Abstract
Biofilm formation, an important virulence trait of Candida species was measured in 107 Candida isolates from 32 candidemic patients by XTT [2,3-bis (2-methoxy-4nitro-5-sulfo-phenyl)-2H-tetra-zolium-5-carboxanilide] activity and compared to biofilm formation of Candida isolates from oropharyngeal lesions of 19 AIDS patients. Biofilm formation by XTT varied among species and C. albicans; C. lusitaniae and C. krusei produced more biofilm than the other Candida species. C. tropicalis was the most dominant species isolated from blood followed by C. albicans, and other non-albicans species whereas only C. albicans was recovered from oral lesions. Importantly, though Biofilm formation was variable within a species it was stable in sequential isolates during chronic infection. Sequential isolates exhibited identical Karyotype pattern or RAPD patterns unless patients were co-infected with more than one strain. High biofilm formation was associated with slow growth rate but not with adherence. Murine infection studies demonstrated that in mice, degree of biofilm formation was associated with enhanced virulence as mice infected both with no and low biofilm formers survived longer than mice infected with high biofilm formers (p≤0.001). We conclude that biofilm formation is a stable but strain specific characteristic that can greatly vary among C. albicans and non-albicans strains, and plays an important role in persistence of infection.
Keywords: Candida, Biofilm, PFGE, XTT
1. Introduction
Several Candida species are commensals and colonize skin and mucosal surfaces of humans. Critically ill patients and otherwise immunocompromised patients are more prone to develop both superficial and life threatening Candida infections [1]. Candida infections also constitute the most common fungal infection in AIDS patients (HIV) [2]. These patients predominantly develop oropharyngeal candidiasis (OPC), which can lead to malnutrition and interfere with absorption of medication. In countries like India, where HAART is not universally available, OPC is still common in HIV infected individuals. Although C. albicans remains the most commonly isolated yeast in the US, non-albicans species are emerging and other countries report higher numbers among their patient specimens [3]. In that respect a recent report from Candida blood stream isolates at All India Institute of Medical Science (AIIMS), one of the major tertiary care centers in India indicated that the proportion of non-albicans Candida was higher than reported in the US and Europe [4] .
Candida infections are commonly associated with biofilm formation (BF) that can occur both on mucosal surfaces and on plastic surfaces of indwelling devices. These biofilm consist of matrix-enclosed micro-colonies of yeast, hyphae and pseudohyphae, arranged in a complex structure [5, 6]. Biofilm are inherently resistant to antifungal agents including amphotericin B (AMB) and fluconazole (FLU) [6-9], and thus affected devices generally need to be removed [10]. Among clinical Candida strains BF can vary and depends on the Candida species [11]. However, the question if variability in BF is an independent virulence factor that affects outcome of infection is still not fully answered. The objective of this study was to investigate BF in clinical Candida isolates from blood and oral lesions from patients at AIIMS. In addition, BF of individual strains during chronic disease was studied. Last, the contribution of BF to virulence was examined in murine infection models.
2. Materials and Methods
2.1. Candida Isolates and study population
A total of 126 Candida isolates were obtained at various days post diagnosis from blood of 32 candidemic patients, and mucosal swabs of 19 HIV patients with OPC at AIIMS (Table 1). The identification of Candida species was done by germ tube test, chlamydospore formation on cornmeal agar and sugar assimilation test, API20C (bioMerieux, Marcy Etoile, France). Most of the candidemic patients were admitted to the intensive care unit (ICU). All HIV patients were clinic patients. Data on patients were collected according to the AIIMS internal review board regulations. MIC to FLU and AMB were determined for planktonic cells according to CLSI M27A guidelines and their biofilm grown counterparts as previously published protocol [12].
Table 1.
Clinical summary and outcome for individual Candidemia patient
| Pt # | Species | Duration of stay (AIIMS) (days) |
Day of 1st (+) isolate |
No. of isolates |
Days recovered |
Flu MIC μgml−1 |
AF start (day) |
Diagnosis | Outcome |
|---|---|---|---|---|---|---|---|---|---|
| P1 | Ck | 19 | 1 | 5 | 0,4,7,12,14 | 64 | 8 | AP & RF | D |
| P2 | Ct | 28 | 23 | 3 | 0,3,4 | 0.5-1 | None | Ludwig's angina, | D |
| P3 | Ct | 180 | 141 | 3 | 0,2,19 | 32 | 65 | ALL | S |
| P4 | Cpe | 45 | 4 | 4 | 0,10,36,40 | 16 | FLU | Pancreas CA | D |
| P5 | UI | 21 | 10 | 4 | 0,0,3,7 | 4 | 15 | Perforative peritonitis | D |
| P6 | Cp | 168 | 120 | 5 | 0,3,7,10,14 | 8 | 82 | Cerebral atropy | D |
| P7 | Ca | 45 | 41 | 3 | 0,1,1 | 0.5 | 42 | Osteoarthritis | S |
| P8 | Ca | 40 | 31 | 3 | 0,4,5 | 1 | 31 | Fungal sepsis, CHF | D |
| P9 | Ct | 203 | 28 | 13 | 0-85 | 2-8 | 69 | ascending myelitis | S |
| P10 | Clu | 62 | 57 | 6 | 0-18 | 0.5 | None | Fungal sepsis, DIC | D |
| P11 | Ct | 31 | 9 | 3 | 0,1,5 | 1 | 5 | CHD, endocarditis | D |
| P12 | Ca | 37 | 6 | 2 | 0,4 | 0.25 | 6 | AP, RF, sepsis | D |
| P13 | Ca | 50 | 16 | 4 | 0,4,5,5 | 0.25 | 8 | HF, ascitis | S |
| P14 | Ct | 5 | 3 | 2 | 0,2 | 1 | None | DSS | D |
| P15 | Ca,Ct,Cp | 22 | 5 | 3 | 0,0,12 | 0.5-1 | VORI | ALL | S |
| P16 | Cg, Cp | 30 | 17 | 2 | 0,1 | 1 | 16 | AP | D |
| P17 | Ca | 56 | 1 | 3 | 0,5,11 | 0.5 | 8 | Fungal endocarditis | S |
| P18 | Ct | 47 | 15 | 2 | 0,3 | 0.5-1 | 13 | Pelvic abcess, MTB | S |
| P19 | Cp | 60 | 19 | 2 | 0,11 | 8-16 | 1 | RF,candidiasis, | D |
| P20 | Cp | NA | NA | 2 | 0,4 | 4-8 | FLU | sepsis | S |
| P21 | Ca | 47 | 48 | 2 | 0,2 | 1 | None | Gall bladder CA | D |
| P22 | Ct,Cg | 50 | 7 | 3 | 0,0,0 | 0.5-8 | 33 | Polytroma | D |
| P23 | Ca,Ct | 35 | 17 | 3 | 0,1,5 | 0.5-2 | 17 | Billiary peritonitis | D |
| P24 | Ct | NA | NA | 2 | 0,1 | 2 | AMB | MTB | S |
| P25 | Ct | 53 | 22 | 2 | 0,6 | 0.5-1 | 30 | Fungal endocarditis | D |
| P26 | Cg | 79 | 55 | 3 | 0,8,15 | 16 | 51 | Cervix CA | D |
| P27 | Ct | 26 | 8 | 6 | 0,3,7,7,9,18 | 1 | 15 | DSS | D |
| P28 | Cp | 70 | 41 | 3 | 0,4,11 | 0.5 | 26 | Tetraplegia | D |
| P29 | Ct | 35 | 28 | 3 | 0,2,7 | 1 | 28 | CHD | S |
| P30 | Ct | NA | NA | 2 | 0,11 | 0.5 | NA | NA | - |
| P31 | Ct | 24 | 17 | 2 | 0,6 | 2 | 9 | CHD, Pneumonia | D |
| P32 | Ct | 4 | 3 | 2 | 0,4 | 0.5 | 4 | Multiorgan failure, AP | D |
Note: ICU -Intensive care unit, AF- antifungal,, AMB- amphotericin B, FLU- fluconazole, VORI- voriconazole, AP- acute pancreatitis, RF- renal failure, ALL- acute lymphoid leukemia, CA- cancer, CHF- chronic heart failure, DIC- disseminated intravascular coagulation, CHD- chronic heart disease, HF- hepatic failure, DSS- dengue shock syndrome, MTB- mycobacterium tuberculosis, NA- not available, S- survived, D- died
2.2. Determination of Biofilm production
A semi- quantitative measure of BF and viability was detected by XTT [2,3-bis (2-methoxy-4nitro-5-sulfo-phenyl)-2H-tetra-zolium-5-carboxanilide] reduction assay in 107 isolates. In addition biofilm mass was directly measured by the crystal violet assay in 72 isolates as described[13, 14]. C. albicans strain SC5314 was used as a control strain in each experiment. C. albicans BF was compared on three different surfaces, polystyrene, polypropylene (Greiner Bio One) and silicon elastomers (Cardiovascular Instrument Corp) as described above [13]. Briefly, 96 well plates were used for biofilm formation on polystyrene and polypropylene. To observe biofilm on silicon, silicon elastomers were cut of equal size and used to grow biofilm in 96 well plates. Growth conditions for BF on all three surfaces were the same. “High” and “low” biofilm formers (HBF and LBF) were defined as isolates that exhibited XTT activity greater than or less than the geometrical mean (OD of 0.50). No biofilm formers (NBF) are with optical density of < 0.1.
2.3. Genotype analysis by RAPD and PFGE
For RAPD DNA was isolated as described previously [15], and RAPD was done using M13 primer (5′ GAGGGTGGCGGTTCT 3′). For PFGE, chromosomal DNA plugs were prepared using 5×108 Candida cells as described [16]. Chromosomal separation was done on 1% pulse-field-certified agarose gel (SeaKem) in a CHEF DRIII pulse-field electrophoresis system (Bio-Rad) in 0.5 X Tris–boric acid–EDTA at 14 °C. The electrophoresis conditions were a switch-time of (i) 120 s for 24 h (ii) 240s for 36 hr, at 4.5V and an angle of 112° each. Saccharomyces cerevisiae chromosomal DNA was used as size marker. DNA gel was stained with ethidium bromide and photographed after destaining for 15 min.
2.4. Phenotypic characterization of yeast isolates
Growth was measured by absorbance at 490 nm at different time interval after growth in RPMI (pH 7) at 150 rpm, and 37 °C. For adhesion assays 103 Candida cells were added into 96-well polystyrene plates (corning) and incubated in RPMI at 37 °C for 2 hours. Adhesion was determined by cell counts using a Photo Zoom inverted light microscope (Cambridge Instrument, MA). The number of Candida cells attached to the bottom of each well was averaged per 40-power field. The assay was done in triplicates.
2.5. Mice study
In vivo pathogenesis of HBF, LBF and NBF C. albicans isolates was studied in female BALB/c mice (6-12 weeks, body weight 18-20 gm) obtained from NCI (Bethesda, MD). Ten mice per group were injected intra-venous with HBF, LBF and NBF C.albicans (grown for 12h in SDA at 30°C) strains with similar doubling time with 3×106 cells in 100 μl sterile PBS via tail vain [17]. Dilutions of the inoculums (all blastophores) were back plated onto SDA plates to assure that comparable numbers of yeast cells were injected. Fungal burden was obtained d-3 in lung, liver and kidney. For survival 5 mice were observed daily for signs of disease till death. Moribund mice were sacrificed according to standard procedures. Each experiment was repeated once. At death organs were immediately excised and processed for organ fungal burden and histopathology. Formalin fixed organs were stained with periodic acid Schiff stain.
2.6. Statistical analysis
The statistical tests used were X2 and student's t test. Survival differences were analyzed using Kaplan-Meier survival curve, SPSS version 8.0 (SPSS Inc., Chicago IL). P value < 0.05 was considered significant.
3. Results
3.1. Patient characteristics
BF was determined in 126 Candida isolates from 51 patients. Nineteen isolates were cultured from the mucosal lesion of 19 HIV patients with OPC and 107 isolates were grown from the blood of 32 in-patients. Of the 32 patients, 28 were admitted to an ICU, 26 were on mechanical ventilator and exhibited risk factors for BSI including 53 % had indwelling central venous catheters. The mortality was 68 % (Table 1 and 2). Associated risk factors and outcome of the patients did not correlate with extent of BF. Eleven (34%) candidemic patients were on antifungal prophylaxis. In 14 (44%) patients therapy was started after infection. In 3 of these patients infection was cleared and in 11, infection persisted. Thus the majority of patients exhibited no or partial response to anti-fungal therapy (AFT). AFT varied and included both single and combined regimens. The HIV patients with OPC were treated as out patients and thus less sick. The majority of HIV patients exhibited low CD4+ counts but were not on HAART.
Table 2.
Underlying Candidemia and HIV patients associated variables
| Variables | Candidemia Patients (N=32) |
HIV patients (N=19) |
|---|---|---|
| Median age | ± 51 (2m-85 yr) | ± 34 (27-62 yr) |
| Male | 17 | 15 |
| Female | 15 | 4 |
| ICU admission | 27 (84%) | NA |
| Ventilator | 26 (81%) | NA |
| Intubated | 15 (47%) | NA |
| Foleys catheter | 27 (84%) | NA |
| Central venous catheter | 17 (53%) | NA |
| Diabetes | 3 (9%) | NA |
| Cancer patients | 5 (16%) | NA |
| Prior surgery | 13 (41%) | NA |
| Prophylactic antifungal | 11 (34%) | 1 (5%) |
| Organ transplant | 1 (3%) | NA |
| Dialysis | 3 (9%) | NA |
| Premature birth | 1 (3%) | NA |
| Old age (>60 yr) | 11(34%) | NA |
| Median CD4 count | NA | 169 ( 14-774) |
| Antiretroviral therapy | NA | 6 (32%) |
3.2. Species distribution and antifungal susceptibility and molecular typing
Various Candida species were identified with C. tropicalis (Ct, 38%) being the most common species followed by C. albicans (Ca, 21%), C. parapsilosis (Cp, 13%), C. glabrata (Cg, 3%), C. krusei (Ck, 3%), C. pelliculosa (Cpe, 3%), C. lusitaniae (Clu, 3%), mixed infection (MI, 13%), unidentified (UI, 3%). All isolates from oral lesions of HIV, OPC patients were C. albicans except in two patients co-infection of C. albicans with C. tropicalis and C. krusei were documented. For HIV patients all the C. albicans except one were sensitive (MIC 0.125-4 μgml−1) and one C. krusei isolate was resistant (MIC >64 μgml−1) to FLU. All 107 blood isolates were susceptible to AMB (MIC <1 μgml−1), and most to FLU (MIC 0.25-8 μgml−1). Eleven isolates exhibited dose dependence (MIC, 16-32 μgml−1) and included 4 C. pelliculosa, 1 C. parapsilosis and 3 each of C. tropicalis and C. glabrata. As expected all C. krusei isolates were resistant to FLU. The majority of Candida isolates grown as biofilm were resistant to antifungals. Only 17% exhibited dose dependent sensitivity to FLU and 33% were sensitive to AMB.
3.3. Degree of BF in patients was dependent on the Candida species
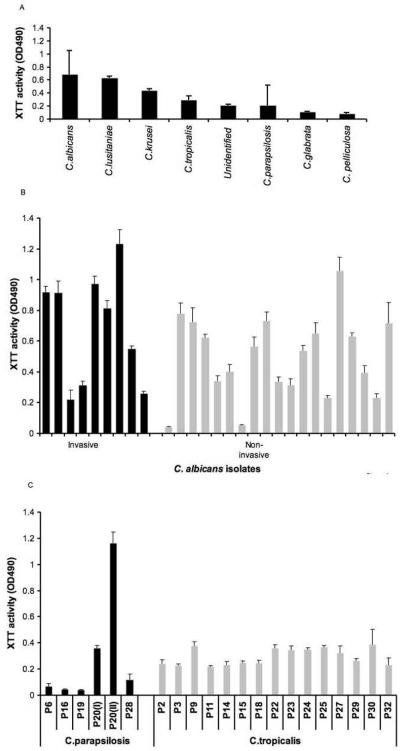
BF in blood Candida isolates differed considerably by XTT method and was dependent on species (Fig. 1a). BF was highest in C. albicans and comparable to C. lusitaniae and C. krusei followed by C. tropicalis C. parapsilosis, one unidentified strain, C. glabrata and C.pelliculosa. C. albicans was the highest biofilm producer, also by crystal violet analysis and results for the two methods were significantly correlated (correlation coefficient, r=0.72, p=0.02), C. glabrata was a low or no biofilm producer by both methods. BF was highly variable among C. albicans strains isolated both from blood and mucosa (Fig. 1b) but did not differ significantly (P=0.11). BF was also variable in C. parapsilosis but comparable among C. tropicalis (Fig. 1c). However for C. tropicalis strains crystal violet analysis exhibited a more variable BF than the XTT method. Four patients were infected with more than one Candida species. In two patients two different Candida species were grown from a single blood sample and in both scenarios C.albicans was the species with higher BF (data not shown).
Fig. 1.
BF is dependent on Candida species. (A) BF is highly variable among clinical Candida species (B) BF was highly variable among C. albicans isolates from both candidemic (invasive-black bar) and oropharyngeal lesion of HIV positive patients (non-invasive-gray bar) (C) Like C. albicans, BF was variable among C. parapsilosis but was relatively stable among C. tropicalis isolates from candidemic patients.
3.4. BF in sequential Candida isolates from same patients
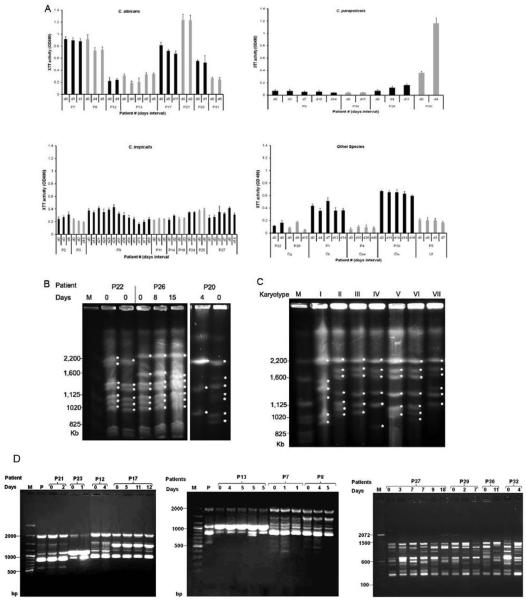
A total of 107 sequential blood isolates were available from 32 patients. Duration between the first and last isolation ranged from 0 to 85 days (Table 1). BF remained stable between most sequential isolates with mean % variability of 11 (range 0.2 to 30%), even in isolates that were recovered 85 days apart (Fig. 2a). In contrast, BF in sequential isolates was more variable (mean variability 56%) if the patient was co-infected with 2 species (P15, P16, P22, P23) or infection with different strains (P20). No significant change in BF in sequential isolates after the initiation of AFT was documented. Hence we conclude BF is stable during chronic infection and both HBF and LBF can co-exist in the same host.
Fig. 2.
A) BF was comparable among serial isolates isolated up to 85 days apart (C. tropicalis-P9). In some cases co-infection with different strains of the same species (C. parapsilosis-P20) resulted in variation of BF whereas others exhibited comparable BF (C. glabrata-P22, P26). Black and gray bar indicates serial isolates from individual patients. (B) Differences in BF of serial C. parapsilosis were associated with karyotype variability and infection with two strains. Serial C. glabrata isolates exhibited in karyotypic variability but no differences in BF unless infection with different strains of same species (P20). (C) Seven different karyotype patterns were found among C. albicans OPC isolates from HIV patients. (D) RAPD profile of sequential isolates by M13 primer are shown for i) C. albicans, P21, P23, P12, P17, P13, P7, P8 and ii) C. tropicalis, P27, P29, P30, P32. Note that this method demonstrated persistence of original strain for C. albicans isolates and more heterogenecity than karyotyping. For C. tropicals RAPD patterns were very heterogenous analogous to C. glabrata.
3.5 Molecular characterization
Genotype characterization of biofilm forming Candida strains by PFGE and/or RAPD demonstrated that the same Candida strain persists during chronic infection in most cases. Only sequential C. tropicalis strains gave variable RAPD patterns and by PFGE did not exhibit enough chromosome bands. Sequential isolates with stable biofilm exhibited identical genotype unless co-infection with two different strain of same species (C. parapsilosis, P20 I & II). Sequential C. parapsilosis isolates with genotype changes exhibited significant differences in their BF, RAPD and PFGE typing, which confirmed that they were distinct strains (Fig. 2b and d(iii)). For C. glabrata, genotypic variability was documented in sequential isolates, all of which produced minimal or no biofilm (Fig. 2b). C. albicans isolates from different patients with identical genotype (RAPD and PFGE) exhibited variable BF. In HIV patients with OPC, 7 different karyotypes were identified in 17 C. albicans isolates (Fig. 2c). The majority of OPC isolates exhibited karyotype III (n=7, 41%), which was also exhibited by all C. albicans isolates from candidemic patients, however RAPD analysis differentiated these strains but showed identical RAPD in sequential isolates. Hence, for most Candida species stability in BF among serial isolates was associated with stability in their Karyotype pattern.
3.6. Association of BF with phenotypic characteristics of Candida
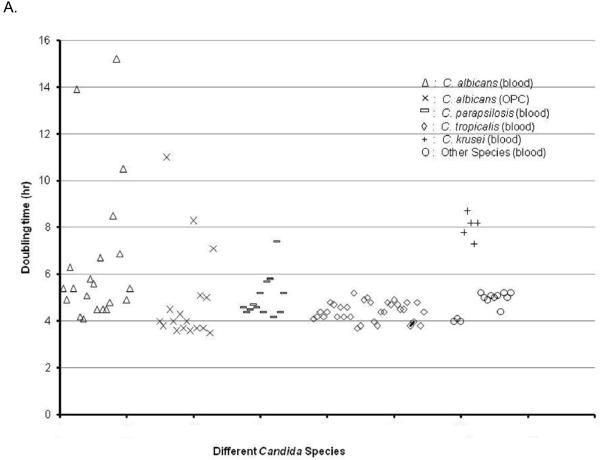
We compared adherence of HBF with LBF Candida strains as well as adherence among different Candida species. No statistical difference in adherence of blastospore after 2 hr was found between HBF vs LBF or between different Candida species. Most Candida strains exhibited comparable growth rates irrespective of species except C. krusei, which all grew slower and some C. albicans strains which were also slow growers (doubling time > 5 hr). Among C. albicans a trend of higher BF was observed in slow growers (0.83 ± 0.24 vs 0.69 ± 0.10, p=0.08) when compared to fast growers. No LBF Candida isolate exhibited a slow growth rate (Fig. 3). Hyphae formation after prolonged culture in RPMI was more extensive in HBF in comparison to LBF. NBF have no hyphae formation and have the least adherence to the surface (OD < 0.1). In addition, we observed that “slow growers” and HBF strains after 24hrs at 37°C produced more filaments in comparison to fast growers, but there was no association with adherence of their blastospores, which was more pronounced in LBF strains. We also compared BF on three different plastic surfaces and determined that BF was the most pronounced on polypropylene followed by polystyrene and silicon (data not shown).
Fig. 3.
Variability in BF was associated with variability in growth. Doubling time for most Candida strains were comparable. Among C. albicans strains slow growers were found.
3.7. Correlation of BF with virulence
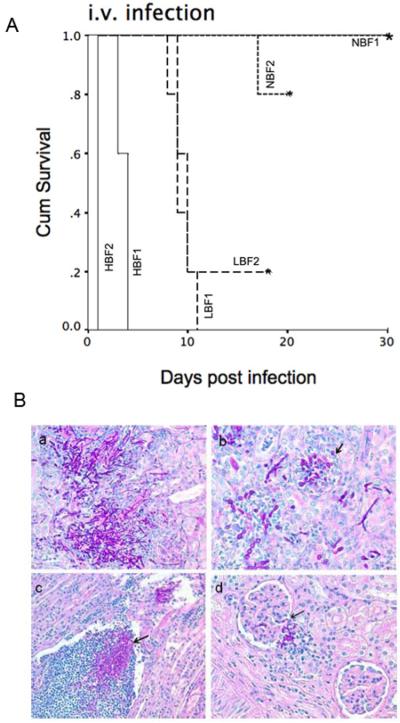
A retrospective chart review determined that in this patient cohort no patient characteristic or potential risk factor was associated with BF or mortality however mortality was too high to draw conclusion. Hence, we examined the virulence of C. albicans strains that differ in BF in a murine infection model. For these studies we selected two C.albicans strains each for HBF, LBF and NBF with similar growth rate and compared their virulence in an intra-venous murine infection model. We selected strains which were identified as HBF, NBF and LBF on all three plastic surfaces. In mice infected i.v. the median survival time differed significantly (p<0.001 by log rank) and correlated with BF (median time of survival was 1 and 4 days vs 10 and 10.8 days vs 20 and > 40 days, p< 0.001) for 2 HBF, 2 LBF and 2 NBF strains respectively (Fig. 3a). Fungal burden in kidney, liver and lung at the time of death of HBF infected mice was detected in all three organs (log 5.84 ± 0.10, log 3.87± 0.16 and log 2.78 ± 0.36, respectively), whereas LBF infected mice at the time of death had log 6 ± 0.66 CFU in the kidney but no yeast was detectable in liver and lung. NBF infected mice had no detectable CFU at day 7. Histopathological data demonstrated massive accumulation of yeast and hyphal elements in cortex, medulla and the papilla associated with degraded glomerulus and renal tubules in HBF infected mice. In contrast, kidney of LBF infected mice were showing infiltration of fungal element and leukocytes mainly in the collecting ducts of the papilla, and only few in cortex and medullary region with small inflammatory foci. Renal clearance could be observed in NBF infected mice and the kidney appeared healthy (Fig. 3b). This result strongly supports the conclusion that variability in C. albicans BF plays an important role in host pathogen interaction and their outcome.
4. Discussion
BF is presumed to promote persistence of infection and thus we investigated BF in serial Candida isolates of patients with candidemia and of isolates of patients with OPC in a major medical center in India. Our data suggests that (i) BF depends on species and in C. albicans and C. parapsilosis species is highly variable but less variable in C. tropicalis (ii) Degree of BF is a specific trait of a Candida isolate that is not associated with differences in adherence but with slow growth. (iii) Most importantly this trait is inherited and thus remains stable in sequential isolates. (iv) No association between individual host factors and the capacity for BF in the Candida spp. was observed and both HBF and LBF can co-exist in the same host. (v) In animal murine models degree of BF correlates with virulence, whereas gene expression of biofilm associated genes is more variable.
Candida biofilms may contribute both to the pathogenesis of superficial and systemic candidiasis as they are notoriously resistant to antifungal drugs. Candida species are now the fourth most common cause of nosocomial BSIs [18]. Although C. albicans remains the most common species recovered from blood in Europe and the US [19] emergence of non-albicans species has been documented [20]. In this and in earlier studies C. tropicalis was the predominant species in BSI in India [4].
We investigated BF in Candida isolates of patients with candidemia and OPC. Patients in this study had many predisposing factors for disseminated candidiasis including longer stay in ICU and indwelling medical devices. Patients with OPC were all HIV clinic patients that were infected with C. albicans. The strength of our study is that it is the first to investigate BF in blood serial isolates derived from the same patient, which yields important information on the stability of this virulence-associated trait. Our data demonstrates that BF is dependent on Candida species and generally highest in C. albicans isolates followed by C. lusitaniae and C. krusei. Most studies have found similar differences of BF among the Candida species [11, 21]. Of note is that BF formation in C. glabrata isolates in this study was found to be among the lowest similar to the result of other studies [22], whereas BF in C. glabrata isolates from urine was found to be much higher [13]. This could potentially suggest that certain host niches in some Candida species promote selection of HBFs.
BF among C.albicans varied significantly both by the XTT and crystal violet method and ranged from high to low both in blood and OPC isolates similar to previous studies in urine [13] and blood isolates [23]. One study reported comparable BF in 26 Candida strains derived from OPC [24] and another study demonstrated that Candida strains from BSI produce more biofilm than those from noninvasive infections [21] neither of which was observed in our study. Variability of BF was also found in C. parapsilosis but only by crystal violet in C. tropicalis isolates. The meaning of these findings is unclear and warrants further investigation. We found under direct microscopy that C. albicans strains exhibit differences in ratio of blastospore, hypha and pseudohyphae, which may explain BF variability, rapid death and pronounced tissue invasion. Although the growth rate was similar between isolates we noted that all slow growers were HBF even though some HBF exhibited a normal growth rate. This is consistent with other reports showing that biofilm-associated cells that are embedded in the deeper layers, show suppressed growth rates, thereby being more resistant to antifungal drugs [25].
Because of extensive co-morbidity candidemia was often persistent despite appropriate AFT and thus several serial isolates could be isolated. As previously reported most biofilms exhibited enhanced resistance to AFT. BF was stable in serial isolates from individual patients even after the initiation of AFT. Identical genotypes were demonstrated in most serial isolates except C. glabrata, C. tropicalis isolates and in one patient with C. parapsilosis infection, which supports conclusions of other studies that the same strain usually persists during chronic infection [26]. We demonstrate that significant differences in BF of two serial isolates are the result of co-infection with more than one species. This finding also demonstrates that both LBF and HBF can co-exist in the same niche and we hypothesize for C. glabrata the host may select for HBF in certain niches (urine and vagina) [13, 27] and LBF in blood [28].
Candidemia ranks among the infections with highest mortality rates [1, 29]. The impact of BF in Candida strains on mortality of candidemic patients concluded based on retrospective multivariate analysis that high BF is associated with poor outcome [11]. This conclusion has not yet been verified in a prospective study. Outcome of candidemia is affected by many factors and the mortality in our patient population was too high to draw such conclusions. However in a murine infection model we demonstrated that BF affects outcome. Infection with 6 different C. albicans HBF, LBF or NBF strains with comparable growth rates demonstrated that decreased survival correlated with degree of BF. Histological analysis suggested that despite comparable fungal burden in the kidney, HBF results in deeper tissue invasion as many fungal cells invade the glomerular capsule, leading to degraded glomeruli and also renal tubules in HBF infected mice. Of note is that is conceivable that the rapid mortality could be the result of more pronounced hyphae formation in HBF strains, which may promote BF and possibly shock. In contrast, LBF infected mice presented with a less invasive infection that was predominantly limited to the papilla and did not result in tissue destruction.
In summary, we conclude that BF represents a phenotypic characteristic of a Candida strain that is inherited and thus stable during chronic infection. BF is more variable in some species especially C. albicans and C. parapsilosis. Our data from murine infection models greatly supports the notion that enhanced BF could potentially be an independent risk factor for prolonged disease and bad outcome. Prospective patient studies may be warranted in addition to studies that investigate the regulatory pathways that control BF and hyphae transition to identify more virulent C. albicans strains and optimize treatment regimens of disseminated candidiasis.
Fig. 4.
(A) Survival of BALB/c mice (n=5 per group) after intravenous challenge with 3×106 blastospore of high, low and no biofilm formers (HBF, LBF, NBF) C. albicans differs significantly (p<0.001, log rank) (B) Histopathological examination of kidney. (a) HBF; massive accumulation of yeast cells, hyphal element and few leukocytes in calyx (b) HBF; kidney cortex showing infiltration of yeast and hyphal elements and degraded glomerulus. (c) LBF; papilla showing leukocytosis and fungal infiltration in collecting duct (d) LBF; little fungal infiltration in cortex and showing intact gromerulus. (Periodic acid Schiff stain was used).
Acknowledgement
We thank Dr. Bitzer for help with the histological analysis. This work was support by grant AI059681-05 to B.C.F., pilot funds from CFAR grant (AI 051519) and by the AIDS International Training and Research Program (program director, Vinayaka Prasad [NID D43-TW001403]) to I.X. and F.H. Furthermore; F.H. was supported by ICMR (3/1/59/MPD/2003-JRF).
Abbreviations
- BF
biofilm
- HBF
high biofilm former
- NBF
no biofilm former
- LBF
low biofilm former
References
- 1.Harbarth S, Ferriere K, Hugonnet S, Ricou B, Suter P, Pittet D. Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch Surg. 2002;137:1353–1359. doi: 10.1001/archsurg.137.12.1353. discussion 1359. [DOI] [PubMed] [Google Scholar]
- 2.Fidel PL., Jr. Candida-host interactions in HIV disease: relationships in oropharyngeal candidiasis. Adv Dent Res. 2006;19:80–84. doi: 10.1177/154407370601900116. [DOI] [PubMed] [Google Scholar]
- 3.Blot S, Vandijck D, Vandewoude K. Risk factors for Candida non-albicans candidemia. Diagn Microbiol Infect Dis. 2008 doi: 10.1016/j.diagmicrobio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Xess I, Jain N, Hasan F, Mandal P, Banerjee U. Epidemiology of candidemia in a tertiary care centre of north India: 5-year study. Infection. 2007;35:256–259. doi: 10.1007/s15010-007-6144-6. [DOI] [PubMed] [Google Scholar]
- 5.Kuhn DM, Chandra J, Mukherjee PK, Ghannoum MA. Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immun. 2002;70:878–888. doi: 10.1128/iai.70.2.878-888.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J Bacteriol. 2001;183:5385–5394. doi: 10.1128/JB.183.18.5385-5394.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Ribot JL. Candida albicans biofilms: more than filamentation. Curr Biol. 2005;15:R453–455. doi: 10.1016/j.cub.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Ramage G, VandeWalle K, Bachmann SP, Wickes BL, Lopez-Ribot JL. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002;46:3634–3636. doi: 10.1128/AAC.46.11.3634-3636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmann SP, Ramage G, VandeWalle K, Patterson TF, Wickes BL, Lopez-Ribot JL. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrob Agents Chemother. 2003;47:3657–3659. doi: 10.1128/AAC.47.11.3657-3659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rex JH, Walsh TJ, Sobel JD, Filler SG, Pappas PG, Dismukes WE, Edwards JE. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:662–678. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 11.Tumbarello M, Posteraro B, Trecarichi EM, Fiori B, Rossi M, Porta R, de Gaetano Donati K, La Sorda M, Spanu T, Fadda G, Cauda R, Sanguinetti M. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J Clin Microbiol. 2007;45:1843–1850. doi: 10.1128/JCM.00131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramage G, Vande Walle K, Wickes BL, Lopez-Ribot JL. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother. 2001;45:2475–2479. doi: 10.1128/AAC.45.9.2475-2479.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jain N, Kohli R, Cook E, Gialanella P, Chang T, Fries BC. Biofilm formation by and antifungal susceptibility of Candida isolates from urine. Appl Environ Microbiol. 2007;73:1697–1703. doi: 10.1128/AEM.02439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Yan Z, Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003;149:353–362. doi: 10.1099/mic.0.25932-0. [DOI] [PubMed] [Google Scholar]
- 15.Meyer W, Lieckfeldt E, Kuhls K, Freedman EZ, Borner T, Mitchell TG. DNA- and PCR-fingerprinting in fungi. Exs. 1993;67:311–320. doi: 10.1007/978-3-0348-8583-6_28. [DOI] [PubMed] [Google Scholar]
- 16.Jain N, Wickes BL, Keller SM, Fu J, Casadevall A, Jain P, Ragan MA, Banerjee U, Fries BC. Molecular epidemiology of clinical Cryptococcus neoformans strains from India. J Clin Microbiol. 2005;43:5733–5742. doi: 10.1128/JCM.43.11.5733-5742.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krueger KE, Ghosh AK, Krom BP, Cihlar RL. Deletion of the NOT4 gene impairs hyphal development and pathogenicity in Candida albicans. Microbiology. 2004;150:229–240. doi: 10.1099/mic.0.26792-0. [DOI] [PubMed] [Google Scholar]
- 18.Pfaller MA, Diekema DJ, Jones RN, Sader HS, Fluit AC, Hollis RJ, Messer SA. International surveillance of bloodstream infections due to Candida species: frequency of occurrence and in vitro susceptibilities to fluconazole, ravuconazole, and voriconazole of isolates collected from 1997 through 1999 in the SENTRY antimicrobial surveillance program. J Clin Microbiol. 2001;39:3254–3259. doi: 10.1128/JCM.39.9.3254-3259.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almirante B, Rodriguez D, Park BJ, Cuenca-Estrella M, Planes AM, Almela M, Mensa J, Sanchez F, Ayats J, Gimenez M, Saballs P, Fridkin SK, Morgan J, Rodriguez-Tudela JL, Warnock DW, Pahissa A. Epidemiology and predictors of mortality in cases of Candida bloodstream infection: results from population-based surveillance, barcelona, Spain, from 2002 to 2003. J Clin Microbiol. 2005;43:1829–1835. doi: 10.1128/JCM.43.4.1829-1835.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Samonis G, Kofteridis DP, Saloustros E, Giannopoulou KP, Ntziora F, Christidou A, Maraki S, Falagas ME. Candida albicans versus non-albicans bloodstream infection in patients in a tertiary hospital: An analysis of microbiological data. Scand J Infect Dis. 2008;40:414–419. doi: 10.1080/00365540701765657. [DOI] [PubMed] [Google Scholar]
- 21.Shin JH, Kee SJ, Shin MG, Kim SH, Shin DH, Lee SK, Suh SP, Ryang DW. Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J Clin Microbiol. 2002;40:1244–1248. doi: 10.1128/JCM.40.4.1244-1248.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawser SP, Douglas LJ. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun. 1994;62:915–921. doi: 10.1128/iai.62.3.915-921.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumbarello M, Bevilacqua N, Federico G, Morace G, Cauda R, Tacconelli E. Fluconazole-resistant Candida parapsilosis fungemia in a patient with AIDS. Clin Infect Dis. 1996;22:179–180. doi: 10.1093/clinids/22.1.179. [DOI] [PubMed] [Google Scholar]
- 24.Jin Y, Samaranayake LP, Samaranayake Y, Yip HK. Biofilm formation of Candida albicans is variably affected by saliva and dietary sugars. Arch Oral Biol. 2004;49:789–798. doi: 10.1016/j.archoralbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Baillie GS, Douglas LJ. Effect of growth rate on resistance of Candida albicans biofilms to antifungal agents. Antimicrob Agents Chemother. 1998;42:1900–1905. doi: 10.1128/aac.42.8.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shin JH, Park MR, Song JW, Shin DH, Jung SI, Cho D, Kee SJ, Shin MG, Suh SP, Ryang DW. Microevolution of Candida albicans strains during catheter-related candidemia. J Clin Microbiol. 2004;42:4025–4031. doi: 10.1128/JCM.42.9.4025-4031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray D, Goswami R, Banerjee U, Dadhwal V, Goswami D, Mandal P, Sreenivas V, Kochupillai N. Prevalence of Candida glabrata and its response to boric acid vaginal suppositories in comparison with oral fluconazole in patients with diabetes and vulvovaginal candidiasis. Diabetes Care. 2007;30:312–317. doi: 10.2337/dc06-1469. [DOI] [PubMed] [Google Scholar]
- 28.Nikawa H, Nishimura H, Makihira S, Hamada T, Sadamori S, Samaranayake LP. Effect of serum concentration on Candida biofilm formation on acrylic surfaces. Mycoses. 2000;43:139–143. doi: 10.1046/j.1439-0507.2000.00564.x. [DOI] [PubMed] [Google Scholar]
- 29.Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–1177. doi: 10.1086/378745. [DOI] [PubMed] [Google Scholar]