Abstract
We describe clinical, morphologic, and immunohistochemical features of 21 cases of solitary fibrous tumor presenting in the oral cavity. There were 9 male and 12 female patients with a median age of 51 years (range 37–83). The most common locations included the buccal mucosa (the most common site), lip, maxillary or mandibular vestibule and tongue. Histopathologic examination showed well-circumscribed tumors with two well-defined patterns: the classic pattern with densely cellular areas alternating with hypocellular areas in a variably collagenous, vascular stroma and a more uniformly sclerotic pattern with only subtle classic areas. The spindle-shaped neoplastic cells consistently showed immunoreactivity for antibodies directed against CD34. Five of nineteen cases (26%) were reactive for CD99 and 19 of 19 for Bcl-2. Follow-up information was available in 17 cases and averaged 54 months, with no evidence of recurrence or metastasis in any of these patients. Awareness that solitary fibrous tumor may present in the oral cavity is important so that confusion with other spindle cell neoplasms can be avoided. We also briefly describe the differential diagnosis and compare this series, the largest single series of intraoral SFT, to cases previously reported in the literature.
Keywords: Solitary fibrous tumor, Oral cavity, CD34, Differential diagnosis
Introduction
In 1931, Klemperer and Rabin [1] described a series of localized solitary pleural tumors arising from the subpleural areolar tissue, and this report is thought to be the first description of what we now refer to as the solitary fibrous tumor (SFT). Since 1931, this entity has been referred to as fibrous mesothelioma, subpleural fibroma and localized fibrous tumor of the pleura. The histogenesis of the SFT has been controversial, with evidence supporting a mesothelial origin being presented by Stout and Murray [2], while an undifferentiated mesenchymal cell histogenesis was preferred by England et al. [3].
In support of a mesenchymal origin, spindle cell tumors bearing a close resemblance to this localized pleural tumor have been described in a variety of anatomic sites that are not lined by mesothelium [4]. These include the orbit [5], nasal cavity [6] salivary glands [7] and meninges [8]. SFT is now the term most widely used to designate these tumors. After revision of its pleural tumor classification by the World Health Organization (WHO) in 1999, SFT was finally classified as an independent entity and was excluded from the mesothelioma subgroups [9]. In the mid-1990s, the diagnosis of hemangiopericytoma (HPC) was called into question [10] [11], and in 2002, the WHO acknowledged that the majority of tumors formerly diagnosed as HPC could be reclassified as any number of other soft tissue tumors including SFTs. Many pathologists now believe that the diagnosis of HPC should be used only for truly pericytic lesions, such as the sinonasal HPC [12].
The histological spectrum of SFT is broad, with appearances often varying from field-to-field within one tumor, thus contributing to diagnostic difficulties. Chan [4] suggested several diagnostic criteria for SFT, namely:
Circumscription
Alternating hypercellular foci and hypocellular sclerotic foci
Short spindly or ovoid cells with scanty and poorly defined cytoplasm
Few mitotic figures (<4/10 HPF)
Intimate intertwining of thin or thick collagen fibrils with spindle cells
CD34 positivity of spindled cells
The aim of this study is to report 21 new cases of SFT of the oral cavity, to evaluate the diagnostic features of these tumors, and to correlate these features with immunohistochemical findings.
Materials and methods
Twenty-one patients with oral and pharyngeal SFTs were identified from the databases of the Division of Oral and Maxillofacial Pathology, Harvard School of Dental Medicine through Pathology Services Inc, Cambridge, MA (N = 4), the Department of Pathology, Brigham and Women’s Hospital, MA, Boston (N = 4), Pacific Oral and Maxillofacial Pathology Laboratory, San Francisco, CA, Oral Pathology Diagnostic Services, San Diego, CA (N = 8), Oral and Maxillofacial Pathology Biopsy Service of the Ohio State University, Columbus, Ohio (N = 4) and Division of Pathology, Texas A&M Health Science Center, Baylor College of Dentistry, Dallas, Texas (N = 1). For each case, clinical information was obtained from accession forms and follow-up was obtained from contributors. Review of all cases was carried out by Drs. S·B Woo (Department of Pathology, Brigham and Womens Hospital, Boston, MA) and CD Fletcher (Department of Pathology, Brigham and Women’s Hospital, Boston, MA) and Dr. Esther O'Regan, Visiting Scholar, Brigham and Womens Hospital, Boston, MA. The diagnosis was confirmed by both microscopic features on hematoxylin-eosin staining and by immunohistochemical analysis. Three original cases were deemed to represent other entities and were excluded from the study. Representative sections of the cases were studied immunohistochemically with antibodies to CD34, CD99, bcl-2, EMA, S-100 protein, smooth muscle actin and AE1/AE3 using the avidin-biotin-peroxidase complex method with appropriate controls (Table 1).
Table 1.
Antibodies used for immunohistochemical studies
| Antibody | Clone | Source | Pre-treatment | |
|---|---|---|---|---|
| CD34 | Mouse anti-human IgG1/κ | QBEnd 10 | Dako, Carpinteria, California | None |
| CD99 | Mouse anti-human IgG1 | O13 | Signet, Dedham, Massachusetts | PC |
| Bcl-2 | Mouse anti-human IgG1/κ | 124 | Dako | PC |
| Epithelial membrane antigen (EMA) | Mouse anti-human IgG2a/κ | E29 | Dako | None |
| α-smooth muscle actin (SMA) | Mouse anti-human IgG2a | 1A4 | Sigma, St. Louis, Missouri | None |
| S-100 protein | Rabbit anti-human Ig | Polyclonal | Dako | None |
| Cytokeratin | Mouse anti-human IgG1/κ | AE1/AE3 | Dako | Pr |
PC pressure cooker antigen retrieval in 10 mM citrate buffer pH6.0, Pr digestion with 0.1% protease from Bacillus licheniformus (10 min at 37°C)
Results
Clinical Findings
The clinical features of the 21 patients are summarized in Table 2. There were 12 men and 9 women, with a median age of 51 years (range 37–83). The most common sites were buccal mucosa (38%), vestibule (19%), lip (14%), tongue (9%), gingiva/alveolar mucosa (9%) and one each in the pharynx and infra-temporal fossa. The tumors showed a predilection for the right side in 17 of 21 cases (81%). All tumors were surgically excised. The tumor was completely excised in only three of the cases. In the other 18 cases, the margin status was positive. Follow-up data was available in 17 cases, and the period of follow-up ranged from 3 months to 144 months (mean 54 months, median 48 months). At time of follow-up, all 17 patients were alive with no evidence of disease.
Table 2.
Clinical features of 21 patients with oral SFT
| Case Number | Age (yrs) | Sex | Site | Laterality | Size (cm) | Follow-up |
|---|---|---|---|---|---|---|
| 1 | 83 | F | Vestibule | R | 1.2 | LTF |
| 2 | 57 | M | Tongue | R | 2.3 | NED at 36 months |
| 3 | 47 | M | Pharynx | R | 5.4 | NED at 3 months |
| 4 | 51 | F | Buccal mucosa | R | 1.5 | LTF |
| 5 | 60 | M | Lip | R | 2.5 | NED at 84 months |
| 6 | 69 | M | Vestibule | R | 0.9 | LTF |
| 7 | 46 | M | Vestibule | R | 4.0 | NED at 21 months |
| 8 | 58 | M | Alveolar mucosa | R | 1.0 | NED at 58 months |
| 9 | 51 | F | Buccal mucosa | L | 1.5 | NED at 9 months |
| 10 | 43 | F | Alveolar mucosa | R | 2.0 | NED at 78 months |
| 11 | 57 | M | Buccal mucosa | R | 2.1 | NED at 62 months |
| 12 | 43 | F | Buccal mucosa | R | 2.0 | NED at 48 months |
| 13 | 74 | M | Vestibule | R | 2.5 | NED at 102 months |
| 14 | 43 | F | Buccal mucosa | R | 0.8 | NED at 90 months |
| 15 | 47 | F | Tongue | L | 1.0 | NED at 57 months |
| 16 | 46 | F | Buccal mucosa | R | 2.5 | NED at 30 months |
| 17 | 54 | M | Lip | R | 0.7 | NED at 27 months |
| 18 | 48 | M | Lip | L | 1.5 | LTF |
| 19 | 64 | M | Infratemporal fossaa | L | 3.5 | NED at 39 months |
| 20 | 44 | M | Buccal mucosa | R | 1.8 | NED at 28 months |
| 21 | 37 | F | Buccal mucosa | R | 1.0 | NED at 144 months |
LTF lost to follow up, NED No evidence of disease, R right, L Left, N/A not available
aThis mass protruded slightly into the maxillary vestibule, but the bulk of it was in the infratemporal fossa
Histopathologic Findings
Grossly, the tumors ranged from 0.7 cm to 5.4 cm with 86% (18/21) of tumors 2.5 cm or less in greatest dimension.
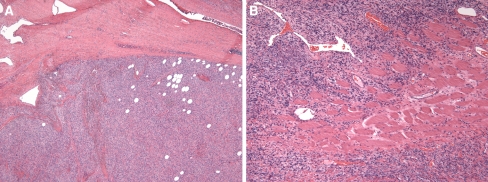
The microscopic findings are presented in Table 3. All tumors exhibited circumscription and two were partially or completely encapsulated (Case 1 and 3, respectively) (Fig. 1a). Skeletal muscle trapping at the periphery was evident in two cases, and in one of those cases, muscle was seen deep within the substance of the tumor, and not just at the periphery (Fig. 1b). All cases were characterized by a proliferation of spindled cells in a variably vascular and collagenized stroma.
Table 3.
Histopathologic features of twenty-one patients with intraoral SFT
| Case number | Circumscribed | Pattern | Stroma | Mitoses | Other features | |
|---|---|---|---|---|---|---|
| Y/N | Classic/Classic cellular/Sclerotic | Myxoid | Hyalinized | Number per 10 hpf | Giant cells/Fat/Mast cells/Lymphocytes | |
| 1 | Ya | CC | − | + | 1 | – |
| 2 | Y | C | + | − | 0 | G/L |
| 3 | Ya | C | + | + | 0 | G/F/M/L |
| 4 | Y | S | + | + | 0 | M/L |
| 5 | Y | S | + | + | 0 | G/L |
| 6 | Y | S | − | + | 0 | G/F/L |
| 7 | Y | CC | − | − | 0 | L |
| 8 | Y | S | + | + | 0 | M/L |
| 9 | Y | C | − | − | 0 | G/M/L |
| 10 | Y | S | − | + | 0 | M/L |
| 11 | Y | C | + | − | 2 | G/F/L |
| 12 | Y | C | + | + | 0 | L |
| 13 | Y | S | + | + | 4 | L |
| 14 | Y | S | − | + | 0 | F/L |
| 15 | Y | CC | − | + | 0 | L |
| 16 | Y | C | + | + | 2 | L |
| 17 | Y | C | + | + | 0 | G/L |
| 18 | Y | C | + | + | 0 | L |
| 19 | Y | S | − | + | 0 | L |
| 20 | Y | Cb | + | − | 0 | L |
| 21 | Y | C | + | + | 2 | G/L |
C Classic, CC Classic cellular, S Sclerotic, G Giant cells, F Fat, M Mast cells, L Lymphocytes
aFull or partial capsule
bClassic pattern with sharp demarcation of hyper- and hypocellular myxoid areas; this unusual case is further described in the results section
Fig. 1.
a SFT with typical well-circumscribed appearance. Also shown here is fat entrapped within the tumor b Muscle entrapment is a feature seen in SFTs
Pattern
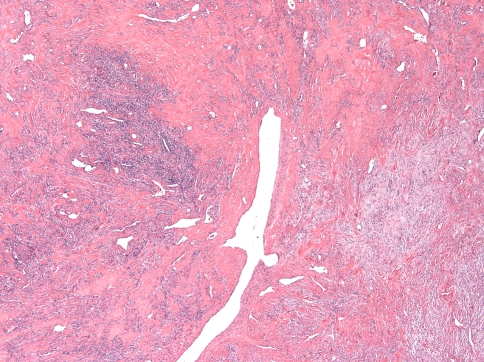
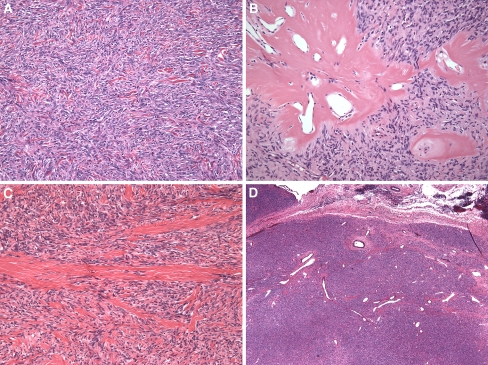
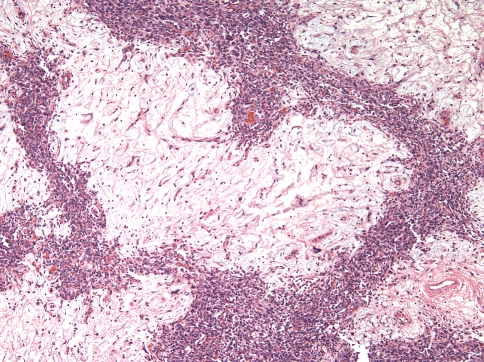
Two patterns were discerned. In the interest of not continuing to perpetuate the term HPC for these lesions, what used to be described as “HPC-like” will be referred to here as “classic pattern” The first and more common (13 of 21 cases, 62%) was the “classic SFT” pattern, defined by the consistent presence of numerous variably ectatic thin-walled vessels, sometimes with a staghorn configuration, in a background of a cellular spindle cell proliferation with hyper- and hypo-cellular areas (Fig. 2). The spindled cells often formed whorls around small capillaries in a vaguely storiform pattern in hypercellular areas (Fig. 3a). Delicately collagenized hypocellular and myxoid areas were often seen around larger dilated vessels that occasionally exhibited perivascular hyalinization that often spread beyond the confines of the perivascular spaces (Case 12, 16) (Fig. 3b). In some areas, the collagen fascicles were longer and streaming. Hyalinized collagen in the form of “ropey collagen” or collagen nodules was seen (Fig. 3c). The term amianthoid fibers have also been used to describe this feature [4, 13]. Three of the thirteen cases had a predominantly hypercellular pattern (Cases 1, 7 and 15), and in these cases the hypocellular areas were composed of sclerotic collagen, rather than the looser, delicate collagen or myxoid areas seen in the other ten tumors (Fig. 3d). Myxoid zones dominated in one case (Case 18) (Fig. 4).
Fig. 2.
Typical alternations of hypercellular and hypocellular areas in a sclerotic background
Fig. 3.
a Characteristic whorls found in the more hypercellular areas b Dense perivascular hyalinized material found in many of the classic cases c Ropey collagen, a consistent feature of the classic pattern d Typical cellular appearance seen in three of the thirteen classic cases
Fig. 4.
Case 18 showed a predominantly myxoid pattern
Case 20 showed the typical storiform spindle cell proliferation and vasculature but was unusual in that these areas of hypercellularity were sharply demarcated from the hypocellular areas, the latter being uniformly myxoid (Fig. 5).
Fig. 5.
Case 20 is an unusual case, showing sharp demarcation between hypercellular and hypocellular areas
The less common “sclerotic” pattern (8 of the 21 cases, 38%) was one of prominent fibrosis, with only focal hypercellular or myxoid areas (Fig. 6). This pattern was predominantly composed of sweeping fascicles of dense collagen; the classic SFT pattern was noted only focally and staghorn vessels were inconspicuous.
Fig. 6.
Thick eosinophilic collagen bundles typical of the sclerotic appearance of some SFTs
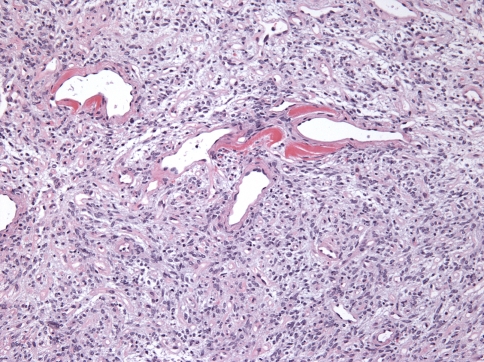
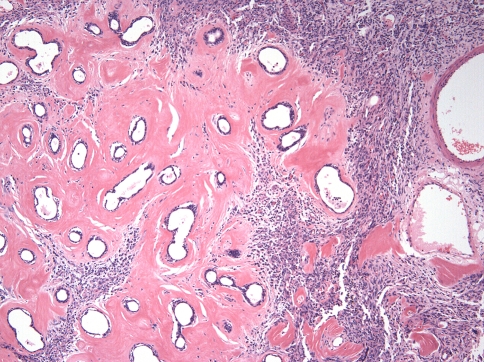
Minor salivary glands were involved in Cases 18 and 21. Only a few remnant acini were noted and there was prominent periductal hyalinization in both cases (Fig. 7).
Fig. 7.
Remnant minor salivary ducts with prominent peri-ductal hyalinization
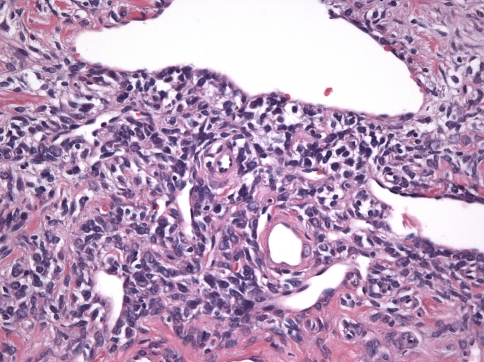
Cytologic Features
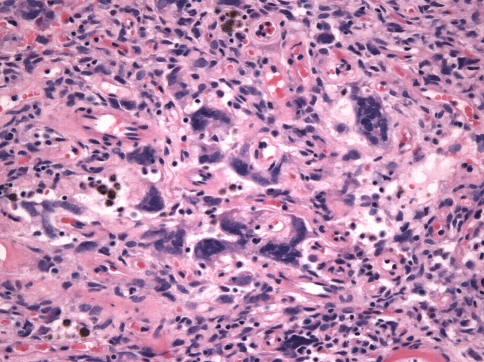
The constituent cells were either spindle-shaped or ovoid, with scant cytoplasm and indistinct cell borders. Nuclei were ovoid, fusiform or even cigar-shaped in some cases and had dispersed chromatin with small to inconspicuous nucleoli (Fig. 8). Giant cells were present in 43% of cases (in 6/13 of tumors with the “classic” pattern and 3/8 of tumors with the “sclerotic” pattern) although they were abundant in only one tumor (Case 17), which exhibited numerous multinucleated giant cells, some with nuclear irregularity and coarse chromatin (Fig. 9). Overall, there were 0–3 mitoses per 10 high power fields. Mast cells were seen in nine cases (43%). Scattered lymphocytes were observed in all but one case, but only one case (Case 11) showed distinct foci of lymphocytes. Mature lipocytes within the tumor were seen in four cases (19%).
Fig. 8.
High power view showing the cytological features of SFT
Fig. 9.
Multi-nucleated giant cells were seen in almost half of the cases
Immunohistochemistry
The results of the immunohistochemical reactions are reported in Table 4. In all but one case CD34 positivity within the cytoplasm was at the 3+ level of intensity. All cases were also positive for bcl-2. Five cases (24%) were immunopositive for CD99, at an intensity of 2+ or less. No immunoreactivity was seen with antibodies directed against AE1/AE3, EMA, SMA and S100.
Table 4.
Immunohistochemistry results
| Case number | CD34 | CD99 | Bcl-2 | EMA | SMA | S-100 protein | AE1/AE3 keratin |
|---|---|---|---|---|---|---|---|
| 1 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 2 | 3+ | N/A | N/A | N/A | neg | neg | neg |
| 3 | 3+ | N/A | N/A | N/A | neg | neg | neg |
| 4 | 1+ | neg | 1+ | neg | neg | neg | neg |
| 5 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 6 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 7 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 8 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 9 | 3+ | 1+ | 3+ | neg | neg | neg | neg |
| 10 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 11 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 12 | 3+ | 1+ | 3+ | neg | neg | neg | neg |
| 13 | 3+ | 1+ | 3+ | neg | neg | neg | neg |
| 14 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 15 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 16 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 17 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 18 | 3+ | 1+ | 3+ | neg | neg | neg | neg |
| 19 | 3+ | neg | 3+ | neg | neg | neg | neg |
| 20 | 3+ | 2+ | 3+ | neg | neg | neg | neg |
| 21 | 3+ | neg | 3+ | neg | neg | neg | neg |
Discussion
The current consensus is that SFT is a mesenchymal neoplasm of fibroblastic and not mesothelial origin [12, 14, 15]. The non-pleural tumors that resembled HPC as described by Stout [2] are now mostly believed to represent extra-pleural SFTs and many have abandoned HPC as a diagnostic term in favor of the term SFT [12]. There is a residual group of tumors that currently retain the diagnosis of HPC and these include sinonasal HPC, which demonstrates cells with true pericytic properties [16]. This group of tumors of pericytic origin in future will likely be reclassified as myopericytomas [6, 12].
A review of the literature revealed 58 cases of SFT in the oral cavity to date, the vast majority of which have been reported as single cases [17–22, 23–30, 19, 31–40, 41–44]. This series of 21 patients is the largest series of intraoral SFT published to date. Table 5 summarizes some of the findings in the literature and compares them to those of the current study. Intraoral SFT occur with equal sex predilection, over a wide age range with a predilection for the sixth decade, and in a variety of sites [17, 18, 28, 45, 46] Concurring with previous reports, the current study found that the buccal mucosa remained the most frequent intraoral site of occurrence, followed by lip and tongue. This case series showed a marked predilection of oral SFTs for the right side (81%). Of note, Alawi et al. found in their series of intraoral SFTs that 80% of cases occurred on the left side [18]. This side predilection in both case series probably reflects sample size, rather than being a truly significant clinical feature.
Table 5.
Comparison between current series and previously reported cases
| Current series (N = 21) | Previously reported cases (N = 56)a | |
|---|---|---|
| Gender ratio M:F | 12:9 | 24:32 |
| Age in years (range) | Mean 53.4, Median 51 (37–83) | Mean 53.7, Median 54.5 (19–94) |
| Site N (%) | ||
| Buccal mucosa | 8 (38.1) | 33 (58.9) |
| Vestibule | 4 (19.2) | – |
| Lip | 3 (14.3) | 2 (3.6) |
| Tongue | 2 (9.5) | 9 (16.1) |
| Alveolar Mucosa | 2 (9.5) | 3 (5.3) |
| Oropharynx | 1(4.7) | 1 (1.8) |
| Infratemporal fossa | 1 (4.7) | – |
| Retromolar | – | 2 (3.6) |
| Palate | – | 4 (7.1) |
| Floor of mouth | – | 2 (3.6) |
| Mean Size in cm (range) | 1.98 (0.7–5.4) | 2.4 (0.7–7.5) |
aClinical information was unavailable in two of the 58 previously reported cases
While we have no reports of a history of trauma in any of these patients, there have been several previous publications recounting histories of trauma and it has been suggested that trauma may impact the site predilection for SFT within the oral cavity [18, 28]. Alawi et al. addressed this in 2001, referring to the identification by Bucala et al. of a CD34- positive, fibroblast-like cell recruited to sites of trauma and functioning as a tissue repair mediator [18, 47]. Alawi raises the possibility that these cells may have a role in at least propagating these tumors at sites of trauma.
In our series, the most common histopathologic form of SFT was the “classic” pattern that exhibited all the criteria delineated by Chan [4]. Within this pattern, three of our cases were primarily hypercellular, with less conspicuous hypocellular/myxoid changes. The differential diagnoses for this variant include leiomyoma, cellular nerve sheath tumor, myofibroma, nodular fasciitis, a low-grade sarcoma [17] and monophasic synovial sarcoma [48].
In case 20, the hypocellular areas were extremely myxoid and sharply demarcated from the hypercellular spindle cell proliferation, an unusual finding. It is unclear whether this warrants separate classification as a “myxoid” form of SFT. The differential diagnoses of the more myxoid forms of SFT include benign nerve sheath tumors, low-grade myxofibrosarcoma, low-grade liposarcoma, myxoid synovial sarcoma and myxoid spindle cell lipoma [49].
A “sclerotic” pattern of SFT was seen in eight of the 21 cases (38%). In this pattern, there were subtle areas of hypercellularity and myxoid change, with the bulk of the tumor composed of dense collagen with interspersed spindle cells and occasional staghorn vessels. The differential diagnosis for this includes the desmoplastic fibroma, sclerotic fibroma and myofibroma [17].
An interesting observation in two cases in the current study was involvement of minor salivary gland, which has not been previously reported. In both cases, the tumor had a lobular architecture with remnant salivary gland tissue embedded within the substance of the tumor, suggesting the tumor arose within the parenchyma of minor glands. Both cases were classic SFTs and exhibited marked periductal hyalinization. SFT involving the major salivary glands has been reported [7, 50, 51].
Mature adipocytes were seen in four cases (19%) in our series and this has been previously reported [6, 52]. These cases share features with the fat-containing variant of SFT [12, 52]. When adipocytes are present, spindle cell lipoma and myxoid liposarcoma must be considered.
Thirty-eight percent of our cases contained multinucleated giant cells, as has been reported [5] and these were prominent in case 21. Guillou et al. discussed the possible overlap between SFT and giant cell angiofibromas (GCA), and suggested that GCAs are in fact a giant cell-rich variant of SFT. They showed that GCA has a wider distribution than initially thought, involving not only the orbit (where they tend to occur) but also other sites in the head and neck [13].
The demonstration of mast cells in SFTs has been reported previously [18, 36, 30]; we noted mast cells in 38% of our cases. However, mast cells are a feature of other soft tissue lesions including schwannomas [53], spindle cell lipomas [54], neurofibromas [55] and vascular tumors [56]. It has been hypothesized that in some soft tissue tumors, mast cells are involved in the development of sclerosis by releasing proteases in the intercellular matrix and around blood vessels [57]. Of note, in the current study, the mast cells were readily identified in the sclerotic pattern.
Scattered lymphocytes were present in 90% of cases in the current series but in only one case was the infiltrate present multi-focally in clusters. It is not an unusual feature on reviewing the literature. At least a sparse lymphocytic infiltrate has been described by Alawi et al., while Lo Muzio et al. described the presence of a chronic inflammatory infiltrate in some cases [18, 40].
Chan considered positive immunoreactivity for CD34 one of the fundamental criteria for diagnosis of an extrapleural SFT [4]. CD34 antigen is a 110-kDa transmembrane cell surface glycoprotein found on myeloid progenitor cells but also noted in other tissues and neoplasms such as dermatofibrosarcoma protruberans, Kaposi sarcoma, epithelioid sarcoma, gastrointestinal stromal tumors as well as the SFT. [58, 59]. CD34 has also been reported to be positive in occasional leiomyomas and leiomyosarcomas [59]. Studies have reported between 80-100% CD34 positivity rate in SFTs, with the percentage difference likely being related to antibody type [59–61]. In the current series 100% of cases were CD34-and bcl-2-positive, with the vast majority being strongly positive (3+). Only one case showed weak (1+) immunoreactivity for CD34. While bcl-2-positive immunoreactivity may be helpful in differentiating SFT from mesothelioma, it is less helpful for extra-pleural sites because other mesenchymal tumors (such as schwannoma, gastrointestinal stromal tumor, synovial sarcoma) may also be bcl-2-positive [62]. CD99 is variably immunopositive in SFTs, with reports describing as many as 70% of cases positive [63, 15, 18]. The current study found CD99 to be positive in a mere 26% of cases. On review of the literature, it is clear that CD99 immunopositivity in SFTs may be variable, and is not even reported in some cases series [28, 46]. None of the 21 cases showed features that may have represented malignant SFT, such as marked cytological atypia, necrosis, ≥4 mitoses per 10 high power fields or infiltrative margins. [64]. The characteristic immunophenotype for the SFT does not help differentiate between benign and malignant types [61]. Overall, there are no histopathologic or immunophenotypical features that separate SFTs arising in the head and neck from those arising at other sites.
Follow-up information available for 17 cases (average 54 months), showed no recurrence and no metastases. Alawi et al. in their case series also saw no recurrence or metastasis in the 10 cases where follow-up data was available [18]. While it is believed that lesions located in the mediastinum, abdomen, pelvis and retroperitoneum may behave aggressively [65] it appears that the intraoral SFT tends to follow a more benign non-recurring course, similar to SFTs that occur in the limbs.
Conclusion
This report of 21 cases of SFT is the largest study of intraoral SFTs in the English literature and confirms findings reported by other investigators. Two patterns were noted. The more common classic pattern consisted of small capillary-like vessels as well as staghorn vessels with mixed hyper- and hypo-cellular areas. The less common sclerotic pattern was characterized primarily by dense bands of collagen with only subtle hypercellular areas. The spindled tumor cells were consistently positive for CD34 and also for Bcl-2.
Acknowledgements
We would like to thank Christopher Fletcher MD who helped review the cases, and all contributing doctors who provided the material for this study.
References
- 1.Klemperer P, Rabin CB. Primary neoplasm of the pleura: a report of 5 cases. Arch Pathol (Chic) 1931;11:28. [Google Scholar]
- 2.Stout AP, Murray MR. Localized pleural mesothelioma: investigation of its characteristics and histogenesis by method of tissue culture. Arch Pathol (Chic) 1942;34:14. [Google Scholar]
- 3.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. Am J Surg Pathol. 1989;13(8):19. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Chan JK. Solitary fibrous tumour—everywhere and a diagnosis in vogue. Histopathology. 1997;31(6):568–576. doi: 10.1046/j.1365-2559.1997.2400897.x. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa T, Matsuno Y, Shimoda T, Hasegawa F, Sano T, Hirohashi S. Extrathoracic solitary fibrous tumors: their histological variability and potentially aggressive behavior. Hum Pathol. 1999;30(12):1464–1473. doi: 10.1016/S0046-8177(99)90169-7. [DOI] [PubMed] [Google Scholar]
- 6.Mentzel T, Bainbridge TC, Katenkamp D. Solitary fibrous tumour: clinicopathological, immunohistochemical, and ultrastructural analysis of 12 cases arising in soft tissues, nasal cavity and nasopharynx, urinary bladder and prostate. Virchows Arch. 1997;430(6):445–453. doi: 10.1007/s004280050054. [DOI] [PubMed] [Google Scholar]
- 7.Guerra MF, Amat CG, Campo FR, Perez JS. Solitary fibrous tumor of the parotid gland: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94(1):78–82. doi: 10.1067/moe.2002.121990. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki SO, Fukui M, Nishio S, Iwaki T. Clinicopathological features of solitary fibrous tumor of the meninges: An immunohistochemical reappraisal of cases previously diagnosed to be fibrous meningioma or hemangiopericytoma. Pathol Int. 2000;50(10):808–817. doi: 10.1046/j.1440-1827.2000.01120.x. [DOI] [PubMed] [Google Scholar]
- 9.Travis WD, Colby TV, Corrin B. Histological typing of tumours of lung and pleura. 3. Verlag: Springer; 1999. [Google Scholar]
- 10.Fletcher CDM. Hemangiopericytoma—a dying breed? Reappraisal of an ‘entity’ and its variants: a hypothesis. Curr Diagn Pathol. 1994;1(19):19–23. doi: 10.1016/S0968-6053(06)80005-0. [DOI] [Google Scholar]
- 11.Nappi ORJ, Pettinato G, Wick MR. Hemangiopericytoma: histopathological pattern or clinicopathological entity. Semin Diagn Pathol. 1995;12(3):12. [PubMed] [Google Scholar]
- 12.Fletcher CD. The evolving classification of soft tissue tumours: an update based on the new WHO classification. Histopathology. 2006;48(1):3–12. doi: 10.1111/j.1365-2559.2005.02284.x. [DOI] [PubMed] [Google Scholar]
- 13.Guillou L, Gebhard S, Coindre JM. Orbital and extraorbital giant cell angiofibroma: a giant cell-rich variant of solitary fibrous tumor? Clinicopathologic and immunohistochemical analysis of a series in favor of a unifying concept. Am J Surg Pathol. 2000;24(7):971–979. doi: 10.1097/00000478-200007000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Dervan PA, Tobin B, O’Connor M. Solitary (localized) fibrous mesothelioma: evidence against mesothelial cell origin. Histopathology. 1986;10(8):867–875. doi: 10.1111/j.1365-2559.1986.tb02584.x. [DOI] [PubMed] [Google Scholar]
- 15.Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology. 2006;48(1):63–74. doi: 10.1111/j.1365-2559.2005.02290.x. [DOI] [PubMed] [Google Scholar]
- 16.Thompson LD, Miettinen M, Wenig BM. Sinonasal-type hemangiopericytoma: a clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Am J Surg Pathol. 2003;27(6):737–749. doi: 10.1097/00000478-200306000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Ordonez B, Koutlas IG, Strich E, Gilbert RW, Jordan RC. Solitary fibrous tumor of the oral cavity: an uncommon location for a ubiquitous neoplasm. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(5):589–593. doi: 10.1016/S1079-2104(99)70139-3. [DOI] [PubMed] [Google Scholar]
- 18.Alawi F, Stratton D, Freedman PD. Solitary fibrous tumor of the oral soft tissues: a clinicopathologic and immunohistochemical study of 16 cases. Am J Surg Pathol. 2001;25(7):900–910. doi: 10.1097/00000478-200107000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Shin JH, Sung IY, Suh JH, Yang SO, Jeong YK, Lee JH, et al. Solitary fibrous tumor in the buccal space: MR findings with pathologic correlation. AJNR Am J Neuroradiol. 2001;22(10):1890–1892. [PMC free article] [PubMed] [Google Scholar]
- 20.Macarenco RS, Bacchi CE, Domingues MA. Solitary fibrous tumor with atypical histological features occurring in the palatine tonsil: an uncommon neoplasm in an uncommon site. J Oral Pathol Med. 2006;35(10):602–605. doi: 10.1111/j.1600-0714.2006.00460.x. [DOI] [PubMed] [Google Scholar]
- 21.Iwai S, Nakazawa M, Yoshikawa F, Amekawa S, Sakuda M. Solitary fibrous tumor of the buccal mucosa: report of a case with immunohistochemical studies. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88(4):461–465. doi: 10.1016/S1079-2104(99)70062-4. [DOI] [PubMed] [Google Scholar]
- 22.Talacko AA, Aldred MJ, Sheldon WR, Hing NR. Solitary fibrous tumour of the oral cavity: report of two cases. Pathology. 2001;33(3):315–318. doi: 10.1080/00313020120062910. [DOI] [PubMed] [Google Scholar]
- 23.Shine N, Nor nurul Khasri M, Fitzgibbon J, O’Leary G. Solitary fibrous tumor of the floor of the mouth case report and review of the literature. Ear Nose Throat J. 2006;85(7):437–439. [PubMed] [Google Scholar]
- 24.Yamashita Y, Satoh T, Goto M. Solitary fibrous tumour of the tongue: a case report with immunohistochemical studies. Int J Oral Maxillofac Surg. 2002;31(6):681–683. doi: 10.1054/ijom.2001.0201. [DOI] [PubMed] [Google Scholar]
- 25.Jham BC, Salles JM, Soares JM, Sousa Ade A, Moraes GM, Ribeiro CA, et al. Solitary fibrous tumour of the buccal mucosa: case report and review of the literature. Br J Oral Maxillofac Surg. 2007;45(4):323–325. doi: 10.1016/j.bjoms.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Dunfee BL, Sakai O, Spiegel JH, Pistey R. Solitary fibrous tumor of the buccal space. AJNR Am J Neuroradiol. 2005;26(8):2114–2116. [PMC free article] [PubMed] [Google Scholar]
- 27.Piattelli A, Fioroni M, Rubini C. Solitary fibrous tumour of the tongue. Oral Oncol. 1998;34(5):431–434. doi: 10.1016/S1368-8375(98)00042-6. [DOI] [PubMed] [Google Scholar]
- 28.Lukinmaa PL, Hietanen J, Warfvinge G, Sane J, Tuominen S, Henriksson V, et al. Solitary fibrous tumour of the oral cavity: clinicopathological and immunohistochemical characterization of three cases. J Oral Pathol Med. 2000;29(4):186–192. doi: 10.1034/j.1600-0714.2000.290407.x. [DOI] [PubMed] [Google Scholar]
- 29.Vargas PA, Alves FA, Lopes MA, Siqueira SA, Menezes LF, Aldred VL, et al. Solitary fibrous tumour of the mouth: report of two cases involving the tongue and cheek. Oral Dis. 2002;8(2):111–115. doi: 10.1034/j.1601-0825.2002.1c769.x. [DOI] [PubMed] [Google Scholar]
- 30.Hirano M, Tanuma J, Shimoda T, Sugihara K, Tsuneyoshi M, Kitano M. Solitary fibrous tumor in the mental region. Pathol Int. 2001;51(11):905–908. doi: 10.1046/j.1440-1827.2001.01290.x. [DOI] [PubMed] [Google Scholar]
- 31.Ide F, Saito I. Oral solitary fibrous tumour. Oral Dis. 2002;8(6):314–315. doi: 10.1034/j.1601-0825.2002.02885.x. [DOI] [PubMed] [Google Scholar]
- 32.Harada T, Matsuda H, Maruyama R, Yoshimura Y. Solitary fibrous tumours of the lower gingiva: a case report. Int J Oral Maxillofac Surg. 2002;31(4):448–450. doi: 10.1054/ijom.2001.0192. [DOI] [PubMed] [Google Scholar]
- 33.Shimoyama T, Horie N, Ide F. Solitary fibrous tumor of the palate: a case report and review of the literature. J Oral Maxillofac Surg. 2004;62(7):895–897. doi: 10.1016/j.joms.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 34.Gonzalez-Garcia R, Gil-Diez Usandizaga JL, Hyun Nam S, Rodriguez Campo FJ, Naval-Gias L. Solitary fibrous tumour of the oral cavity with histological features of aggressiveness. Br J Oral Maxillofac Surg. 2006;44(6):543–545. doi: 10.1016/j.bjoms.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Wu SL, Vang R, Clubb FJ, Jr, Connelly JH. Solitary fibrous tumor of the tongue: report of a case with immunohistochemical and ultrastructural studies. Ann Diagn Pathol. 2002;6(3):168–171. doi: 10.1053/adpa.2002.33903. [DOI] [PubMed] [Google Scholar]
- 36.Veltrini VC, Etges A, Magalhaes MH, Araujo NS, Araujo VC. Solitary fibrous tumor of the oral mucosa–morphological and immunohistochemical profile in the differential diagnosis with hemangiopericytoma. Oral Oncol. 2003;39(4):420–426. doi: 10.1016/S1368-8375(02)00148-3. [DOI] [PubMed] [Google Scholar]
- 37.Brunnemann RB, Ro JY, Ordonez NG, Mooney J, El-Naggar AK, Ayala AG. Extrapleural solitary fibrous tumor: a clinicopathologic study of 24 cases. Mod Pathol. 1999;12(11):1034–1042. [PubMed] [Google Scholar]
- 38.Kurihara K, Mizuseki K, Sonobe J, Yanagihara J. Solitary fibrous tumor of the oral cavity: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(2):223–226. doi: 10.1016/S1079-2104(99)70276-3. [DOI] [PubMed] [Google Scholar]
- 39.Shnayder Y, Greenfield BJ, Oweity T, DeLacure MD. Malignant solitary fibrous tumor of the tongue. Am J Otolaryngol. 2003;24(4):246–249. doi: 10.1016/S0196-0709(03)00007-3. [DOI] [PubMed] [Google Scholar]
- 40.Lo Muzio L, Mascolo M, Capodiferro S, Favia G, Maiorano E. Solitary fibrous tumor of the oral cavity: the need for an extensive sampling for a correct diagnosis. J Oral Pathol Med. 2007;36(9):538–542. doi: 10.1111/j.1600-0714.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- 41.Schirmang TC, Davis LM, Nigri PT, Dupuy DE. Solitary fibrous tumor of the buccal space: treatment with percutaneous cryoablation. AJNR Am J Neuroradiol. 2007;28(9):1728–1730. doi: 10.3174/ajnr.A0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fusconi M, Ciofalo A, Greco A, Pulice G, Macci M, Mariotti M, et al. Solitary fibrous tumor of the oral cavity: case report and pathologic consideration. J Oral Maxillofac Surg. 2008;66(3):530–534. doi: 10.1016/j.joms.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 43.Manor E, Bodner L. Chromosomal aberrations in oral solitary fibrous tumor. Cancer Genet Cytogenet. 2007;174(2):170–172. doi: 10.1016/j.cancergencyto.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, An JS, Lee ES, Kwon SY, Kim YS. Comparison of sporadic sclerotic fibroma and solitary fibrous tumor in the oral cavity. Yonsei Med J. 2007;48(3):535–539. doi: 10.3349/ymj.2007.48.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suster S, Nascimento AG, Miettinen M, Sickel JZ, Moran CA. Solitary fibrous tumors of soft tissue. A clinicopathologic and immunohistochemical study of 12 cases. Am J Surg Pathol. 1995;19(11):1257–1266. doi: 10.1097/00000478-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Ganly I, Patel SG, Stambuk HE, Coleman M, Ghossein R, Carlson D, et al. Solitary fibrous tumors of the head and neck: a clinicopathologic and radiologic review. Arch Otolaryngol Head Neck Surg. 2006;132(5):517–525. doi: 10.1001/archotol.132.5.517. [DOI] [PubMed] [Google Scholar]
- 47.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Molecular medicine (Cambridge, Mass) 1994;1(1):71–81. [PMC free article] [PubMed] [Google Scholar]
- 48.Tsuneyoshi M, Daimaru Y, Enjoji M. Malignant hemangiopericytoma and other sarcomas with hemangiopericytoma-like pattern. Pathol Res Pract. 1984;178(5):446–453. doi: 10.1016/S0344-0338(84)80004-7. [DOI] [PubMed] [Google Scholar]
- 49.Saint Aubain Somerhausen N, Rubin BP, Fletcher CD. Myxoid solitary fibrous tumor: a study of seven cases with emphasis on differential diagnosis. Mod Pathol. 1999;12(5):463–471. [PubMed] [Google Scholar]
- 50.Mohammed K, Harbourne G, Walsh M, Royston D. Solitary fibrous tumour of the parotid gland. J Laryngol Otol. 2001;115(10):831–832. doi: 10.1258/0022215011909099. [DOI] [PubMed] [Google Scholar]
- 51.Thompson M, Cheng LH, Stewart J, Marker A, Adlam DM. A paediatric case of a solitary fibrous tumour of the parotid gland. Int J Pediatr Otorhinolaryngol. 2004;68(4):481–487. doi: 10.1016/j.ijporl.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 52.Guillou L, Gebhard S, Coindre JM. Lipomatous hemangiopericytoma: a fat-containing variant of solitary fibrous tumor? Clinicopathologic, immunohistochemical, and ultrastructural analysis of a series in favor of a unifying concept. Hum Pathol. 2000;31(9):1108–1115. doi: 10.1053/hupa.2000.9777. [DOI] [PubMed] [Google Scholar]
- 53.Klijanienko JCJ, Lagacé R. Cytohistologic correlations in schwannomas (neurilemmomas) ia, “cellular, and epithelioid variants., Aug;34(8):517–22. DC. Cytohistologic correlations in schwannomas (neurilemmomas), including “ancient,”cellular and epitheliois variants. Diagn Cytopathol. 2006;34(8):6. doi:10.1002/dc.20320. [DOI] [PubMed]
- 54.Pitt MA, Roberts ISD, Curry A. Spindle cell and pleomorphic lipoma: an ultrastructural study. Ultrastruct Pathol. 1995;19(6):6. doi: 10.3109/01913129509014622. [DOI] [PubMed] [Google Scholar]
- 55.Viskochil DH. It takes two to tango: mast cell and Schwann cell interactions in neurofibromas. J Clin Invest. 2003;112(12):1791–1793. doi: 10.1172/JCI20503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glowacki J, Mulliken JB. Mast cells in hemangiomas and vascular malformations. Pediatrics. 1982;70(1):48–51. [PubMed] [Google Scholar]
- 57.Bigotti GCA, Mottolese M, Di Filippo F. Selective location of palisaded myofibroblastoma with amianthoid fibres. J Clin Pathol. 1991;44(9):4. doi: 10.1136/jcp.44.9.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suster SFC. Immunoreactivity for the human hematopoietic progenitor cell antigen (CD34) in lipomatous tumors. Am J Surg Pathol. 1997;21(2):6. doi: 10.1097/00000478-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 59.Rijn M, Lombard CM. Rouse RV. Expression of CD34 by solitary fibrous tumors of the pleura mediastinum and lung. Am J Surg Pathol. 1994;18(8):814–820. doi: 10.1097/00000478-199408000-00008. [DOI] [PubMed] [Google Scholar]
- 60.Westra WHgW, Rosai J. Solitary fibrous tumor Consistent CD34 immunoreactivity and occurrence in the orbit. Am J Surg Pathol. 1994;18(10):7. doi: 10.1097/00000478-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 61.Hanau CA, Miettinen M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol. 1995;26(4):440–449. doi: 10.1016/0046-8177(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 62.Chilosi MFF, Dei Tos AP, Lestani M, Morassi ML, Martignoni G, Sorio C, Benedetti A, Morelli L, Doglioni C, Barberis M, Menestrina F, Viale G. Bcl-2 expression in pleural and extrapleural solitary fibrous tumours. J Pathol. 181;4:6. doi: 10.1002/(SICI)1096-9896(199704)181:4<362::AID-PATH764>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 63.Renshaw AA. O13 (CD99) in spindle cell tumors. Reactivity with hemangiopericytoma, solitary fibrous tumor, synovial sarcoma, and meningioma but rarely with sarcomatoid mesothelioma. Appl Immunohistochem. 1995;3:250–256. [Google Scholar]
- 64.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22(12):1501–1511. doi: 10.1097/00000478-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 65.Fletcher CDM, Unni KK, Mertens F. World Health Organization classification of tumours. Pathology and genetics of tumors of soft tissue and bone. Lyon: IARC Press; 2002. p. 87.