Abstract
The remarkable infectiousness of Francisella tularensis suggests that the bacterium efficiently evades innate immune responses that typically protect the host during its continuous exposure to environmental and commensal microbes. In our studies of the innate immune response to F. tularensis, we have observed that, unlike the live vaccine strain (LVS) of F. tularensis subsp. holarctica, F. tularensis subsp. novicida U112 opsonized in pooled human serum activated the NADPH oxidase when incubated with human neutrophils. Given previous observations that F. tularensis fixes relatively small quantities of complement component C3 during incubation in human serum and the importance of C3 to neutrophil phagocytosis, we hypothesized that F. tularensis subsp. novicida may fix C3 in human serum more readily than would LVS. We now report that F. tularensis subsp. novicida fixed approximately six-fold more C3 than did LVS when incubated in 50% pooled human serum and that this complement opsonization was antibody-mediated. Furthermore, antibody-mediated C3 deposition enhanced bacterial uptake and was indispensable for the neutrophil oxidative response to F. tularensis subsp. novicida. Taken together, our results reveal important differences between these two strains of F. tularensis and may, in part, explain the low virulence of F. tularensis subsp. novicida for humans.
Keywords: FRANCISELLA TULARENSIS, NEUTROPHILS, COMPLEMENT ACTIVATION, COMPLEMENT C3, HUMANS, PHAGOCYTOSIS, RESPIRATORY BURST
1. Introduction
Francisella tularensis is a small Gram-negative coccobacillus that causes tularemia, a highly infectious and life-threatening zoonosis of humans. Infection can be acquired via aerosol, insect bites, or inoculation onto broken skin or mucous membranes, and infections are typically seen in people with exposure to infected animals or contaminated soil or water [1]. The low infectious dose and the high mortality of pneumonic tularemia sparked interest in the use of the organism as an agent of biowarfare, leading to its weaponization during the Cold War [2]. Consequently, F. tularensis has been deemed a category A agent of bioterrorism and a priority for research.
F. tularensis is divided into several subspecies. F. tularensis subsp. tularensis is the most virulent to humans and is found in North America, whereas subsp. holarctica is less virulent and can be found throughout the Northern Hemisphere [1]. Two other subspecies, novicida and mediasiatica, rarely cause disease in humans. Given the virulence and infectiousness of the wild-type tularensis and holarctica strains, much of the existing research has been performed using an attenuated live vaccine strain (LVS, derived from F. tularensis subsp. holarctica) and subsp. novicida (FtN), both of which cause a lethal invasive infection of mice [3]. They share with the more virulent strains a Francisella pathogenicity island and the ability to replicate within macrophages of many species, including humans [1], but the basis for their decreased virulence is currently unclear. Furthermore, F. tularensis subsp. novicida is distinct antigenically from subsp. tularensis and subsp. holarctica [4] and apparently lacks a capsule-like material that the other subspecies possess ([5] and M. A. Apicella, unpublished observations). Thus, although the various subspecies are similar genetically and share some virulence factors, differences remain that are sufficient to render some strains highly virulent for humans whereas others rarely cause disease. The determinants of these differences in virulence are largely undefined.
Previous studies from our group have shown that LVS fails to stimulate the oxidative burst in human neutrophils (polymorphonuclear leukocytes, PMN) [6]. Intriguingly, we have observed that unlike LVS, F. tularensis subsp. novicida (FtN) stimulates the PMN NADPH oxidase. It has been reported that LVS fixes small quantities of complement component C3 to its surface during incubation in human serum [7], and given the importance of complement opsonization to PMN phagocytosis [8, 9], we hypothesized that FtN would fix more C3 than does LVS when incubated in pooled human serum (PHS). We further hypothesized that uptake of FtN and the subsequent PMN oxidative burst would be dependent upon this increased C3 deposition.
2. Materials and Methods
2.1. Materials
Endotoxin-free phosphate buffered saline (PBS) and Hank’s buffered salt solution (HBSS) with or without divalent cations were from Mediatech, Inc. (Herndon, VA). Endotoxin-free water, normal saline, and human serum albumin were from Baxter International Inc. (Deerfield, IL). Bovine serum albumin (BSA), luminol, and 10% neutral buffered formalin were from Sigma-Aldrich Corp. (St. Louis, MO). Clinical grade dextran was from Pharmacosmos (Holbaek, Denmark). Difco cysteine heart agar (CHA) was from Becton Dickinson (Franklin Lakes, NJ). Sensitized sheep erythrocytes, gelatin veronal buffer, and purified human complement component C3 were from Complement Technology, Inc. (Tyler, TX). Fluorescent labeled secondary antibodies were from Jackson Immunoresearch (West Grove, PA),
2.2. Human Sera
Pooled human serum (PHS) was obtained from approximately 10 healthy donors without a history of tularemia. Immune serum from four patients diagnosed with tularemia was the gift of Dr. Gregory Storch (Washington University School of Medicine, St. Louis, MO). Immune sera were heat-inactivated (at 56 °C for 30 min) and pooled with a resulting agglutinating titer of 1:640, determined as previously described with slight modification [10]. Serum from a patient with agammaglobulinemia was the gift of Dr. Peter Densen, University of Iowa, Iowa City, IA. Functional activity of the classical pathway of complement (CH50) was assessed as described previously [11]. For some experiments, aliquots of PHS were depleted of antibody to FtN. Formalin-treated FtN (3 × 109/ml) were incubated in PHS for 30 min on ice. Bacteria were centrifuged and incubation with bacteria was repeated. Aliquots of serum were frozen at −80 °C until use.
2.3. PMN isolation
PMN were isolated from healthy donors as described previously using dextran sedimentation followed by Ficoll-Paque Plus (Amersham Biosciences, Piscataway, NJ) density-gradient separation [12].
2.4. Bacteria
F. tularensis subsp. holarctica strain LVS (ATCC strain 29684) was obtained from Dr. Michael Apicella, University of Iowa and F. tularensis subsp. novicida U 112 was obtained from Dr. Colin Manoil at the University of Washington (www.francisella.org). LVS and FtN were grown for 48 and 24 hours, respectively, on CHA/9% sheep blood plates at 37 °C in 5% CO2. Bacteria were formalin fixed in 10% buffered formalin for 30 min. Periodate treatment was carried out as previously described [13].
2.5. Opsonization
Washed francisellae (1.2 × 109 bacteria/ml) were suspended in 50% PHS, incubated with mixing at 37 °C, and washed twice. Incubation in serum did not affect the viability of the Francisella strains used in this study (not shown). Pretreatment of bacteria in immune serum was carried out for 30 min at 37 °C at a dilution of immune serum that did not result in agglutination (1:600-1:1000). Bacteria were pelleted and resuspended in 50% pooled PHS as a source of complement and incubated at 37 °C for 30 min as described above. In some experiments, calcium-dependent complement opsonization via the classical and mannose-binding lectin pathways was inhibited by adding magnesium and EGTA (final concentrations 10 mM and 8 mM, respectively) to 50% PHS in PBS without cations [14].
2.6. Measuring C3 and immunoglobulin deposition
To quantify C3, opsonized bacteria were incubated with FITC-conjugated goat anti-human-C3 antibodies (Cappel) for 60 min, washed, and assayed for fluorescence in a Novostar fluorometer (BMG Labtech, Offenberg, Germany) at equivalent concentrations. IgG and IgM opsonization were determined similarly using FITC-conjugated goat anti-human IgG and IgM antibodies (Cappel, MP Biomedicals, Irvine, CA). Fluorescence was normalized to the signal obtained from serum-treated bacteria incubated with normal goat FITC-conjugated antibody. For measurement of C3 deposition by immunoblotting, washed bacteria were boiled in sample buffer in reducing conditions and separated by 4–12 % SDS-PAGE (Invitrogen, Carlsbad California). Proteins were transferred to polyvinylidene difluoride membranes (Perkin Elmer, Boston, MA), and C3 was detected using an HRP-conjugated goat antibody to human C3 (Cappel). Immunoblots were developed using enhanced chemiluminescence and viewed on a Typhoon 9410 imager (GE Healthcare, Piscataway, NJ).
2.7. Phagocytosis and ROS production
To minimize adherence of PMN to polystyrene plates during infection, wells were coated with poly (2-hydroxyethyl methacrylate) (Polyhema, Sigma) [15]. PMN were diluted to 1 × 107/ml in HBSS with 0.1% HSA and 250 μM luminol. An equal volume of buffer or bacteria (5 × 108 million/ml) in HBSS buffer was then added simultaneously to the PMN, and the plate was placed into a Novostar luminometer for assay of ROS production by luminol-enhanced chemiluminescence (LCL) [16]. All assays were performed in triplicate. For each sample, peak ROS was defined as the highest arbitrary light unit (ALU) value achieved during 60 minutes of incubation. The peak values were then normalized to the highest peak value seen in each experiment (defined as 1), in order to facilitate statistical comparisons across independent experiments.
After ROS production had peaked (45–60 min), PMN were fixed in 0.5% paraformaldehyde (Electron Microscopy Sciences, Hatfield PA). In order to distinguish between intracellular and extracellular bacteria, immunofluorescent labeling was performed before and after permeabilization as previously described for F. tularensis LVS-infected macrophages [17]. Extracellular bacteria were labeled using rabbit anti-Francisella antiserum (Becton Dickinson), washed, and incubated with affinity-purified donkey anti-rabbit tetramethylrhodamine isothiocyanate-conjugated F(ab′)2 secondary antibody. Cells were then permeabilized in methanol/acetone and stained with rabbit anti-Francisella antiserum followed by affinity-purified donkey anti-rabbit FITC-conjugated F(ab′)2 antibody. Thus, extracellular bacteria fluoresced red, whereas all bacteria fluoresced green. Images were viewed using an Axioplan2 fluorescence microscope (Carl Zeiss).
2.8. Statistical analysis
Three or more independent experiments were compared using repeated measures analysis of variance using Prism 5 (GraphPad Software, San Diego, CA). Two groups were compared using a paired t test. In the figure legends, the number of independent experiments analyzed is indicated as “N”.
3. Results
3.1. Fixation of complement component C3 by LVS and FtN during incubation in pooled human serum
We recently reported that LVS incubated in PHS fails to stimulate an oxidative burst when incubated with human PMN [6]. We now show that in contrast to LVS, PHS-treated live FtN stimulated an oxidative burst in PMN (Fig. 1A). Specifically, whereas LVS stimulated oxidant activity identical to cells exposed to buffer, both FtN and periodate-treated LVS (PI-LVS) stimulated a significant oxidative response (Fig. 1A and [6]). It has previously been reported that LVS fixes small quantities of complement component C3 [7], and given the importance of C3 to opsonization for PMN phagocytosis [8, 9], we hypothesized that FtN and PI-LVS may fix greater quantities of C3 than LVS. Using fluorescently tagged anti-C3 antibody, we observed that LVS fixed significantly less C3 than live FtN or PI-LVS during incubation in 50% PHS for 5–60 min (Fig. 1B). To detect C3 that might be inaccessible to anti-C3 antibody on intact bacteria, we also subjected serum-treated bacteria to SDS-PAGE in reducing conditions followed by immunoblotting with anti-C3 antibody (Fig. 1C). The total quantity of C3b and iC3b was assayed by measuring the density of the C3β chain, as this 75 kD protein is shared by these fragments. Consistent with the data in Fig. 1B, C3 was barely detectable on LVS compared with FtN or PI-LVS; densitometry of the β-chain indicated that live LVS fixed 87% less C3b and iC3b than live FtN (P = < .001, n = 4). Furthermore, the α′-chain of C3 and its fragments can be seen readily on FtN and PI-LVS but are undetectable on live F. tularensis LVS. These findings confirm and extend the observations of Sandstrom et al. [7], demonstrating that F. tularensis LVS incubated in PHS fixes small quantities of C3 compared to FtN. Our results also show that the resistance of LVS to C3 deposition can be reversed by treatment of the bacteria with periodate (or formalin, not shown).
Fig. 1.
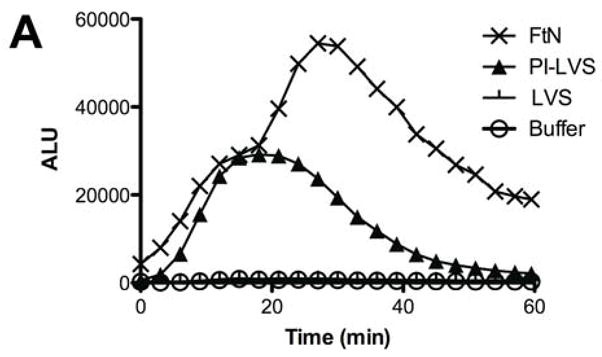
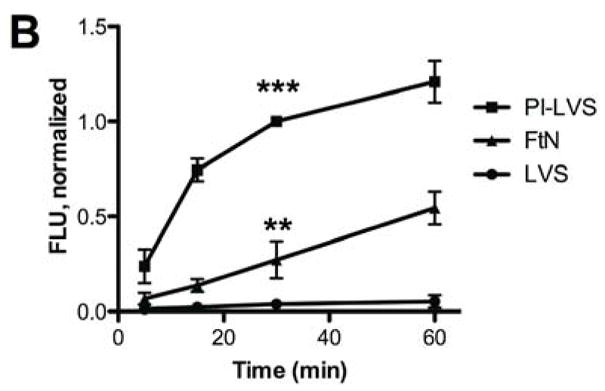
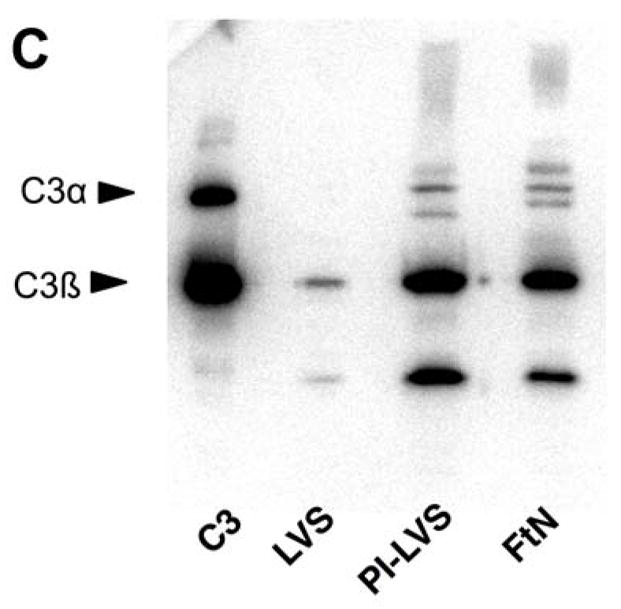
FtN stimulated LCL in human PMN and fixed complement component C3. (A) Bacteria were incubated in 50% PHS for 30 min, washed and incubated with human PMN. Control PMN were incubated in buffer without bacteria. Reactive oxidant species production was measured using LCL every 30 seconds. ALU, arbitrary light units. Results are representative of five experiments. (B) Bacteria were incubated in 50% PHS, and opsonization was stopped at the indicated times by adding ice-cold buffer with ethylenediaminetetraacetic acid (final concentration, 10 mM). Bacteria were then washed and incubated with anti-C3 fluorescent antibody. FLU, fluorescence light units. FLU were normalized based on the fluorescence of PI-LVS at 30 min. **, P < 0.01 compared to LVS at 30 min. ***, P < 0.001 compare to LVS at 30 min. N= 3. (C) Bacteria incubated in 50% PHS for 30 min were washed and subjected to immunoblotting for C3. Immunoreactive proteins corresponding to C3α and C3β are marked on the left. The α′-chain of C3 and its fragments can be seen on the blot as higher molecular weight bands that migrate more slowly than the C3 β-chain because they remain covalently bound to their bacterial targets. C3, purified complement component C3. The blot is representative of four independent experiments.
3.2. The role of antibody in C3 fixation by FtN
Because the classical and mannose-binding lectin pathways of complement require calcium, only the alternative pathway of complement is active in the presence of magnesium and the calcium chelator, EGTA [14]. In the presence of these additives, C3 deposition on FtN was decreased 80% and approximated that bound to LVS (Fig. 2A), demonstrating that full complement opsonization of FtN was calcium-dependent. One possible explanation for the calcium-dependence of C3 deposition is that antibody in PHS bound to FtN and mediated complement activation via the classical pathway. Indeed, Balagopal et al. detected antibody to FtN in their PHS [18]. Similarly, we observed that FtN incubated in 50% PHS bound four-fold more IgG than did LVS (Fig. 2B) but did not detect any binding of IgM to FtN in excess of that bound to LVS (Fig. 2C).
Fig. 2.
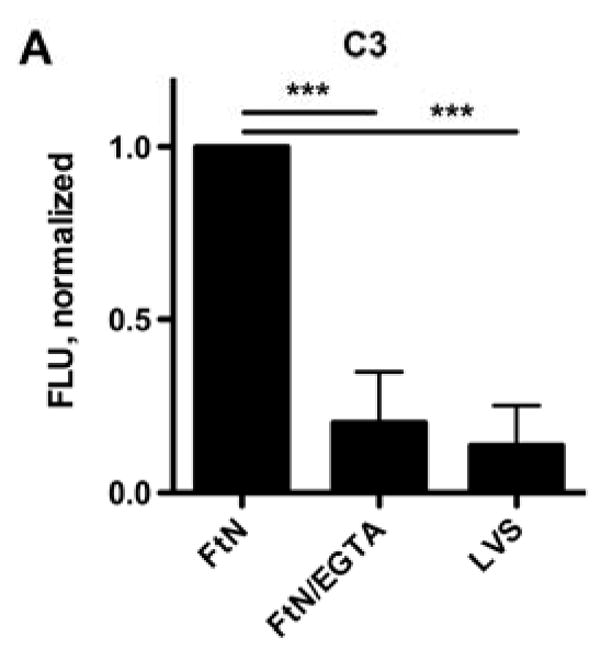
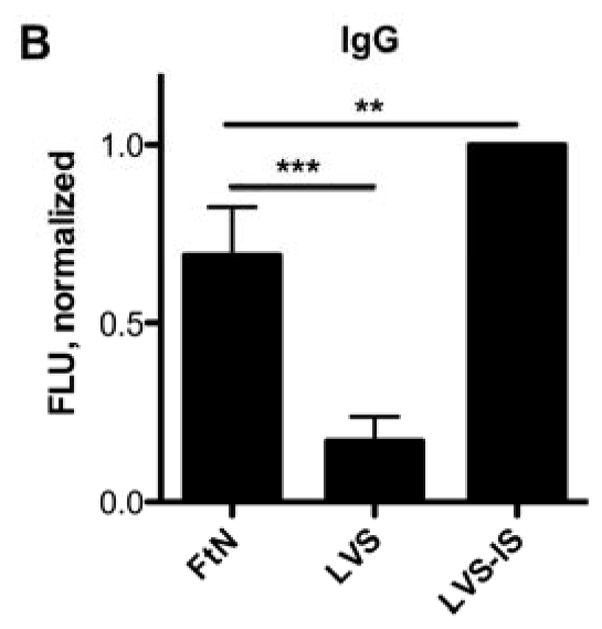
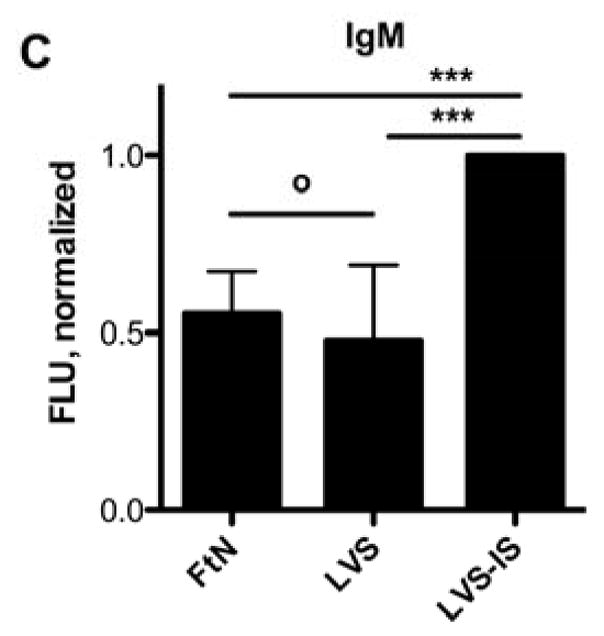
The majority of C3 fixation of FtN was calcium-dependent, and FtN bound more antibody in PHS than LVS. (A) Bacteria were incubated in 50% PHS for 30 min. The FTN/EGTA sample was supplemented with magnesium and EGTA to chelate calcium during serum incubation (see methods). C3was normalized to the value for FtN. N = 8. Identically-treated bacteria were assayed for IgG (B) and IgM (C) binding using immunofluorescent detection of bound antibody. Immunoglobulin FLU are normalized for the fluorescence value of LVS that had been incubated in a sub-agglutinating concentration of tularemia immune serum prior to incubation in 50% PHS (LVS-IS, see methods). N = 5. For all three panels, results are expressed as the mean ± the standard deviation (SD). °, P > 0.05; **, P < 0.01; ***, P < 0.001.
To test the role of specific antibody in C3 opsonization of FtN, we incubated live FtN with serum that had been depleted of FtN-reactive antibodies (dPHS, see methods). In these conditions, IgG deposition on FtN was decreased by more than 90% (P < .001) compared to mock-depleted serum (Fig. 3A). In contrast, we detected no decrease in IgM binding (not shown). In parallel, C3 deposition by dPHS decreased 54% (P < .001) (Fig. 3A). In order to define more precisely the role of antibody in complement opsonization, we opsonized FtN in serum from a patient with agammaglobulinemia (AGS) and assessed C3 deposition. C3 deposition by AGS was 66% (95% CI, 40 to 92%) less than that seen on FtN incubated in PHS (Fig. 3B). Our sample of AGS had not lost its complement activity during storage, as its CH50 was equivalent to that of our stocks of PHS (data not shown). Furthermore, AGS was able to confer efficient C3 deposition when bacteria were coated with antibody from PHS. Namely, when FtN bacteria were first incubated in heat-inactivated PHS, C3 deposition by AGS was restored (Fig. 3B). Taken together, our data indicate that most of the C3 deposition on FtN was mediated by FtN-reactive antibody in PHS.
Fig. 3.
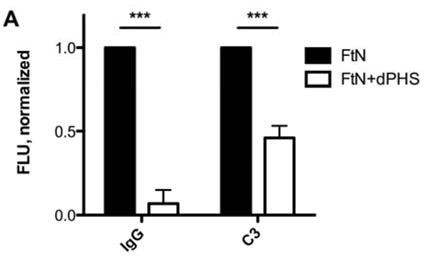
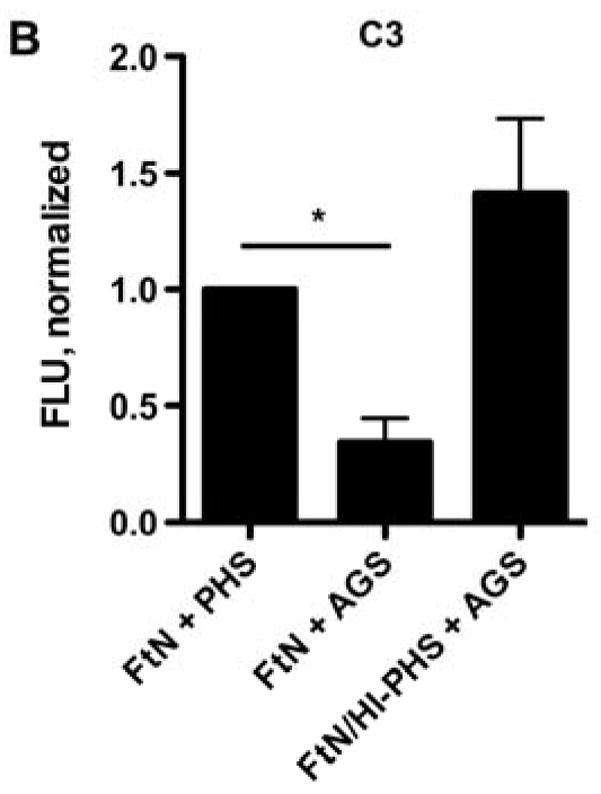
The majority of C3 fixation by FtN incubated in 50% PHS was antibody-dependent. (A) FtN were incubated for 30 min in 50% PHS that had been depleted of anti-FtN antibody (dPHS, see methods) and assayed as above for IgG (left) and C3 (right) binding. N = 3. Results are normalized to the values seen with FtN incubated in 50% PHS. (B) FtN was incubated in either 50% AGS or 40% PHS for 30 min and assessed for C3 binding (Because AGS had been dialyzed at the time of its collection to remove antibiotics, it had been diluted approximately 10%. Thus, 50% AGS was compared with 40% PHS). The rightmost sample was incubated in HI-PHS prior to incubation in AGS (FtN/HI-PHS + AGS). Results are normalized to the value seen with FtN incubated in 40% PHS. N = 3. Results are expressed as the mean ± SD. *, P < 0.05; ***, P < 0.001.
3.3. The role of C3 fixation in phagocytosis of FtN by PMN
We hypothesized that differences in C3 deposition on FtN would result in differences in uptake. As shown in Fig. 4, the phagocytic index of LVS, which fixed relatively small quantities of C3, was approximately 15% that of FtN. Furthermore, incubation of FtN in serum supplemented with EGTA and Mg++ decreased bacterial uptake by over 80% (Fig. 4), paralleling the effect of calcium depletion upon C3 deposition (Fig. 2A). Similarly, FtN incubated in AGS, which fixed small quantities of C3 (Fig. 3B), was inefficiently ingested compared to bacteria incubated in PHS or to FtN incubated in HI-PHS prior to incubation in AGS (data not shown). Thus, uptake of francisellae by human PMN correlated closely with the degree of C3 deposition on the bacteria.
Fig. 4.
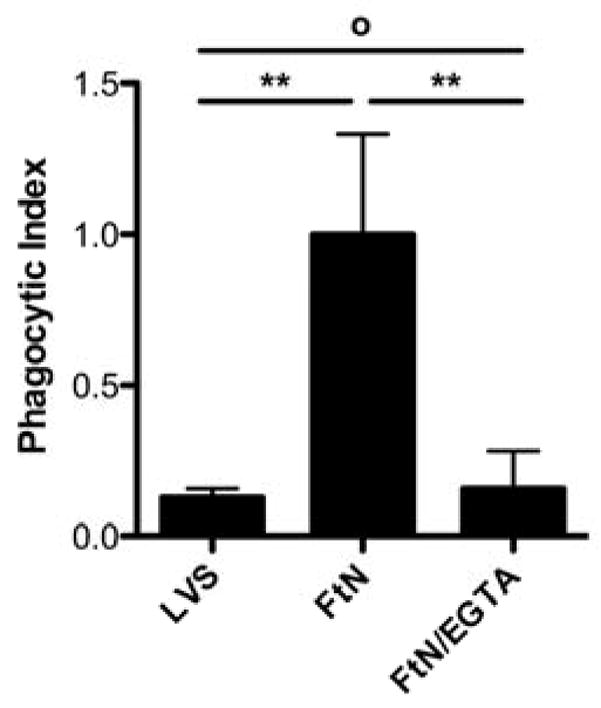
Optimal uptake of FtN by human PMN required calcium-dependent pathways of complement opsonization. PMN infected as described in Fig. 2A were assayed for intracellular bacteria after LCL had peaked (45–60 min). Phagocytic index was defined as the average number of ingested bacteria per PMN (with at least 100 PMN counted per sample). Uptake of LVS that had been incubated in 50% PHS for 30 min is included for comparison. N = 3. Results are expressed as the mean ± SD. °, P > 0.05; **, P < 0.01. When FtN are not opsonized in serum, bacterial uptake is negligible in identical infection conditions (phagocytic index < 0.02, data not shown).
3.4. The role of C3 fixation by FtN in stimulation of the PMN oxidative response
Given the importance of C3 opsonization to phagocytosis of FtN by PMN (Fig. 4), we hypothesized that the oxidative response would also be decreased when bacteria fixed decreased quantities of C3. Indeed, the oxidative response of human PMN to FtN was reduced to that of buffer-treated cells when bacteria were not incubated in serum or when serum was heat-inactivated (not shown). Similarly, blockade of calcium-dependent complement opsonization with Mg++/EGTA, which decreased C3 deposition (Fig. 2A) and phagocytosis (Fig. 4) by ~80%, decreased the induction of peak LCL by 61% (95% CI, 39 to 82%) (Fig. 5A and 5B). We further hypothesized that if C3 deposition on FtN were dependent upon antibodies in PHS (Fig. 3), then FtN incubated in AGS would fail to stimulate an oxidative burst. Indeed, the peak LCL burst stimulated by FtN incubated in AGS was decreased 79% (95% CI, 56 to 100%) compared to that stimulated by FtN incubated in PHS (Fig. 5C). When antibody binding to FtN was restored by first incubating the bacteria in heat-inactivated PHS before adding AGS, LCL was similarly restored (Fig. 5C). In summary, antibody-mediated deposition of C3 on FtN was indispensible for the oxidative response of human PMN to FtN.
Fig. 5.
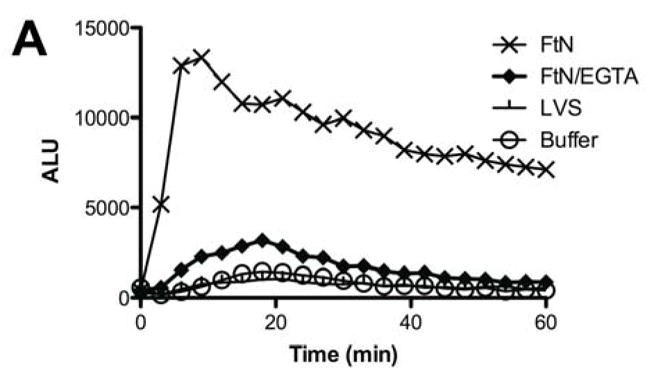
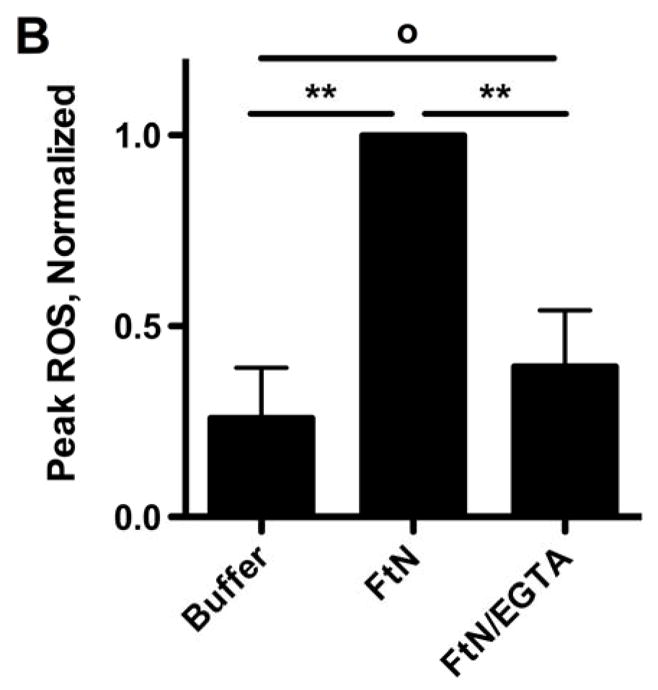
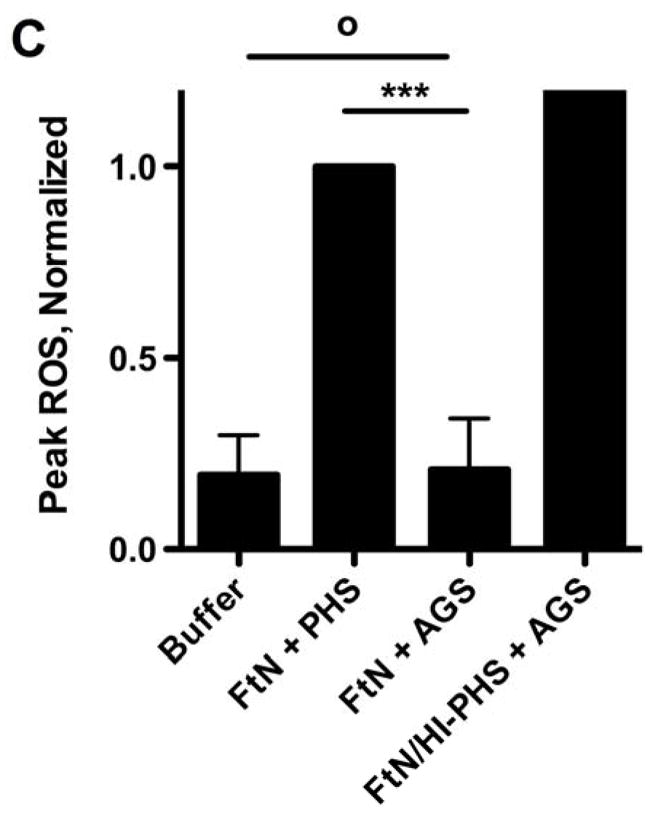
The PMN LCL response to FtN required antibody-mediated C3 deposition. (A) Opsonized bacteria were incubated with human PMN at an MOI of 50:1 for 60 min. LCL was measured as in Fig. 1A. This result is representative of three independent experiments. (B) Peak ROS was determined for each sample in the three experiments represented by Fig. 5A and normalized to the peak FtN. Results are expressed as the mean ± SD. (C) FtN were opsonized in AGS or PHS as described in Fig. 3B prior to incubation with PMN, and peak LCL was determined. Uninfected PMN were incubated in identical buffer without bacteria. N = 3. Results are expressed as the mean ± SD. °, P > 0.05; **, P < 0.01; ***, P < 0.001.
3.5. Comparison between immune antibody opsonization of LVS and antibody opsonization of FtN
Given the role of antibody in conferring complement fixation on FtN in PHS, we hypothesized that LVS incubated in human tularemia immune serum would fix increased quantities of C3 and stimulate uptake and ROS production. To test this hypothesis, we incubated LVS bacteria in a sub-agglutinating concentration of tularemia immune serum prior to incubation in 50% PHS as a source of intact complement (LVS-IS). LVS-IS bound similar quantities of IgG as FtN incubated in 50% PHS (Fig. 2B). By contrast, greater quantities of IgM were deposited on LVS-IS than on FtN (Fig. 2C). However, despite the presence of equal or greater quantities of immunoglobulin, LVS-IS did not fix more C3 than LVS exposed to PHS (Fig. 6A). Furthermore, uptake of LVS-IS by PMN was not increased above that of LVS (not shown), and LVS-IS failed to stimulate increased LCL compared to buffer or to LVS that had been incubated in 50% PHS (Fig. 6B). Thus, even when similar or greater quantities of immunoglobulin were bound to LVS as compared to FtN, C3 binding, uptake, and ROS production were not increased.
Fig. 6.
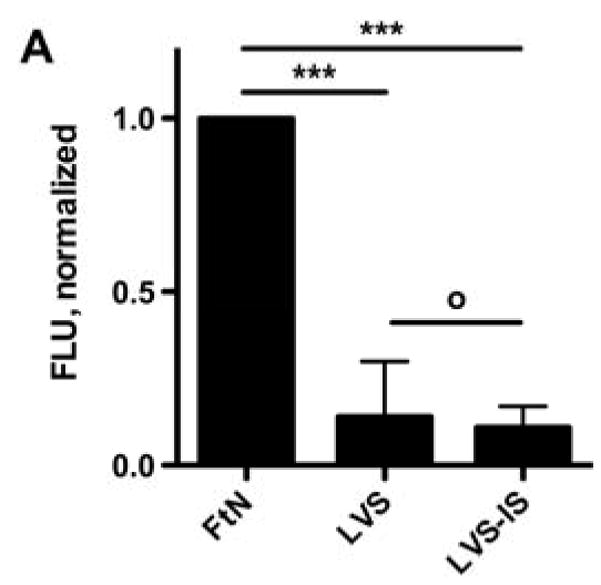
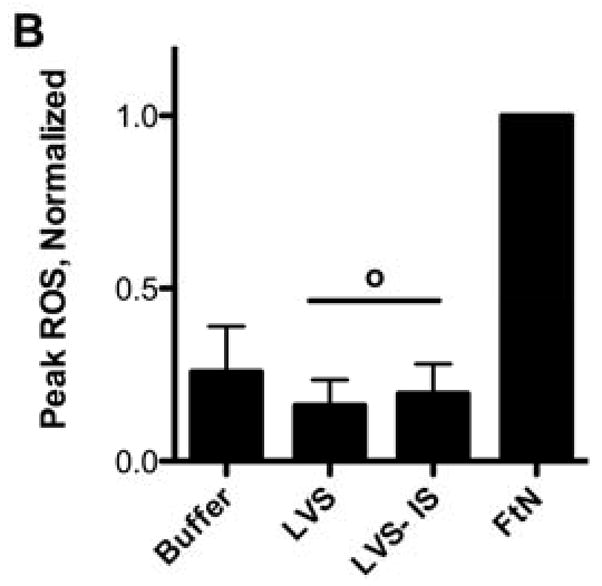
LVS incubated in sub-agglutinating concentrations of tularemia immune serum failed to fix C3 (A) or stimulate PMN LCL (B). LVS-IS had been incubated in a sub-agglutinating concentration of tularemia immune serum, washed, and then incubated in 50% PHS as a source of active complement. C3 binding was determined as described in Fig. 2A. PMN were incubated with PMN and peak LCL determined as described in Fig. 4A. N = 3. °, P > 0.05; ***, P < 0.001.
4. Discussion
Complement is a crucial component of the innate immune system that mediates direct antimicrobial activity, elaboration of inflammatory mediators, and opsonization [19]. Consistent with its remarkable infectiousness and invasiveness, F. tularensis resists the direct antimicrobial activity of human serum and C3 deposition [7, 20]. Our results confirm the observation that LVS fixes relatively small quantities of C3 to its surface, and we extend these observations to show that FtN binds antibody in PHS and that this antibody stimulates C3 deposition and enhances phagocytosis. Furthermore, classical pathway-mediated C3 deposition was indispensible to the ability of FtN to stimulate ROS production by human PMN in vitro.
Our observation that LVS and FtN bound different quantities of antibody after incubation in PHS is consistent with the findings of Balagopal et al., who detected antibodies to FtN in the PHS used in their studies [18]. Furthermore, Owen et al. demonstrated that FtN is antigenically distinct from F. tularensis subsps. tularensis and holarctica using a variety of serologic assays [4]. The basis for this antigenic difference is uncertain, but it is known that the O-antigen of FtN is different from that found in other F. tularensis subspecies [21]. Furthermore, F. tularensis LVS is reported to elaborate a capsule-like material [22], which F. novicida apparently lacks ([5] and M. Apicella, unpublished observations), which could potentially shield antigens from recognition by pre-existing antibody. The identities of the antigens on FtN that are recognized by antibodies in PHS are unknown.
Clay et al. recently identified an important role for the O-antigen of the francisellae in resisting C3 deposition and killing by human complement [20]. In contrast to our findings, they did not detect increased quantities of C3 on FtN in comparison to LVS that had been incubated in PHS. Importantly, however, Clay et al. used 10% PHS in their C3 deposition assays, whereas we used 50%, which would have resulted in significantly more antibody deposition on FtN. Consistent with a role for the dose of antibody on the bacterium, we have found that incubation of LVS and FtN in 10% PHS resulted in greatly attenuated differences in C3 deposition between these strains (not shown). Ultimately, the extent to which these antibodies can confer complement deposition in vivo is unknown.
Unlike previous investigators, we did not find that immune serum enhanced the oxidative response of PMN to LVS [23]. In many previous studies of PMN phagocytosis of Francisella using tularemia immune serum, the concentration of serum used during infection was in excess of 2%, and in some instances, as high as 75% [23–25]. Because a diagnostic titer is typically ≥ 1:160 [2], incubation in serum at these concentrations could potentially result in agglutination, depending upon the concentration of bacteria and duration of incubation. Because we chose to focus on the role of complement opsonization in phagocyte responses, we chose the lowest dilution of immune serum at which we did not detect agglutination. In these conditions, although we detected both IgG and IgM on LVS, we observed no enhancement of C3 deposition. By contrast, a similar amount of IgG from PHS was sufficient to enhance complement opsonization on FtN. There are two possible explanations for the distinct consequences of antibody binding by FtN and LVS. First, the pre-existing antibodies to FtN present in human serum likely differ in quantity, specificity, and isotype from antibodies that result from the immune response during infection. Furthermore, the agglutination phenomenon may be a particular feature following the adaptive humoral immune response that complicates in vitro studies of phagocytosis in certain conditions. Second, LVS may possess a relative resistance to antibody-mediated complement deposition. For instance, it is possible that, as is the case for other bacteria [26], capsule impairs C3 deposition on F. tularensis LVS. Although oxidation of surface carbohydrates with periodate (Fig. 1B and C) or fixation with formalin (data not shown) reversed the resistance to C3 deposition, the specific basis for the relative resistance of LVS to complement opsonization remains uncertain. Recently, Ben Nasr et al. detected factor H on the surface of LVS after incubation in serum, which may inactivate the C3 convertase and thus impair C3 deposition [27]. However, whether FtN binds less factor H than LVS is unknown.
Antibody contributes to phagocytosis directly by virtue of its ability to engage Fc receptors on phagocytes, and it also contributes indirectly via its ability to stimulate complement deposition. Our results suggest that this indirect effect of antibody upon the complement cascade is necessary for efficient uptake of FtN and stimulation of the PMN oxidative response. In general, C3 and antibody seem to stimulate phagocytosis in a synergistic manner, with the existing evidence suggesting that C3 deposition is particularly important for uptake by PMN [28]. Indeed, for at least some bacteria, uptake by human PMN correlates closely with the quantity of C3 deposition [8, 9]. Our results suggest that FtN is more susceptible than is LVS to PMN-mediated defense by virtue of its more efficient complement opsonization and uptake, and it is intriguing to speculate that the avirulence of FtN for humans derives in part from the phenomena we have observed. Furthermore, complement activation in vivo may have effects upon other aspects host defense beyond opsonophagocytosis. Early liberation of proinflammatory complement-derived anaphylatoxins C3a and C5a could recruit and activate phagocytes early in the host response. Intriguingly, Hall et al. observed strikingly different populations of infected cells after inhalational infection of mice with FtN, LVS, and the virulent Schu S4 strain [29]. Namely, 24 hours after infection, 27% of cells infected with FtN in the lung were PMN, whereas infected PMN in mice exposed to LVS or Schu S4 were rare. Although the number of PMN infected with LVS and Schu S4 increased at day 3, the relative predominance of PMN among infected cells was maintained in mice infected with FtN. Although it is not clear whether differences in opsonophagocytosis between FtN and LVS would be apparent in mouse serum with mouse PMN, it is possible that at least some of the difference in host cell infection seen by Hall et al. is due to increased complement-mediated opsonization of FtN.
In summary, our data identify a potentially important difference between the host response to two commonly studied strains, FtN and LVS, and suggest that effective opsonization of FtN may contribute to its lack of virulence for humans.
Acknowledgments
This material is based upon work supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. Jason H. Barker is supported by a Veterans Administration Career Development Award. This work was also supported by funds from the National Institutes of Health (P01-AI44642 to W. M. N., J. P. W., and L-A. H. A and R01-AI073835 to L-A. H. A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLendon MK, Apicella MA, Allen LAH. Francisella tularensis: Taxonomy, genetics, and immunopathogenesis of a potential agent of biowarfare. Annu Rev Microbiol. 2006;60:167–185. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O’Toole T, Parker G, Perl TM, Russell PK, Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 3.Lyons CR, Wu TH. Animal models of Francisella tularensis infection. Ann N Y Acad Sci. 2007;1105:238–265. doi: 10.1196/annals.1409.003. [DOI] [PubMed] [Google Scholar]
- 4.Owen CR, Buker EO, Jellison WL, Lackman DB, Bell JF. Comparative studies of Francisella tularensis and Francisella novicida. J Bacteriol. 1964;87:676–683. doi: 10.1128/jb.87.3.676-683.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elkins KL, Cowley SC, Bosio CM. Innate and adaptive immunity to Francisella. Ann N Y Acad Sci. 2007;1105:284–324. doi: 10.1196/annals.1409.014. [DOI] [PubMed] [Google Scholar]
- 6.McCaffrey RL, Allen LAH. Pivotal Advance: Francisella tularensis LVS evades killing by human neutrophils via inhibition of the respiratory burst and phagosome escape. J Leukoc Biol. 2006;80:1224–1230. doi: 10.1189/jlb.0406287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandstrom G, Lofgren S, Tarnvik A. A capsule-deficient mutant of Francisella tularensis LVS exhibits enhanced sensitivity to killing by serum but diminished sensitivity to killing by polymorphonuclear leukocytes. Infect Immun. 1988;56:1194–1202. doi: 10.1128/iai.56.5.1194-1202.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engels W, Endert J, Van Boven CP. A quantitative method for assessing the third complement factor (C3) attached to the surface of opsonized Pseudomonas aeruginosa: interrelationship between C3 fixation, phagocytosis and complement consumption. J Immunol Methods. 1985;81:43–53. doi: 10.1016/0022-1759(85)90120-6. [DOI] [PubMed] [Google Scholar]
- 9.Verbrugh HA, van Dijk WC, van Erne ME, Peters R, Peterson PK, Verhoef J. Quantitation of the third component of human complement attached to the surface of opsonized bacteria: opsonin-deficient sera and phagocytosis-resistant strains. Infect Immun. 1979;26:808–814. doi: 10.1128/iai.26.3.808-814.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown SL, McKinney FT, Klein GC, Jones WL. Evaluation of a safranin-O-stained antigen microagglutination test for Francisella tularensis antibodies. J Clin Microbiol. 1980;11:146–148. doi: 10.1128/jcm.11.2.146-148.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giclas PC. In: Current Protocols in Immunology. Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Struber W, editors. John Wiley and Sons, Inc; Hoboken, New Jersey: 2006. pp. 13.1.1–13.1.5. [Google Scholar]
- 12.Borregaard N, Heiple JM, Simons ER, Clark RA. Subcellular localization of the b-cytochrome component of the human neutrophil microbicidal oxidase: translocation during activation. J Cell Biol. 1983;97:52–61. doi: 10.1083/jcb.97.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clemens DL, Lee BY, Horwitz MA. Francisella tularensis enters macrophages via a novel process involving pseudopod loops. Infect Immun. 2005;73:5892–5902. doi: 10.1128/IAI.73.9.5892-5902.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine DP, Marney SRJ, Colley DG, Sergent JS, Des Prez RM. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972;109:807–809. [PubMed] [Google Scholar]
- 15.Kettritz R, Xu YX, Kerren T, Quass P, Klein JB, Luft FC, Haller H. Extracellular matrix regulates apoptosis in human neutrophils. Kidney Int. 1999;55:562–571. doi: 10.1046/j.1523-1755.1999.00280.x. [DOI] [PubMed] [Google Scholar]
- 16.Allen RC. Phagocytic leukocyte oxygenation activities and chemiluminescence: a kinetic approach to analysis. Methods Enzymol. 1986;133:449–493. doi: 10.1016/0076-6879(86)33085-4. [DOI] [PubMed] [Google Scholar]
- 17.Schulert GS, Allen LAH. Differential infection of mononuclear phagocytes by Francisella tularensis: role of the macrophage mannose receptor. J Leukoc Biol. 2006;80:563–571. doi: 10.1189/jlb.0306219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balagopal A, Macfarlane AS, Mohapatra N, Soni S, Gunn JS, Schlesinger LS. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74:5114–5125. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volanakis JE. In: The Human Complement System in Health and Disease. Volanakis JE, Frank MM, editors. M. Dekker; New York: 1998. pp. 9–32. [Google Scholar]
- 20.Clay CD, Soni S, Gunn JS, Schlesinger LS. Evasion of complement-mediated lysis and complement C3 deposition are regulated by Francisella tularensis lipopolysaccharide O antigen. J Immunol. 2008;181:5568–5578. doi: 10.4049/jimmunol.181.8.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinogradov E, Conlan WJ, Gunn JS, Perry MB. Characterization of the lipopolysaccharide O-antigen of Francisella novicida (U112) Carbohydr Res. 2004;339:649–654. doi: 10.1016/j.carres.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 22.Cherwonogrodzky JW, Knodel MH, Spence MR. Increased encapsulation and virulence of Francisella tularensis live vaccine strain (LVS) by subculturing on synthetic medium. Vaccine. 1994;12:773–775. doi: 10.1016/0264-410x(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 23.Lofgren S, Tarnvik A, Carlsson J. Demonstration of opsonizing antibodies to Francisella tularensis by leukocyte chemiluminescence. Infect Immun. 1980;29:329–334. doi: 10.1128/iai.29.2.329-334.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lofgren S, Tarnvik A, Bloom GD, Sjoberg W. Phagocytosis and killing of Francisella tularensis by human polymorphonuclear leukocytes. Infect Immun. 1983;39:715–720. doi: 10.1128/iai.39.2.715-720.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proctor RA, White JD, Ayala E, Canonico PG. Phagocytosis of Francisella tularensis by Rhesus monkey peripheral leukocytes. Infect Immun. 1975;11:146–151. doi: 10.1128/iai.11.1.146-151.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joiner KA. Complement evasion by bacteria and parasites. Annu Rev Microbiol. 1988;42:201–230. doi: 10.1146/annurev.mi.42.100188.001221. [DOI] [PubMed] [Google Scholar]
- 27.Ben Nasr A, Klimpel GR. Subversion of complement activation at the bacterial surface promotes serum resistance and opsonophagocytosis of Francisella tularensis. J Leukoc Biol. 2008;84:77–85. doi: 10.1189/jlb.0807526. [DOI] [PubMed] [Google Scholar]
- 28.Ehlenberger AG, Nussenzweig V. The role of membrane receptors for C3b and C3d in phagocytosis. J Exp Med. 1977;145:357–371. doi: 10.1084/jem.145.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun. 2008;76:5843–5852. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


