane sites involved in granule product translocation. Intragranular structures appearing as round profiles in single routine 80 nm sections of eosinophils (Figure 2C) were revealed by the 3D models to result from cross-sections of intragranular tubules rather than vesicles (Figure 8H–K and Movie 2). Altogether, these findings have the important functional implication that proteins may be specifically segregated within granules before being sent to the cell surface through vesicular compartments.
Figure 8. Tomographic slices and three dimension (3D) models from an emptying specific granule.

(A–F) The tomographic volume shows intragranular subcompartments in conjunction with mobilized content. Circles indicate the same subcompartment surrounding part of the electron-dense content that is relocated to the granule outer membrane. Note in (C and D) that the electron density of this membrane changes at the site of contact with the membranous intragranular subcompartment. The arrows point to a forming Eosinophil Sombrero Vesicle (EoSV). Seventy and five serial single virtual slices as in (F) were extracted from the tomogram, outer granule membrane was partially traced in red and intragranular vesiculotubular structures were outlined in blue as in (G) so as to generate 3D models. (H–K) 3D models of the same granule show intragranular membrane domains (blue) organized as a flattened tubular network and tubules. In (J and K), the model has been rotated to provide another view. An area of continuity between the intragranular membranous network and the limiting granule membrane is indicated in (J, arrows). The slices (∼4 nm of thickness) were extracted from 3D reconstructions of a 400 nm eosinophil section analyzed by automated electron tomography at 200 kV. The numbers on the upper left corner indicate the slice number through the tomographic volume. Cells were stimulated with eotaxin as in Figure 1, chemically fixed and processed for transmission electron microscopy. Scale bar, 500 nm (A–F), 450 nm (G), 400 nm (H), 180 nm (I) and 150 nm (J, K). Also see Movie 1 and Movie 2 in the movie gallery gr, granule.
Discussion
The specific (also called secretory or crystalline) granules of eosinophils are notable not only for their ultrastructural morphology but also because they contain several dozen preformed cytokine proteins as well as four cationic proteins (5). In this work, we have provided new insights into the structural mechanisms involved in the mobilization and release of proteins from within specific granules of activated eosinophils. As evidenced by EM, three different known agonists of eosinophil ‘degranulation’, two chemokines (eotaxin and RANTES) and PAF (1,33–36) elicited within 30–60 min identical alterations in the ultrastructure of eosinophil specific granules. Agonist-stimulated eosinophils showed great morphological diversity within their specific granules. Classical signs of PMD, i.e. losses of granule contents in the absence of granule fusions with the plasma membrane, were observed consistent with changes seen in eosinophils at sites of allergic diseases and also in other cells involved in inflammatory responses (37). With each of the three stimuli, quantitative analyses revealed significant increases in numbers of emptying granules after 1 h of stimulation (Figure 1C). Our findings also highlight the fact that not all eosinophil specific granules are uniformly, co-ordinately and simultaneously responsive to stimuli (Figure 1C). Therefore, in contrast to classical regulated secretion characterized by extrusion of entire granules, PMD sustains a pool of intact secretory granules that may contribute to the special capability of eosinophils to rapidly release their products under different or repetitive stimuli. Indeed, eosinophils display a wide range of functions (3), and PMD is a key event in most of them. These in vitro ultrastructural features of PMD, thus, are fully compatible with PMD changes recorded in granules of eosinophils in vivo in concert with many allergic diseases (23–26).
In addition, we have documented earlier stages of PMD. Multiple protrusions from granule surfaces (Figure 4), prior to frank emptying of granules, were indicative of early events in mobilization of granule-stored proteins. These alterations represent the initial formation of vesicular compartments involved in the transport of granule products to the cell surface, as evidenced by the development of a prominent extragranular vesicular system associated with mobilized granules in response to different stimuli (Figures 2B–D and 4B). This is consistent with perigranular vesicles documented in eosinophils and other cells undergoing PMD [reviewed in (28,37)]. In confirmation that vesicles surrounding granules contained granule-derived cargo, we performed immunonanogold ultrastructural localizations for MBP, a dominant preformed cationic protein constituent of eosinophil specific granules (5). In eotaxin-stimulated eosinophils, MBP was mobilized into the matrix of granules and into vesicles apparently arising from (Figure 5C,Di,Dii) and surrounding (Figure 5A,C,Eii) granules. Of note, another granule-derived protein, ECP, has been documented in subcellular fractionation studies to be localized in cytosolic vesicles isolated from the eosinophils of allergic patients specifically during their seasonal allergen exposures (24). Our findings provide the first evidence that granule-derived MBP is mobilized into vesicles for its extracellular release.
While the above findings provided evidence for mobilization of eosinophil granule proteins into extragranular vesicles, mechanisms active within stimulated granules were also revealed with the demonstration of membrane domains within specific granules undergoing PMD. The presence of eosinophil intragranular membranes has been noted only occasionally in prior years in eosinophils from allergic subjects (38), in Crohn's disease (23) and in PAF-stimulated human eosinophils (39). We now demonstrate, as visualized in granules exhibiting agonist-elicited granule emptying, that eosinophil specific granules contain a very extensive membranous vesiculotubular network. The presence within granules of membranes was confirmed by immunonanogold labeling for CD63, a tetraspanin membrane protein. The ultrastructural visualization of preformed membranes within secretory granules in resting eosinophils is probably masked by a large amount of protein compacted within granules. In support of this interpretation are our findings with specific granules isolated from unstimulated eosinophils by subcellular fractionation. Using flow cytometry, these granules were immunostained for surface CD63, as showed by deconvo-lution microscopy on resting eosinophils and their granules (Figure 7C). Of note, after saponin permeabilization of outer granule membranes, flow cytometry demonstrated additional intragranular CD63 staining in isolated granules, indicating that even unstimulated, resting granules contained intragranular membranes. Internal CD63-positive membranes have been recognized in some other lyso-some-related organelles such as platelet alpha granules (40) and MHC class II compartments in dendritic cells (41), and our results provide compelling evidence for the presence of CD63-bearing intragranular membranous structures within eosinophil granules.
The origin of the CD63-bearing membranes within eosinophil granules has not been ascertained. It is likely over time these extensive intragranular membranous compartments are refreshed from endocytic recycling, from granule membranes and/or from biosynthetic pathways, but this remains to be delineated. In the short (30–60 min) time frame of our observed agonist-elicited mobilization of granule contents for secretion, it is highly likely that the pre-existing vesiculotubular network within granules was critically involved. Studies with BFA were informative. Brefeldin A inhibited granule emptying (Figure 3A). This was not likely attributable principally to inhibition of trafficking from Golgi-derived or other exogenous vesicles, because BFA acted directly within eosinophil granules to collapse the intragranular membranous vesiculotubular network into ultrastructurally recognizable lipid-rich deposits (Figure 3B,C,E). A potential target of BFA is CtBP/BARS, an enzyme that regulates vesicular membrane curvature and induces fission of tubular networks (42). CtBP/BARS protein is present within eosinophil granules (our preliminary data based on immunoblotting of isolated granules with anti-BARS antibody, courtesy of Dr Daniela Corda). Thus, it is likely that sites of BFA action in inhibiting PMD release of eosinophil granule-derived proteins are the vesiculotubular networks within the granules themselves.
Electron tomographic analyses were especially revealing in demonstrating, for the first time, that eosinophil intragranular membranes constitute an extensive and complex tubular network (Figure 8 and movie 1). Roles for intragranular vesiculotubular networks in the active mobilization of granule proteins were indicated by ultrastructural demonstration of granule contents within membrane-delimited structures (Figure 6) as well as tomographic images showing mobilization of granule contents to the granule surface (Figure 8A–F). These findings suggest that proteins can be sorted within granule subcompartments before reaching the outer granule membrane in order to be delivered to the cell surface. Thus, ET added new dimensions to our EM studies, providing additional strong evidence for sequestration and translocation of products within eosinophil granules. A functional implication of this finding is that it adds support to the occurrence of selective release of products from eosinophils, as indicated previously by us (19) and other groups (18). Structural connections between the intragranular membranous network and granule limiting membrane, as visualized by ET (Figure 8J), may further contribute to the mobilization of granule proteins for their release into vesicles derived from granule surfaces. In fact, a prominent system of both small spherical and large tubular vesicles (EoSVs) can be formed from eosinophil granules (unpublished data). Eosinophil Sombrero Vesicles are likely released by a tubulation process which requires substantial amounts of membranes and seems to involve the intragranular networks (unpublished data).
Thus, our studies have helped delineate mechanisms that underlie the responses of eosinophil granules as they undergo PMD. Within specific granules are extensive tubulovesicular networks that mediate the mobilization of preformed granule proteins for their release into granule-derived vesicles. Eosinophil specific granules are elaborate organelles whose internal tubulovesicular networks are important for the capacity of eosinophils to secrete, by vesicular transport, their content of preformed and granule-stored cytokines and cationic proteins.
Materials and Methods
Eosinophil isolation, stimulation and viability
Granulocytes were isolated from the blood of healthy donors as described (43), and eosinophils were purified by negative selection using human eosinophil-enrichment cocktail (StemSep™, StemCell Technologies, Vancouver, Canada) and the magnetic antibody cell-sorting (MACS)-bead procedure (Miltenyi Biotec, Auburn, CA, USA). Experiments were approved by the Committee on Clinical Investigation, and informed consent was obtained from all subjects. Eosinophil purity was >99%. Purified eosinophils (106 cells/mL) were stimulated with recombinant human eotaxin (100 ng/mL; R & D Systems, Minneapolis, MN, USA), RANTES (100 ng/mL; R & D Systems) or PAF (1-O-Hexadecyl-2-acetyl-sn-glycero-3-phosphocholine) (1 μm )(Calbiochem, San Diego, CA, USA) in RPMI-1640 medium plus 0.1% ovalbumin (Sigma, St. Louis, MO, USA) or medium alone at 37 °C, for 30 min or 1 h. In some experiments, eosinophils were pretreated (30 min prior to eotaxin) with BFA (1 μg/mL, Biomol, Plymouth Meeting, PA, USA). Cell viability after stimulation was greater than 95% as determined by ethidium bromide incorporation.
Antibody reagents
Anti-human mouse IgG1 CD63 (clone H5C6) and irrelevant isotype control monoclonal antibodies (BD-Pharmingen, San Diego, CA, USA) were used for light microscopy (5 μg/mL) and EM (2 μg/mL) immunodetection studies. Secondary antibody for immunofluorescence was anti-mouse Alexa 488 or Alexa 594 (1:500; Molecular Probes, Eugene, OR) and for immunoEM was an affinity-purified goat anti-mouse Fab fragment conjugated to 1.4 nm gold (1:100; Nanogold®, Nanoprobes, Stony Brook, NY, USA). Antibodies for CD63 detection in eosinophil granule subcellular fractions by flow cytometry were fluorescein isothiocyanate (FITC)-conjugated rat monoclonal anti-CD63 (clone H5C6) or irrelevant isotype IgG1 control (5 μg/106 cells; BD-Pharmingen). Antibodies for MBP detection in eosinophils by immunoEM (5 μg/mL) were monoclonal mouse anti-human MBP (clone AHE-2) and irrelevant isotype IgG1 control (BD-Pharmingen).
Cell preparation for EM
Purified eosinophils were immediately fixed in a mixture of freshly prepared aldehydes (1% paraformaldehyde and 1.25% glutaraldehyde) in 1 m sodium cacodylate buffer, pH 7.4, for 1 h, at room temperature, washed in the same buffer and centrifuged at 1500 g for 1 min. They were then resuspended in molten 2% agar in 1 m sodium cacodylate buffer, pH 7.4, and quickly recentrifuged. Resultant agar pellets were kept in the same buffer, at 4 °C for further processing. For immunoEM, cells were fixed in fresh 4% paraformaldehyde in 0.02 m PBS, pH 7.4 (CD63 labeling), or 1% paraformaldehyde and 1% glutaraldehyde in 1 m sodium cacodylate buffer, pH 7.4 (MBP labeling), for 30 min at room temperature, washed in PBS and embedded in molten 2% agar, as above. Pellets were immersed in 30% sucrose in PBS overnight at 4 °C, embedded in optimal cutting temperature (OCT) compound (Miles, Elkhart, IN, USA) and stored in −180 °C liquid nitrogen for subsequent use.
Conventional TEM
Agar pellets containing the cells were postfixed in 1% osmium tetroxide in Sym-Collidine buffer, pH 7.4, for 2 h at room temperature. After washing with sodium maleate buffer, pH 5.2, they were stained en bloc in 2% uranyl acetate in 0.05 m sodium maleate buffer, pH 6.0, for 2 h at room temperature and washed in the same buffer as before prior to dehydration in graded ethanols and infiltration and embedding with a propylene oxide-Epon sequence (Eponate 12 Resin; Ted Pella, Redding, CA, USA). After polymerization at 60 °C for 16 h, thin sections were cut using a diamond knife on an LKB ultramicrotome (LKB Instruments, Gaithersburg, MD, USA). Sections were mounted on uncoated 200-mesh copper grids (Ted Pella) before staining with lead citrate and viewed with a transmission electron microscope (P 300; Philips, Eindhoven, the Netherlands) at 60 KV. To quantify the number of specific granules, 95 electron micrographs showing the entire cell profile and nucleus were randomly taken at the magnification of × 12,000 and used at end magnification of ×33,000. A total of 3945 granules were counted. Data were compared by the Mann–Whitney ‘U’ test (p < 0.05).
ImmunoEM
Immunonanogold was performed on cryostat 10 μm sections mounted on glass slides. All steps were done at room temperature as described (44) and modified as follows: cells were incubated in PBS-BSA buffer (0.02 m PBS plus 1% BSA) containing 0.1% gelatin (20 min) followed by PBS-BSA plus 10% normal goat serum (NGS) and incubated with primary antibody (1 h). After blocking with PBS-BSA plus NGS (30 min), cells were incubated with secondary antibody (1 h), washed in PBS-BSA, postfixed in 1% glutaraldehyde (10 min) and incubated with HQ silver enhancement solution (Nanoprobes) (10 min). Cells were immersed in 5% sodium thiosulfate (5 min), postfixed with 1% osmium tetroxide in distilled water (10 min), stained with 2% uranyl acetate in distilled water (5 min), embedded in Eponate and cut as described (44). Two controls were performed: (i) primary antibody was replaced by an irrelevant antibody and (ii) primary antibody was omitted. Specimens were examined as described for conventional TEM.
Electron tomography, 3D reconstruction and modeling
Eponate sections of 200 or 400 nm were collected from eotaxin-stimulated eosinophils for analysis by ET. Tilt series were acquired fully automatically at 200 kV on a Tecnai Sphera microscope (FEI, Eidhoven, the Netherlands) using xplore 3d software (FEI). Digital images of the structures of interest were recorded as they were tilted from −65° to +65° at 1° intervals on a 1 K Gatan 794 slow-scan CCD camera (Gatan Inc., Pleasanton, CA, USA). The tomograms were generated using xplore 3d software (FEI). All tilted images were aligned to a common tilt axis using cross correlation, and the volume was reconstructed by real space-weighted back projection. Five tomograms were analyzed. Modeling was carried out using imod software (45).
Immunofluorescence microscopy
Eosinophils (15 × 106 cells/mL) were gently mixed at 37 °C with a low-gelling temperature 1.25% agarose (Pierce, Rockford, IL, USA) in a 3:1 ratio (cells : agarose) and carefully spread onto slides (20 μL/slide). After agarose was slightly solidified, a perfusion chamber (CoverWell™, Grace Bio-Laboratories, Bend, OR, USA) was affixed over cells and medium added to maintain moisture. After several minutes, to allow for cell attachment and spreading, chambers were removed and cells fixed in 2% paraformadehyde for 5 min at room temperature. Slides were then washed with calcium magnesium-free Hanks' balanced salt solution (HBSS) (HBSS−/−) alone, followed by a 5 min incubation in permeabilization solution (0.1% saponin, 5% milk and 1% NGS in HBSS−/−). Permeabilized cells were incubated with primary antibody for 1 h, washed and incubated with secondary antibody for 45 min at room temperature. Cells were washed (2 ×10 min) before drying and coverslipping. Analyses were performed by both phase contrast and fluorescence microscopy.
Deconvolution microscopy
Fluorescence images were acquired using a Retiga Exi-cooled CCD camera (Burnaby, BC, Canada) coupled to a Provis AX-70 Olympus microscope (Olympus, Melville, NY, USA) and a UPlanApo objective (100 × 1.35). The microscope, Z-motor drive, shutters and camera were controlled by iplab 3.6 for Mac (Scanalytics, VA, USA). The acquired stacks were further processed for deconvolution with volocity 2.6 (Improvision, Lexington, MA, USA).
Flow cytometry
Eosinophil granule fractions were resuspended in RPMI + 0.1% ovalbumin and incubated at 37 °C. Following incubation, cells were fixed with 2% paraformaldehyde (5 min) and permeabilized in 0.1% saponin plus 2.5% NGS and 2.5% human serum before staining with anti-CD63 or control-irrelevant antibodies. Flow cytometric data, acquired using a FACScan (Becton Dickinson, Franklin Lakes, NJ, USA) with cellquest software, was analyzed by comparisons of MFI of unimodal histograms.
Subcellular fractionation
Eosinophils were resuspended in disrupting buffer as described (18) and subjected to nitrogen cavitation under pressure of 800 psi (10 min). Post-nuclear supernatants recovered after centrifugation (400 g, 10 min) were ultracentrifuged (100 000 g, 1 h at 4 °C) in linear Optiprep™ (Axis-Shield PoC AS, Oslo, Norway) gradients (0–45% in disrupting buffer). Fractions (20 × 0.5 mL) were collected with a peristaltic pump.
Acknowledgments
We thank Rita Monahan-Earley, Tracey Sciuto and Patricia Fox (Department of Pathology) for technical assistance, Wim Voorhout and FEI Company (Eidhoven, the Netherlands) for the tomograms and Dr Daniela Corda (Istituto di Ricerche Farmacologiche ‘Mario Negri’, Italy) for anti-BARS antibody. This work was supported by National Institutes of Health grants AI33372, AI20241, AI22571, HL70270. R.C.N. Melo and S.A.C. Perez were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil).
References
- 1.Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–663. doi: 10.1067/mai.2000.105712. [DOI] [PubMed] [Google Scholar]
- 2.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–1779. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 3.Munitz A, Levi-Schaffer F. Eosinophils: ‘new’ roles for ‘old’ cells. Allergy. 2004;59:268–275. doi: 10.1111/j.1398-9995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 4.Adamko DJ, Odemuyiwa SO, Vethanayagam D, Moqbel R. The rise of the phoenix: the expanding role of the eosinophil in health and disease. Allergy. 2005;60:13–22. doi: 10.1111/j.1398-9995.2005.00676.x. [DOI] [PubMed] [Google Scholar]
- 5.Lacy P, Moqbel R. Eosinophil cytokines. Chem Immunol. 2000;76:134–155. doi: 10.1159/000058782. [DOI] [PubMed] [Google Scholar]
- 6.Wong DT, Weller PF, Galli SJ, Elovic A, Rand TH, Gallagher GT, Chiang T, Chou MY, Matossian K, McBride J, Todd R. Human eosinophils express transforming growth factor alpha. J Exp Med. 1990;172:673–681. doi: 10.1084/jem.172.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broide DH, Paine MM, Firestein GS. Eosinophils express interleukin 5 and granulocyte macrophage-colony-stimulating factor mRNA at sites of allergic inflammation in asthmatics. J Clin Invest. 1992;90:1414–1424. doi: 10.1172/JCI116008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beil WJ, Weller PF, Tzizik DM, Galli SJ, Dvorak AM. Ultrastructural immunogold localization of tumor necrosis factor-alpha to the matrix compartment of eosinophil secondary granules in patients with idiopathic hypereosinophilic syndrome. J Histochem Cytochem. 1993;41:1611–1615. doi: 10.1177/41.11.8409368. [DOI] [PubMed] [Google Scholar]
- 9.Fujisawa T, Fukuda S, Atsuta J, Ichimi R, Kamiya H, Sakurai M. Interferon-gamma induces interleukin-3 release from peripheral blood eosinophils. Int Arch Allergy Immunol. 1994;104(Suppl 1):41–43. doi: 10.1159/000236748. [DOI] [PubMed] [Google Scholar]
- 10.Dubucquoi S, Desreumaux P, Janin A, Klein O, Goldman M, Tavernier J, Capron A, Capron M. Interleukin 5 synthesis by eosinophils: association with granules and immunoglobulin-dependent secretion. J Exp Med. 1994;179:703–708. doi: 10.1084/jem.179.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moller GM, de Jong TA, van der Kwast TH, Overbeek SE, Wierenga-Wolf AF, Thepen T, Hoogsteden HC. Immunolocalization of interleukin-4 in eosinophils in the bronchial mucosa of atopic asthmatics. Am J Respir Cell Mol Biol. 1996;14:439–443. doi: 10.1165/ajrcmb.14.5.8624248. [DOI] [PubMed] [Google Scholar]
- 12.Lim KG, Wan HC, Bozza PT, Resnick MB, Wong DT, Cruikshank WW, Kornfeld H, Center DM, Weller PF. Human eosinophils elaborate the lymphocyte chemoattractants. IL-16 (lymphocyte chemoattractant factor) and RANTES. J Immunol. 1996;156:2566–2570. [PubMed] [Google Scholar]
- 13.Ying S, Meng Q, Taborda-Barata L, Corrigan CJ, Barkans J, Assoufi B, Moqbel R, Durham SR, Kay AB. Human eosinophils express messenger RNA encoding RANTES and store and release biologically active RANTES protein. Eur J Immunol. 1996;26:70–76. doi: 10.1002/eji.1830260111. [DOI] [PubMed] [Google Scholar]
- 14.Horiuchi T, Weller PF. Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5. Am J Respir Cell Mol Biol. 1997;17:70–77. doi: 10.1165/ajrcmb.17.1.2796. [DOI] [PubMed] [Google Scholar]
- 15.Hartman M, Piliponsky AM, Temkin V, Levi-Schaffer F. Human peripheral blood eosinophils express stem cell factor. Blood. 2001;97:1086–1091. doi: 10.1182/blood.v97.4.1086. [DOI] [PubMed] [Google Scholar]
- 16.Woerly G, Lacy P, Younes AB, Roger N, Loiseau S, Moqbel R, Capron M. Human eosinophils express and release IL-13 following CD28-dependent activation. J Leukoc Biol. 2002;72:769–779. [PubMed] [Google Scholar]
- 17.Kita H, Adolphson C, Gleich G. Biology of eosinophils. In: Middleton E Jr, Reed CE, Ellis EF, Adkinson NF Jr, Yunginger JW, Busse WW, editors. Allergy: Principles and Practice. 5th. St. Louis: Mosby; 1998. pp. 242–260. [Google Scholar]
- 18.Lacy P, Mahmudi-Azer S, Bablitz B, Hagen SC, Velazquez JR, Man SF, Moqbel R. Rapid mobilization of intracellularly stored RANTES in response to interferon-gamma in human eosinophils. Blood. 1999;94:23–32. [PubMed] [Google Scholar]
- 19.Bandeira-Melo C, Sugiyama K, Woods LJ, Weller PF. Cutting edge: eotaxin elicits rapid vesicular transport-mediated release of preformed IL-4 from human eosinophils. J Immunol. 2001;166:4813–4817. doi: 10.4049/jimmunol.166.8.4813. [DOI] [PubMed] [Google Scholar]
- 20.Dvorak HF, Dvorak AM. Basophilic leucocytes: structure, function and role in disease. Clin Haematol. 1975;4:651–683. [PubMed] [Google Scholar]
- 21.Dvorak AM. Basophils and mast cells: piecemeal degranulation in situ and ex vivo: a possible mechanism for cytokine-induced function in disease. In: RG, editor. Immunol Ser. Vol. 57. 1992. pp. 169–271. [PubMed] [Google Scholar]
- 22.Dvorak AM. Chem Immunol Allergy. Basel: S. Karger; 2005. Ultrastructure of Mast Cells and Basophils. [Google Scholar]
- 23.Dvorak AM, Monahan RA, Osage JE, Dickersin GR. Crohn's disease: transmission electron microscopic studies. II. Immunologic inflammatory response. Alterations of mast cells, basophils, eosinophils, and the microvasculature. Hum Pathol. 1980;11:606–619. doi: 10.1016/s0046-8177(80)80072-4. [DOI] [PubMed] [Google Scholar]
- 24.Karawajczyk M, Seveus L, Garcia R, Bjornsson E, Peterson CG, Roomans GM, Venge P. Piecemeal degranulation of peripheral blood eosinophils: a study of allergic subjects during and out of the pollen season. Am J Respir Cell Mol Biol. 2000;23:521–529. doi: 10.1165/ajrcmb.23.4.4025. [DOI] [PubMed] [Google Scholar]
- 25.Erjefalt JS, Greiff L, Andersson M, Adelroth E, Jeffery PK, Persson CG. Degranulation patterns of eosinophil granulocytes as determinants of eosinophil driven disease. Thorax. 2001;56:341–344. doi: 10.1136/thorax.56.5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahlstrom-Emanuelsson CA, Greiff L, Andersson M, Persson CG, Erjefalt JS. Eosinophil degranulation status in allergic rhinitis: observations before and during seasonal allergen exposure. Eur Respir J. 2004;24:750–757. doi: 10.1183/09031936.04.00133603. [DOI] [PubMed] [Google Scholar]
- 27.Dvorak A, Monahan-Earley RA. Diagnostic Ultrastructural Pathology III A Text-Atlas of Case Studies Emphasizing Endocrine and Hematopoietic Systems. Boca Raton: CRC Press; 1995. [Google Scholar]
- 28.Crivellato E, Nico B, Mallardi F, Beltrami CA, Ribatti D. Piecemeal degranulation as a general secretory mechanism? Anat Rec. 2003;274A:778–784. doi: 10.1002/ar.a.10095. [DOI] [PubMed] [Google Scholar]
- 29.Dvorak AM, Furitsu T, Letourneau L, Ishizaka T, Ackerman SJ. Mature eosinophils stimulated to develop in human cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. I. Piecemeal degranulation of specific granules and distribution of Charcot-Leyden crystal protein. Am J Pathol. 1991;138:69–82. [PMC free article] [PubMed] [Google Scholar]
- 30.Dvorak AM, Ackerman SJ, Furitsu T, Estrella P, Letourneau L, Ishizaka T. Mature eosinophils stimulated to develop in human-cord blood mononuclear cell cultures supplemented with recombinant human interleukin-5. II. Vesicular transport of specific granule matrix peroxidase, a mechanism for effecting piecemeal degranulation. Am J Pathol. 1992;140:795–807. [PMC free article] [PubMed] [Google Scholar]
- 31.Nebenfuhr A, Ritzenthaler C, Robinson DG. Brefeldin A: deciphering an enigmatic inhibitor of secretion. Plant Physiol. 2002;130:1102–1108. doi: 10.1104/pp.011569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahmudi-Azer S, Downey GP, Moqbel R. Translocation of the tetraspanin CD63 in association with human eosinophil mediator release. Blood. 2002;99:4039–4047. doi: 10.1182/blood.v99.11.4039. [DOI] [PubMed] [Google Scholar]
- 33.Moqbel R, Lacy P. Molecular mechanisms in eosinophil activation. Chem Immunol. 2000;78:189–198. doi: 10.1159/000058806. [DOI] [PubMed] [Google Scholar]
- 34.Bandeira-Melo C, Herbst A, Weller PF. Eotaxins. Contributing to the diversity of eosinophil recruitment and activation. Am J Respir Cell Mol Biol. 2001;24:653–657. doi: 10.1165/ajrcmb.24.6.f209. [DOI] [PubMed] [Google Scholar]
- 35.Klein A, Pinho V, Alessandrini AL, Shimizu T, Ishii S, Teixeira MM. Platelet-activating factor drives eotaxin production in an allergic pleurisy in mice. Br J Pharmacol. 2002;135:1213–1218. doi: 10.1038/sj.bjp.0704570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kato M, Kita H, Tachibana A, Hayashi Y, Tsuchida Y, Kimura H. Dual signaling and effector pathways mediate human eosinophil activation by platelet-activating factor. Int Arch Allergy Immunol. 2004;134(Suppl 1):37–43. doi: 10.1159/000077791. [DOI] [PubMed] [Google Scholar]
- 37.Dvorak AM, Weller PF. Ultrastructural analysis of human eosinophils. In: Marone G, editor. Human Eosinophils: Biological and Chemical Aspects. Basel: Karger; 2000. pp. 1–28. [DOI] [PubMed] [Google Scholar]
- 38.Okuda M, Takenaka T, Kawabori S, Ogami Y. Ultrastructural study of the specific granule of the human eosinophil. J Submicrosc Cytol. 1981;13:465–471. [PubMed] [Google Scholar]
- 39.Kroegel C, Dewar A, Yukawa T, Venge P, Barnes PJ, Chung KF. Ultrastructural characterization of platelet-activating factor-stimulated human eosinophils from patients with asthma. Clin Sci (Lond) 1993;84:391–399. doi: 10.1042/cs0840391. [DOI] [PubMed] [Google Scholar]
- 40.Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91:2313–2325. [PubMed] [Google Scholar]
- 41.Barois N, de Saint-Vis B, Lebecque S, Geuze HJ, Kleijmeer MJ. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic. 2002;3:894–905. doi: 10.1034/j.1600-0854.2002.31205.x. [DOI] [PubMed] [Google Scholar]
- 42.Weigert R, Silletta MG, Spano S, Turacchio G, Cericola C, Colanzi A, Senatore S, Mancini R, Polishchuk EV, Salmona M, Facchiano F, Burger KN, Mironov A, Luini A, Corda D. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- 43.Bandeira-Melo C, Gillard G, Ghiran I, Weller PF. EliCell: a gel-phase dual antibody capture and detection assay to measure cytokine release from eosinophils. J Immunol Methods. 2000;244:105–115. doi: 10.1016/s0022-1759(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 44.Feng D, Flaumenhaft R, Bandeira-Melo C, Weller P, Dvorak A. Ultrastructural localization of vesicle-associated membrane protein(s) to specialized membrane structures in human pericytes, vascular smooth muscle cells, endothelial cells, neutrophils, and eosinophils. J Histochem Cytochem. 2001;49:293–304. doi: 10.1177/002215540104900303. [DOI] [PubMed] [Google Scholar]
- 45.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]


