Abstract
Wild birds of several species are dying in large numbers from an idiopathic paralytic disease in the Baltic Sea area. Here, we demonstrate strong relationships between this disease, breeding failure, and thiamine (vitamin B1) deficiency in eggs, pulli, and full-grown individuals. Thiamine is essential for vertebrates, and its diphosphorylated form functions as a cofactor for several life sustaining enzymes, whereas the triphosphorylated form is necessary for the functioning of neuronal membranes. Paralyzed individuals were remedied by thiamine treatment. Moreover, thiamine deficiency and detrimental effects on thiamine-dependent enzymes were demonstrated in the yolk, liver, and brain. We propose that the mortality and breeding failure are part of a thiamine deficiency syndrome, which may have contributed significantly to declines in many bird populations during the last decades.
Keywords: α-ketoglutarate dehydrogenase, avian, extinction, transketolase
In recent years we and others have observed an increasing number of wild birds dying from an idiopathic paralytic disease in different parts of northern Europe. The general course of this disease in full-grown individuals is difficulty in keeping the wings folded along the side of the body, inability to fly, inability to walk, and death. Other symptoms are tremor and seizures. By these main criteria, the occurrence of the paralytic disease was obvious in 451 of 837 specimens (54%) found dying or dead in southern Sweden, and affected specimens were found in 28 of 36 investigated species (78%; Table S1 in the SI Appendix). The earliest report about an unknown paralytic disease in the Baltic Sea area, fitting this symptomology, dates back to 1982 (1). Hence, this disease may have been affecting birds for >25 years without any particular notice or understanding of its importance. Standardized monitoring programs have been tracking the development of bird populations in Sweden since the late 1970s. A recent evaluation of the 3 major programs (2) established that many Swedish bird populations have declined since 1980. Similar population declines have also occurred in other European countries (3–6). Here, we present observational and experimental data linking the paralytic disease to thiamine deficiency. We also propose thiamine deficiency as a possible cause for observed bird population declines, in addition to other proposed causes, such as habitat loss (7, 8) and climate change (9).
Thiamine (T), vitamin B1, is a water-soluble vitamin essential for vertebrates. Inside the cell, T is phosphorylated (10) to thiamine diphosphate (TDP), which functions as a cofactor for at least 5 vital enzymes in the cellular metabolism: transketolase (TK) in the hexose monophosphate shunt, pyruvate dehydrogenase in the glycolysis, α-ketoglutarate dehydrogenase (KGDH) in the citric acid cycle, branched-chain α-keto acid dehydrogenase for the metabolism of amino acids, and, as recently shown, 2-hydroxyacyl-CoA lyase 1 for the α-oxidation of 3-methyl-branched and 2-hydroxy straight chain fatty acids (11, 12). Further phosphorylation produces thiamine triphosphate, which is necessary for the proper functioning of neuronal membranes (13, 14).
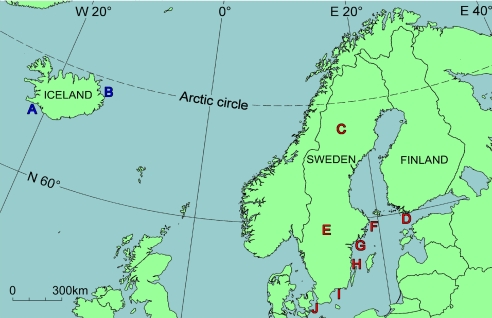
Thiamine deficiency was demonstrated in several bird species by remediation of paralyzed individuals by thiamine treatment, as well as by measurement of thiamine-dependent enzymes and thiamine concentrations. Three species, belonging to different bird orders and differing in food and habitat preferences, breeding biology, and migration patterns, were investigated in detail: the herring gull (Larus argentatus), the common starling (Sturnus vulgaris), and the common eider (Somateria mollissima). For the herring gull and the common eider we also estimated the potential effect of breeding failure and excess mortality on the population size. Samples were collected from 10 regions (labeled A–J in Fig. 1) in northern Europe. The Baltic Sea area was compared with Iceland, where no outbreaks of the paralytic disease have been documented. Incipient thiamine deficiency was, however, indicated also in Iceland by comparison with control domestic chicken (Gallus gallus) and literature data. The material comprised a total of 829 specimens from 83 locations (Fig. S1 a–j in the SI Appendix), traditionally noted for their rich bird life, often bird preservation areas.
Fig. 1.
The 10 investigated regions: A, Southwestern Iceland; B, Eastern Iceland; C, County of Västerbotten; D, County of Södra Finland; E, County of Värmland; F, County of Stockholm; G, County of Södermanland; H, County of Kalmar; I, County of Blekinge; and J, County of Skåne.
Results and Discussion
Description of the Paralytic Disease.
During the typical course of the paralytic disease in full-grown birds, the clinical symptoms generally appear in the following order: (i) Difficulty in keeping the wings folded along the side of the body (hanging wings) when resting. (ii) Loss of ability to fly, while still being able to walk and run. Complete loss of voice. (iii) Loss of appetite, while still drinking and swallowing water without any problem. Labored breathing and gradual loss of strength in the legs. The bird may try to crawl, using its wings and beak. (iv) Complete paralysis of the wings and legs, loss of strength in the beak (Movie S1), and diarrhea. Force-feeding may result in vomiting. (v) Opisthotonus (star-gazing) and tremor. (vi) Ataxia, catatonia, and seizures. (vii) Death. A symptom that seems to occur independently of disease phase is pigmentation changes in the iris (Fig. S2 a–d in the SI Appendix).
The description so far is based mainly on observations of relatively large (≈0.5–1 kg) species (Table S2 in the SI Appendix), but there are occasional observations of the same symptoms also in small (≈5–100 g) species (Text S1 in the SI Appendix). The time between losing the ability to fly and death varies between species and is generally shorter for smaller species. In herring gull, which is a relatively large species, this time is often 10–20 days. The paralytic disease has been observed in various bird species throughout the year (Tables S1 and S2 in the SI Appendix). The numerous observations of affected water birds in breeding colonies do not mean that the paralytic disease is confined to water birds during the breeding season, but rather is likely an effect of a high discovery rate. The herring gull, for example, is a large white bird, which is easily discovered in colonies. The symptoms of the paralytic disease have previously been produced in controlled laboratory experiments with birds by thiamine depletion (15–17). Moreover, these symptoms differ in several ways from those of botulism (Clostridium botulinum poisoning), that also causes paralysis. The differences are listed in Table S3 in the SI Appendix.
Herring Gull (Larus argentatus).
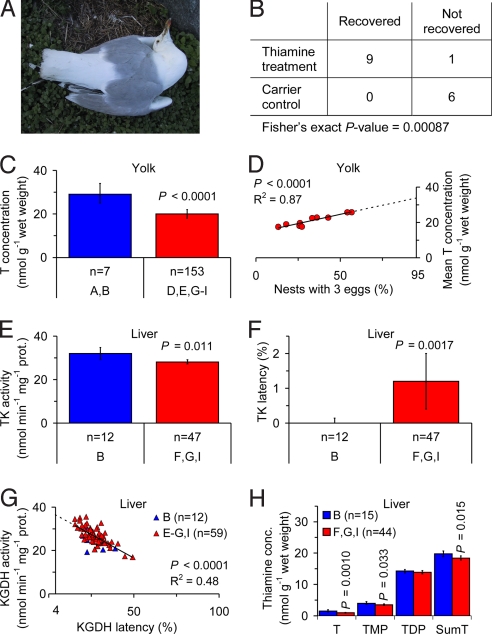
The herring gull is one of the most well known species affected by the paralytic disease (18). Fig. 2A shows a specimen suffering from opisthotonus and hanging wings. In fact, this specimen is dying while incubating.
Fig. 2.
Observations of thiamine deficiency in the herring gull (Larus argentatus). (A) A specimen in the County of Stockholm (region F in Fig. 1) suffering from opisthotonus and difficulty in keeping the wings folded along the side of the body. The specimen is dying while incubating, an abnormal situation. (B) Thiamine treatment of paralyzed full-grown specimens from the Baltic Sea area had a highly significant effect on recovery. (C) Yolk T concentration was 34% lower in the Baltic Sea area (regions D, E, and G–I in Fig. 1) than in Iceland (regions A and B in Fig. 1). (D) Relationship between mean yolk T concentration in the first laid eggs and proportion of nests with 3 eggs in 10 colonies in the Baltic Sea area. Extrapolation to 95% nests with 3 eggs yielded a yolk T concentration of 34 nmol/g wet weight. (E) Liver TK activity in pulli was 12% lower in the Swedish coastal regions together (regions F, G, and I in Fig. 1) than in Iceland (region B in Fig. 1). (F) Liver TK latency (i.e., the proportion of apoenzyme) in pulli was significantly higher in the Swedish coastal regions together (regions F, G, and I in Fig. 1) than in Iceland (region B in Fig. 1). (G) Relationship between liver KGDH activity and latency in pulli. Extrapolation to 4.0% latency, assumed to be normal in healthy individuals, yielded an activity of 36.5 nmol/min per mg protein. (H) Liver thiamine concentrations in pulli. T, TMP, and T+TMP+TDP (SumT) concentrations were 34%, 13%, and 7% lower, respectively, in the Swedish coastal regions together (regions F, G, and I in Fig. 1) than in Iceland (region B in Fig. 1). Error bars (C, E, F, and H) indicate 95% confidence intervals of the mean. P-values are given only when significant. n = number of clutches. Blue, Iceland and red, Baltic Sea area.
A thiamine treatment experiment was performed with 16 paralyzed full-grown specimens from the Swedish Baltic Sea coast. These specimens received an injection in the breast muscle with either a T solution, at a dose of 50 mg T per kg body weight, or a saline. They were then studied for up to 14 days, and during this time all but 1 of the thiamine-treated specimens recovered completely, whereas none of the control specimens showed any sign of improvement. The difference in response between these 2 groups was highly significant (Fig. 2B; Fisher's exact test, P = 0.00087). Specimens of other species were also possible to cure by similar thiamine treatment (Text S1 and Table S2 in the SI Appendix).
Another thiamine treatment experiment was performed with 20 newly hatched pulli from the County of Södermanland (region G in Fig. 1). Ten sibling pairs were divided into a saline control group and a pair-fed thiamine treatment group, orally administered a T solution (1 mL) at a dose of 5 mg T per specimen on day 1 and 3. There was an indisputable difference in behavior between these 2 groups. The thiamine-treated specimens were vigorous, active, and always hungry, whereas the control specimens were lethargic, apathetic, and had reduced appetite, and on days 4 and 5 they started to die.
In egg yolk the T concentration was 28–41% lower in the 5 investigated regions in the Baltic Sea area (regions D, E, and G–I in Fig. 1) compared with the Icelandic control (regions A and B in Fig. 1; Table S4 in the SI Appendix; P < 0.005), and 34% lower in the Baltic Sea area as a whole (Fig. 2C; P < 0.0001). The range of yolk T concentrations of 9.9–41.9 nmol/g (n = 167) indicated that the herring gull is unable to produce eggs with a yolk T concentration lower than ≈10 nmol/g. A possible consequence would be that thiamine deficiency results in fewer eggs per nest than the normal 3 per nest (19). Support for such a relationship was obtained in 2 ways. First, in 1 randomly selected colony in the County of Värmland (region E in Fig. 1) we found a positive relationship between the yolk T concentration in the first laid egg and the final number of eggs in the same nest (Spearman rank correlation, r = 0.61, P = 0.025). Second, in 10 colonies in Sweden there was a positive linear relationship between the mean yolk T concentration in the first laid eggs and the proportion of 3 egg clutches in the colony (Fig. 2D; P < 0.0001). Extrapolation of this relationship to 95% 3 egg clutches, a proportion observed in healthy populations (19), yielded a yolk T concentration of 34 ± 4.1 nmol/g [mean ± 95% confidence interval (CI)] in fair agreement with the Icelandic control (regions A and B in Fig. 1) of 29 ± 4.5 nmol/g (mean ± 95% CI). In fact, these values were not significantly different (Z-test, P = 0.14). There was no strong correlation between yolk redness and yolk T concentration, and there was no indication of eggshell thinning, a phenomenon previously related to certain classical persistent pollutants (Text S2 in the SI Appendix).
In pulli, the TK activity in the liver, an established biomarker of thiamine deficiency (20), was 21% lower in the County of Södermanland (region G in Fig. 1) compared with the Icelandic control (region B in Fig. 1; Table S5 in the SI Appendix; P < 0.0001) and 12% lower in the Swedish coastal regions together (regions F, G, and I in Fig. 1; Fig. 2E; P = 0.011). The liver TK latency, i.e., the proportion of enzyme without the TDP cofactor (apoenzyme), was significantly more dispersed in the County of Södermanland (region G in Fig. 1) and the County of Blekinge (region I in Fig. 1) compared with the Icelandic control (region B in Fig. 1; Table S5 in the SI Appendix; P < 0.05). The larger dispersion in the 2 Swedish regions, combined with higher mean values, demonstrated a higher prevalence of individual clutches with high liver TK latency in these regions. This kind of difference is typical when there is varying degree of disease in a case group, while the control group is healthy. Also, liver TK latency was significantly higher in the Swedish coastal regions together (regions F, G, and I in Fig. 1; Fig. 2F; P = 0.0017). Neither liver TK activity nor latency differed between the County of Värmland (region E in Fig. 1) and the Icelandic control (region B in Fig. 1; Table S5 in the SI Appendix), despite a 34% lower yolk T concentration in the eggs in the County of Värmland (Table S4 in the SI Appendix; P < 0.0001). A possible explanation may be higher food quality for the pulli in the inland than at the coast. For KGDH activity in the liver there was no significant difference among any of the investigated regions, or the Baltic Sea area as a whole (regions E–G and I in Fig. 1), and the Icelandic control (region B in Fig. 1; Table S5 in the SI Appendix). For liver KGDH latency, the only significant difference was a 28% higher latency in the County of Södermanland (region G in Fig. 1) compared with the Icelandic control (region B in Fig. 1; Table S5 in the SI Appendix; P = 0.012). The liver KGDH latency was, however, ≥25% throughout (Table S5 in the SI Appendix), i.e., strongly indicating thiamine deficiency (21, 22) in all investigated regions, including Iceland. Since reference values for nonthiamine deficient birds were missing, we measured KGDH latency in the liver of 36 nonthiamine deficient domestic chicken pulli, which yielded a value of 4.0 ± 0.92% (mean ± 95% CI). This is in fair agreement with published values of liver KGDH latency in nonthiamine deficient rats: 6.5% (22), 5.4% (23), and 4.8% (24). We noted that there was a negative linear relationship between liver KGDH activity and latency in the thiamine deficient rats (22) (R2 = 0.88, P = 0.00062), but no such relationship in nonthiamine deficient rats (22), or in the nonthiamine deficient domestic chicken pulli (Fig. S4 a–c in the SI Appendix). Similar to the thiamine deficient rats, there was a negative linear relationship between liver KGDH activity and latency in the herring gull pulli (Fig. 2G; P < 0.0001). Extrapolation of this relationship to 4.0% latency, observed in the nonthiamine deficient domestic chicken pulli, yielded a liver KGDH activity of 36.5 ± 2.62 nmol/min per mg protein (mean ± 95% CI). The observed KGDH activities in the regions of the Baltic Sea area and Iceland were 24–32% lower than this value (Table S5 in the SI Appendix). The concentration of T, thiamine monophosphate (TMP), and T+TMP+TDP in the liver was 34%, 13%, and 7% lower, respectively, in the Swedish coastal regions together (regions F, G, and I in Fig. 1) compared with the Icelandic control (region B in Fig. 1; Fig. 2H; P = 0.0010, P = 0.033, and P = 0.015). The liver concentration of TDP was significantly different from the Icelandic control (region B in Fig. 1) only in the County of Södermanland (region G in Fig. 1), where it was 10% lower (Table S5 in the SI Appendix). This result agrees well with that of the liver KGDH latency. An important indication that the results really reflect effects of thiamine deficiency was a salient covariation of the effects between regions. The lowest liver TK and KGDH activities, the highest latencies of these enzymes, and the lowest liver T, TMP, TDP, and T+TMP+TDP concentrations were all found in the County of Södermanland (region G in Fig. 1; Table S5 in the SI Appendix). Liver body index (LBI), the liver weight expressed as percentage of the body weight, may be reduced because of thiamine deficiency in both the Baltic Sea area and in Iceland (Text S3 in the SI Appendix). Excess mortality and breeding failure has probably contributed to the population decline in Sweden (Text S4 in the SI Appendix).
A negative linear relationship between liver KGDH activity and latency was also found in full-grown specimens in both the Baltic Sea area (regions G, I, and J in Fig. 1) and in Iceland (regions A and B in Fig. 1; Fig. S7 in the SI Appendix; R2 = 0.81, P < 0.0001). There was an extremely large range of latency values (8–96%), and the many high values indicated advanced thiamine deficiency in these regions. Similar results were obtained also for 2 other gull species (Text S1 in the SI Appendix).
Common Starling (Sturnus vulgaris).
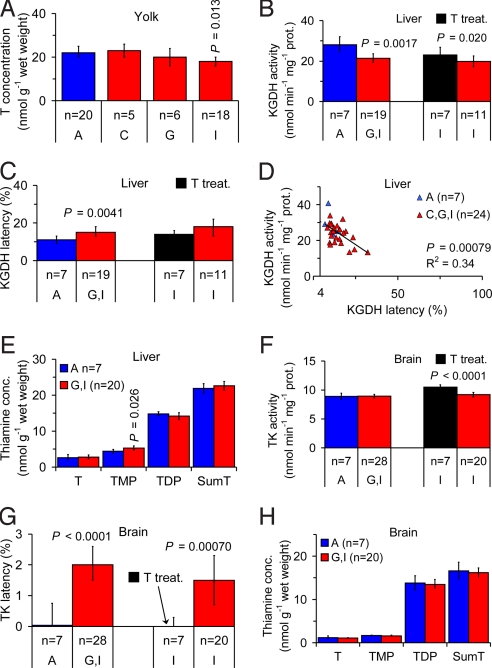
The common starling is a farmland passerine that feeds on invertebrates and has declined in many countries in northern Europe during the last decades (25, 26). Two thiamine treatment experiments were performed in the field, one with egg laying females in the County of Södermanland (region G in Fig. 1) and one with pulli in the County of Blekinge (region I in Fig. 1). In the first experiment, 4 females received an injection in the breast muscle with a T solution, at a dose of 50 mg T per kg body weight, 19–24 days before egg laying. The newly laid eggs were then sampled. In the second experiment, 31 pulli from 7 clutches were orally administered a T solution, at a dose of 50 mg T per kg body weight, and all but 1 specimen were sampled 11 days later.
The eggs from Iceland (region A in Fig. 1), the County of Västerbotten (region C in Fig. 1), and the thiamine-treated females had similar T concentrations in the yolk with a mean of 22–23 nmol/g (Table S6 in the SI Appendix). The good agreement between these 3 groups was a strong indication that these values may, in fact, be normal for nonthiamine deficient common starling eggs. The yolk T concentration was 18% lower in the County of Blekinge (region I in Fig. 1) than in Iceland (region A in Fig. 1; Fig. 3A; P = 0.013), and a gradient was indicated with decreasing yolk T concentrations from north to south in Sweden (regions C, G, and I in Fig. 1; Fig. 3A). The range of yolk T concentrations of 10.8–33.6 nmol/g (n = 93) indicated that the common starling is unable to produce eggs with a yolk T concentration lower than ≈10 nmol/g, similar to the herring gull. There was no correlation between yolk redness and yolk T concentration, and there was no indication of eggshell thinning (Text S2 in the SI Appendix).
Fig. 3.
Observations of thiamine deficiency in the common starling (Sturnus vulgaris). (A) Yolk T concentration, indicating a decreasing gradient from north to south (regions C, G, and I in Fig. 1) in Sweden. (B) Liver KGDH activity in pulli was 24% lower in southern Sweden (regions G and I in Fig. 1) than in Iceland (region A in Fig. 1). Thiamine treatment resulted in 19% higher liver KGDH activity. (C) Liver KGDH latency (i.e., the proportion of apoenzyme) in pulli was 36% higher in southern Sweden (regions G and I in Fig. 1) than in Iceland (region A in Fig. 1). (D) Relationship between liver KGDH activity and latency in pulli. Extrapolation to 4.0% latency, assumed to be normal in healthy individuals, yielded an activity of 30.9 nmol/min per mg protein. (E) Liver thiamine concentrations in pulli. TMP concentration was 20% higher in southern Sweden (regions G and I in Fig. 1) than in Iceland (region A in Fig. 1). (F) Brain TK activity in pulli. Thiamine treatment resulted in 14% higher brain TK activity. (G) Brain TK latency in pulli was significantly higher in southern Sweden (regions G and I in Fig. 1) than in Iceland (region A in Fig. 1). Thiamine treatment resulted in significantly lower brain TK latency. (H) Brain thiamine concentrations in pulli. Error bars (A–C and E–H) indicate 95% confidence intervals of the mean. P-values are given only when significant. n = number of clutches. Blue, Iceland; red, Baltic Sea area; and black, thiamine treatment.
In pulli, liver KGDH activity and latency followed the same gradient in Sweden as the yolk T concentration, with increasing thiamine deficiency from north to south (Table S7 in the SI Appendix). The highest liver KGDH activities were found in Iceland (region A in Fig. 1) and the County of Västerbotten (region C in Fig. 1), which did not differ significantly from each other (Table S7 in the SI Appendix). Southern Sweden as a whole (regions G and I in Fig. 1) had 24% lower activity than Iceland (region A in Fig. 1; Fig. 3B; P = 0.0017), and untreated clutches in the County of Blekinge (region I in Fig. 1) had 16% lower activity than the thiamine-treated clutches (Fig. 3B; P = 0.020). Iceland (region A in Fig. 1) and the County of Västerbotten (region C in Fig. 1) also had the lowest liver KGDH latencies, and did not differ significantly from each other (Table S7 in the SI Appendix). Southern Sweden as a whole (regions G and I in Fig. 1) had 36% higher latency than Iceland (region A in Fig. 1; Fig. 3C; P = 0.0041), whereas the untreated clutches in the County of Blekinge (region I in Fig. 1) did not differ significantly from the thiamine-treated clutches (Fig. 3C; P = 0.17). Investigations of thiamine-dependent enzymes have shown that low supply of T during early development may cause chronically reduced activities, as well as chronically elevated latencies, which are not always restored even if thiamine becomes available later (27–29). Hence, chronically reduced activity and elevated latency, due to low yolk T concentration in the eggs in the County of Blekinge (region I in Fig. 1), may explain why the liver KGDH activity and latency in the thiamine-treated clutches were not restored to similar values as those in Iceland (region A in Fig. 1) and the County of Västerbotten (region C in Fig. 1). The observation of a specimen in the County of Västerbotten (region C in Fig. 1) with a liver KGDH latency of 5.7% shows that such low latencies are possible. Hence, the observed liver KGDH latencies in nonthiamine deficient domestic chicken and rat, described earlier, may be extrapolated to nonthiamine deficient common starling. Similar to the herring gull, there was a negative linear relationship between liver KGDH activity and latency in the common starling pulli (Fig. 3D; P = 0.00079). Extrapolation of this relationship to 4.0% latency, observed in the nonthiamine deficient domestic chicken pulli, yielded a liver KGDH activity of 30.9 ± 3.92 nmol/min per mg protein (mean ± 95% CI). The observed KGDH activities in the County of Södermanland (region G in Fig. 1) and the County of Blekinge (region I in Fig. 1) were 25% and 36% lower, respectively, than this value (Table S7 in the SI Appendix). The concentration of T, TDP, and T+TMP+TDP in the liver did not differ significantly between southern Sweden as a whole (regions G and I in Fig. 1) and Iceland (region A in Fig. 1; Fig. 3E; interpreted below), whereas the TMP concentration was 20% higher in southern Sweden as a whole than in Iceland (region A in Fig. 1; Fig. 3E; P = 0.026). It has been demonstrated in certain tissues that the concentration of TMP may initially increase during early thiamine deficiency, and eventually decrease more than the concentrations of the other forms of thiamine during late stages of thiamine deficiency (30). These observations, together with the existence of a TMPase, which is induced by thiamine deficiency (31), suggest that tissues respond to thiamine deficiency by maximizing the reuse of TMP, which is otherwise excreted. Hence, the high liver TMP concentration in southern Sweden may be compatible with thiamine deficiency. The TK activity in the brain did not differ significantly between southern Sweden as a whole (regions G and I in Fig. 1) and Iceland (region A in Fig. 1; Fig. 3F; P = 0.84), whereas the activity in the County of Blekinge (region I in Fig. 1) was 12% lower than in the thiamine-treated clutches (Fig. 3F; P < 0.0001). In fact, brain TK activity was 12–17% lower in all regions than in the thiamine-treated clutches, including Iceland (Table S7 in the SI Appendix; P < 0.001). Decreases of this size have also been observed in thiamine deficient rats (27, 32, 33). Brain TK latency was significantly higher in southern Sweden as a whole (regions G and I in Fig. 1) compared with Iceland (region A in Fig. 1; Fig. 3G; P < 0.0001) as well as in untreated clutches in the County of Blekinge (region I in Fig. 1) compared with the thiamine-treated clutches (Fig. 3G; P = 0.00070). The observation that thiamine treatment reduced the brain TK latency to zero is important and raises the question why the liver KGDH latency was not also restored to zero by thiamine treatment. A possible explanation is that irreversible effects may occur earlier in the liver than in the brain because homeostasis in the brain has priority over homeostasis in other organs (34), but differences between the 2 enzymes have also been demonstrated (27, 35, 36). The mean concentration of T, TMP, TDP, and T+TMP+TDP in the brain did not differ significantly between southern Sweden as a whole (regions G and I in Fig. 1) and Iceland (region A in Fig. 1; Fig. 3H). Also this result may be explained by permanent high priority to homeostasis in the brain. A very important indication of thiamine deficiency, although, was found in the T+TMP+TDP concentration ratio between the liver and brain. Studies of other vertebrates have shown that this ratio is 2–3 in nonthiamine deficient individuals, i.e., the liver thiamine concentration is 2–3 times higher than that of the brain. When thiamine deficiency was induced in these individuals, the liver thiamine concentration was reduced faster than the brain thiamine concentration, and after 8–15 days the ratio was 1.2 or lower (34, 37, 38). In the common starling pulli this ratio was 1.2–1.4, including Iceland. This indication of thiamine deficiency also in Iceland may explain why the concentration of T, TDP, and T+TMP+TDP in the liver did not differ significantly between southern Sweden as a whole (regions G and I in Fig. 1) and Iceland (region A in Fig. 1; Fig. 3E; described above). LBI may be reduced because of thiamine deficiency in both the Baltic Sea area and in Iceland (Text S3 in the SI Appendix).
Common Eider (Somateria mollissima).
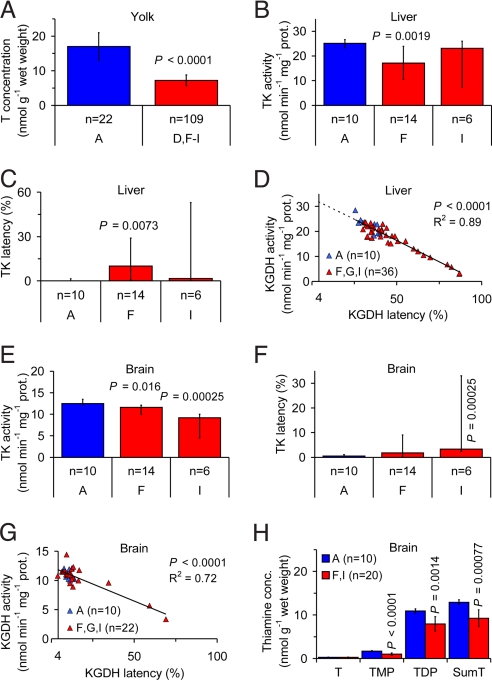
The frequent occurrence of dying or dead pulli, and low breeding output observed by us and others (39), led us to include the common eider in our investigations. In recent years (2004–2007) the abundance of common eider pulli in the County of Södermanland (region G in Fig. 1) a few days after hatching, has been of the magnitude 1 pullus per 100 females.
The concentration of T in the egg yolk was 58–72% lower in 4 of 5 regions in the Baltic Sea area (regions D, F, G, and I in Fig. 1) compared with Iceland (region A in Fig. 1; Table S8 in the SI Appendix; P < 0.0005), and 58% lower in the Baltic Sea area as a whole (Fig. 4A; P < 0.0001). The range of yolk T concentrations of 0.10–27 nmol/g (n = 179) indicated that the common eider is able to produce eggs essentially devoid of thiamine. The possibility of very low yolk T concentrations may explain why the common eider pulli die so soon after hatching, or already in the egg, whereas in the herring gull and common starling, which apparently do not lay eggs with a yolk T concentration below ≈10 nmol/g, the fetuses and pulli survive longer. The County of Södermanland (region G in Fig. 1) had the lowest mean yolk T concentration of all investigated regions, 4.7 nmol/g. This observation agrees well with the almost complete absence of pulli in this region. There was no correlation between yolk redness and yolk T concentration, and there was no indication of eggshell thinning (Text S2 in the SI Appendix).
Fig. 4.
Observations of thiamine deficiency in the common eider (Somateria mollissima). (A) Yolk T concentration was 58% lower in the Baltic Sea area (regions D and F–I in Fig. 1) than in Iceland (region A in Fig. 1). (B) Liver TK activity in pulli was 32% lower in the County of Stockholm (region F in Fig. 1) than in Iceland (region A in Fig. 1). (C) Liver TK latency (i.e., the proportion of apoenzyme) in pulli was significantly higher in the County of Stockholm (region F in Fig. 1) than in Iceland (region A in Fig. 1). (D) Relationship between liver KGDH activity and latency in pulli. Extrapolation to 4.0% latency, assumed to be normal in healthy individuals, yielded an activity of 31.9 nmol/min per mg protein. (E) Brain TK activity in pulli was 7% and 26% lower in the County of Stockholm (region F in Fig. 1) and the County of Blekinge (region I in Fig. 1), respectively, than in Iceland (region A in Fig. 1). (F) Brain TK latency in pulli was significantly higher in the County of Blekinge (region I in Fig. 1) than in Iceland (region A in fig. 1). (G) Relationship between brain KGDH activity and latency in pulli. Extrapolation to 3.8% latency, assumed to be normal in healthy individuals, yielded an activity of 11.9 nmol/min per mg protein. (H) Brain thiamine concentrations in pulli. TMP, TDP, and SumT concentrations were 41%, 27%, and 28% lower, respectively, in Sweden (regions F and I in Fig. 1) than in Iceland (region A in Fig. 1). Error bars (A–C, E, F, and H) indicate 95% CI of the mean (A and H) or median (B, C, E, and F). P-values are given only when significant. n = number of clutches. Blue, Iceland and red, Baltic Sea area.
In pulli, the activity and latency of TK and KGDH in the liver and brain had to be evaluated with nonparametric statistical methods, since the samples from the Baltic Sea area were far from normally distributed. The typical distribution was a cluster of values similar to control and a long narrow tail of values indicating varying degree of thiamine deficiency. This pattern may be explained in the following general way: The cluster represented clutches that were still able to maintain a reasonable degree of homeostasis, whereas the tail represented clutches that were sampled during the presumably short period between the collapse of homeostasis and death. The median liver TK activity was 32% lower in the County of Stockholm (region F in Fig. 1) compared with Iceland (region A in Fig. 1; Fig. 4B; P = 0.0019), but not significantly different in the County of Blekinge (region I in Fig. 1), although 25% of the clutches in the latter region had considerably lower activity compared with Iceland (Table S9 in the SI Appendix). Similarly, the median liver TK latency was significantly higher in the County of Stockholm (region F in Fig. 1) compared with Iceland (region A in Fig. 1; Fig. 4C; P = 0.0073), but not significantly different in the County of Blekinge (region I in Fig. 1), although 25% of the clutches in the latter region had considerably higher latency compared with Iceland (Table S9 in the SI Appendix). The median KGDH activity in the liver was 20% lower in the County of Stockholm (region F in Fig. 1) and 21% lower in the County of Blekinge (region I in Fig. 1) compared with Iceland (region A in Fig. 1; Table S9 in the SI Appendix; P < 0.01). Similarly, the median liver KGDH latency was 14% higher in the County of Stockholm (region F in Fig. 1) and 19% higher in the County of Blekinge (region I in Fig. 1) compared with Iceland (region A in Fig. 1; Table S9 in the SI Appendix; P < 0.05). Similar to the herring gull and common starling, there was a significant negative linear relationship between liver KGDH activity and latency in the common eider pulli (Fig. 4D; R2 = 0.89, P < 0.0001). Extrapolation of this relationship to 4.0% latency, observed in the nonthiamine deficient domestic chickens, yielded a liver KGDH activity of 31.9 ± 1.53 nmol/min per mg protein (mean ± 95% CI). The median liver KGDH activities in Iceland (region A in Fig. 1), the County of Stockholm (region F in Fig. 1), and the County of Blekinge (region I in Fig. 1) were 29%, 43%, and 44% lower, respectively, than this value, and the median liver KGDH latencies in these regions were 36%, 41%, and 43%, respectively (Table S9 in the SI Appendix). These observations demonstrate thiamine deficiency in all of the investigated regions, including Iceland. The concentrations of TMP, TDP, and T+TMP+TDP in the liver were 48%, 33%, and 34% lower, respectively, in the County of Stockholm (region F in Fig. 1) compared with Iceland (region A in Fig. 1; Table S9 in the SI Appendix; P < 0.01), but not significantly different in the County of Blekinge (region I in Fig. 1; Table S9 in the SI Appendix). The concentrations of TDP and T+TMP+TDP were, however, significantly more dispersed in both these regions (regions F and I in Fig. 1) compared with Iceland (region A in Fig. 1; Table S9 in the SI Appendix; P < 0.005). This observation, combined with the lower mean values, indicates varying degree of thiamine deficiency in the Baltic Sea area and a less deteriorated thiamine status in Iceland. The median TK activity in the brain was 7% lower in the County of Stockholm (region F in Fig. 1) and 26% lower in the County of Blekinge (region I in Fig. 1) compared with Iceland (region A in Fig. 1; Fig. 4E; P < 0.05). The median brain TK latency was significantly higher in the County of Blekinge (region I in Fig. 1) compared with Iceland (region A in Fig. 1; Fig. 4F; P = 0.00025), but not significantly different in the County of Stockholm (region F in Fig. 1). The median KGDH activity and latency in the brain did not differ significantly between the County of Stockholm (region F in Fig. 1) or the County of Blekinge (region I in Fig. 1) and Iceland (region A in Fig. 1; Table S9 in the SI Appendix), although 10–25% of the clutches in both regions in the Baltic Sea area (regions F and I in Fig. 1) had considerably lower activity and higher latency than Iceland. Moreover, whereas the median brain KGDH latency in the Baltic Sea area and Iceland was 9.3–13%, the corresponding mean latency in the 36 nonthiamine deficient domestic chicken pulli (same specimens as earlier) was 3.8 ± 1.7% (mean ± 95% CI). Similar to KGDH in the liver, there was a negative linear relationship between KGDH activity and latency in the brain (Fig. 4G; P < 0.0001). Extrapolation of this relationship to 3.8% latency, observed in the nonthiamine deficient domestic chicken pulli, yielded a brain KGDH activity of 11.9 ± 0.477 nmol/min per mg protein (mean ± 95% CI). This value was quite close to the mean KGDH activities in all investigated regions, including Iceland, even though these regions had latencies of 9.3–13% (Table S9 in the SI Appendix). These observations indicate high priority to homeostasis in the brain. The concentrations of TMP, TDP, and T+TMP+TDP in the brain were 41%, 27%, and 28% lower, respectively, in the Baltic Sea area (regions F and I in Fig. 1) compared with Iceland (region A in Fig. 1; Fig. 4H; P < 0.005), whereas the concentration of T did not differ significantly. The T+TMP+TDP concentration ratio between the liver and brain in the investigated regions, including Iceland, was 0.71–0.82. This is extremely low compared with the assumed ratio of 2–3 in nonthiamine deficient individuals (34, 37, 38). There was a positive linear relationship between liver KGDH activity and brain T+TMP+TDP concentration (Fig. S8 in the SI Appendix; P < 0.0001), illustrating the systemic nature of the thiamine deficiency. Excess mortality and breeding failure has probably contributed to the population decline in Sweden (Text S4 in the SI Appendix).
Important Aspects and Conclusions.
In the herring gull, we observed a reduced number of eggs per clutch in the Baltic Sea area, and a correlation between the number of eggs per clutch and the yolk thiamine concentration. We also observed a reduced thiamine concentration ratio between the liver and brain in the common starling and the common eider, and elevated liver KGDH latency throughout the investigated material. Our many observations of advanced thiamine deficiency in the Baltic Sea area and incipient thiamine deficiency in Iceland strongly suggest that also varying degrees of sublethal thiamine deficiency occur among the affected species. Well known effects of sublethal thiamine deficiency are disturbed basal intermediary metabolism, loss of appetite, abnormal behavior, and increased risk of secondary infections owing to immune suppression. Thiamine deficiency may be induced by a causative agent(s) acting directly on the affected individual, and/or by insufficient transfer of thiamine between the trophic levels in the food web. Further investigations focusing on causation are urgently needed. Text S5 in the SI Appendix contains a fuller discussion of these aspects.
In this work, we describe a widespread idiopathic paralytic disease, which we link to thiamine deficiency. We also provide an indication of the temporal and geographical distribution of this phenomenon that may be defined as a thiamine deficiency syndrome. Our observations of the paralytic disease in several wild bird species suggest that many other bird species in northern Europe are also affected. Because the investigated species occupy a wide range of ecological niches and positions in the food web, we are open to the possibility that other animal classes may suffer from thiamine deficiency as well.
Methods
Detailed methods are provided in the SI Appendix as Materials and Methods.
Supplementary Material
Acknowledgments.
This work was supported by Signhild Engkvists Stiftelse and Stiftelsen Olle Engkvist Byggmästare, Vänerns vattenvårdsförbund, Stockholm Vatten, Sörmlands Sparbank, the Stockholm County Council, the County Administrative Board of Södermanland, and the Swedish Environmental Protection Agency (M. Eriksson). Carl Zeiss AB (P. Nordgren, Sweden) supplied the telescope and binoculars.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902903106/DCSupplemental.
References
- 1.Roos G. Falsterbo bird station: August 1981–June 1982. Anser. 1982;21:169–174. [Google Scholar]
- 2.Karlsson L, Ehnbom S, Walinder G. A comparison between ringing totals at Falsterbo, SW Sweden, ringing totals at Ottenby, SE Sweden, and point counts from the Swedish breeding bird census during 20 years (1980–1999) Ornis Svec. 2005;15:183–205. [Google Scholar]
- 3.Donald PF, Green RE, Heath MF. Agricultural intensification and the collapse of Europe's farmland bird populations. Proc R Soc Lond B. 2001;268:25–29. doi: 10.1098/rspb.2000.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaston KJ, Blackburn TM, Goldewijk KK. Habitat conversion and global avian biodiversity loss. Proc R Soc Lond B. 2003;270:1293–1300. doi: 10.1098/rspb.2002.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas JA, et al. Comparative losses of British butterflies, birds, and plants and the global extinction crisis. Science. 2004;303:1879–1881. doi: 10.1126/science.1095046. [DOI] [PubMed] [Google Scholar]
- 6.Burfield I, van Bommel F. Birds in Europe. Population Estimates, Trends and Conservation Status. Cambridge: BirdLife International; 2004. p. 374. BirdLife Conservation Series No 12. [Google Scholar]
- 7.Meeus JHA, Wijermans MP, Vroom MJ. Agricultural landscapes in Europe and their transformation. Landscape Urban Plan. 1990;18:289–352. [Google Scholar]
- 8.Schmiegelow FKA, Machtans CS, Hannon SJ. Are boreal birds resilient to forest fragmentations? An experimental study of short-term community responses. Ecology. 1997;78:1914–1932. [Google Scholar]
- 9.Thomas CD, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. [DOI] [PubMed] [Google Scholar]
- 10.Yoshioka K. Some properties of the thiamine uptake system in isolated rat hepatocytes. Biochim Biophys Acta. 1984;778:201–209. doi: 10.1016/0005-2736(84)90463-2. [DOI] [PubMed] [Google Scholar]
- 11.Foulon V, et al. Purification, molecular cloning, and expression of 2-hydroxyphytanoyl-CoA lyase, a peroxisomal thiamine pyrophosphate-dependent enzyme that catalyzes the carbon-carbon bond cleavage during α-oxidation of 3-methyl-branced fatty acids. Proc Natl Acad Sci. 1999;96:10039–10044. doi: 10.1073/pnas.96.18.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sniekers M, et al. Thiamine pyrophosphate: An essential cofactor for the α-oxidation in mammals – implications for thiamine deficiencies? Cell Mol Life Sci. 2006;63:1553–1563. doi: 10.1007/s00018-005-5603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper JR, Pincus JH. The role of thiamine in nervous tissue. Neurochem Res. 1979;4:223–239. doi: 10.1007/BF00964146. [DOI] [PubMed] [Google Scholar]
- 14.Bettendorff L, Hennuy B, Wins P, Schoffeniels E. Thiamin and derivatives as modulators of rat brain chloride channels. Neuroscience. 1993;52:1009–1017. doi: 10.1016/0306-4522(93)90547-s. [DOI] [PubMed] [Google Scholar]
- 15.Peters RA. The biochemical lesion in vitamin B1 deficiency. Application of modern biochemical analysis in its diagnosis. Lancet. 1936;1:1161–1165. [Google Scholar]
- 16.Swank RL. Avian thiamine deficiency: A correlation of the pathology and clinical behavior. J Exp Med. 1940;71:683–702. doi: 10.1084/jem.71.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilman AP. Larus argentatus. Canada: University of Guelph; 1978. Natural and induced thiamin deficiency in the herring gull. PhD dissertation. [Google Scholar]
- 18.Mörner T, et al. Mass mortality in waterfowl in Sweden. Sven Veterinärtidn. 2005;8–9:11–18. [Google Scholar]
- 19.Paludan K. Denmark: University of Copenhagen; 1951. Contributions to the breeding biology of Larus argentatus and Larus fuscus. PhD dissertation. [Google Scholar]
- 20.McCandless DW, Schenker S, Cook M. Encephalopathy of thiamine deficiency: Studies of intracerebral mechanisms. J Clin Invest. 1968;47:2268–2280. doi: 10.1172/JCI105912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parker WD, et al. Brain mitochondrial metabolism in experimental thiamine deficiency. Neurology. 1984;34:1477–1481. doi: 10.1212/wnl.34.11.1477. [DOI] [PubMed] [Google Scholar]
- 22.Blair VP, et al. Dietary thiamin level influences levels of its diphosphate form and thiamin-dependent enzymic activities of rat liver. J Nutr. 1999;129:641–648. doi: 10.1093/jn/129.3.641. [DOI] [PubMed] [Google Scholar]
- 23.Takahasi K, Nakamura A, Nose Y. Effects of thiamine deficiency and thiamine administration on the thiamine diphosphate-dependent enzymes in rat liver. J Vitaminol. 1971;17:207–214. doi: 10.5925/jnsv1954.17.207. [DOI] [PubMed] [Google Scholar]
- 24.Nose Y, Takahasi K, Nakamura A. Thiamine diphosphate-dependent enzyme status due to thiamine deficiency in rat liver. J Nutr Sci Vitaminol Suppl. 1976;22:51–55. doi: 10.3177/jnsv.22.supplement_51. [DOI] [PubMed] [Google Scholar]
- 25.Feare CJ. Changes in numbers of common starlings and farming practice in Lincolnshire. Brit Birds. 1994;87:200–204. [Google Scholar]
- 26.Feare CJ, Douville de Farnseau P, Peris SJ. The starling in Europe: Multiple approaches to and problem species. Proc Vertebr Pest Conf. 1992;15:83–88. [Google Scholar]
- 27.Gibson GE, Ksiezak-Reding H, Sheu K-FR, Mykytyn V, Blass JP. Correlation of enzymatic, metabolic, and behavioral deficits in thiamin deficiency and its reversal. Neurochem Res. 1984;9:803–814. doi: 10.1007/BF00965667. [DOI] [PubMed] [Google Scholar]
- 28.Roth-Maier DA, Trübswetter N, Stangl GI, Kirchgessner M. Dietary thiamin supply during lactation influences thiamin status in lactating rats and their offspring and the thiamin level in milk. Z Ernährungswiss. 1997;36:169–175. doi: 10.1007/BF01611396. [DOI] [PubMed] [Google Scholar]
- 29.Amcoff P, et al. Hepatic activities of thiamine-dependent enzymes, glucose-6-phosphate dehydrogenase and cytochrome P4501A in Baltic salmon (Salmo salar) yolk-sac fry after thiamine treatment. Aquat Toxicol. 2000;48:391–402. doi: 10.1016/s0166-445x(00)00089-8. [DOI] [PubMed] [Google Scholar]
- 30.Harata N, Iwasaki Y, Ohara Y. Reappraisal of regional thiamine content in the central nervous system of the normal and thiamine-deficient mice. Metab Brain Dis. 1993;8:45–59. doi: 10.1007/BF01000529. [DOI] [PubMed] [Google Scholar]
- 31.Tomiyasu K, Inomata K. Enzyme-cytochemical study of small ganglion cells in experimental thiamine deficiency: Concerning the pain mechanism. Acta Neuropathol. 1991;81:396–400. doi: 10.1007/BF00293460. [DOI] [PubMed] [Google Scholar]
- 32.Dreyfus PM. The regional distribution of transketolase in the normal and the thiamine deficient nervous system. J Neuropath Exp Neur. 1965;24:119–129. doi: 10.1097/00005072-196501000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Geel SE, Dreyfus PM. Thiamine deficiency encephalopathy in the developing rat. Brain Res. 1974;76:435–445. doi: 10.1016/0006-8993(74)90820-8. [DOI] [PubMed] [Google Scholar]
- 34.Balaghi M, Pearson WN. Tissue and intracellular distribution of radioactive thiamine in normal and thiamine-deficient rats. J Nutr. 1966;89:127–132. doi: 10.1093/jn/89.2.127. [DOI] [PubMed] [Google Scholar]
- 35.Butterworth RF, Giguere J-F, Besnard A-M. Activities of thiamine-dependent enzymes in two experimental models of thiamine-deficiency encephalopathy. 2. α-Ketoglutarate dehydrogenase. Neurochem Res. 1986;11:567–577. doi: 10.1007/BF00965326. [DOI] [PubMed] [Google Scholar]
- 36.Butterworth RF. Cerebral thiamine-dependent enzyme changes in experimental Wernicke's encephalopathy. Metab Brain Dis. 1986;1:165–175. doi: 10.1007/BF01001778. [DOI] [PubMed] [Google Scholar]
- 37.Rindi G, de Giuseppe L, Ventura U. Distribution and phosphorylation of oxythiamine in rat tissues. J Nutr. 1963;81:147–154. doi: 10.1093/jn/81.2.147. [DOI] [PubMed] [Google Scholar]
- 38.Batifoulier F, Verny M-A, Besson C, Demigne C, Remesy C. Determination of thiamine and its phosphate esters in rat tissues analyzed as thiochromes on a RP-amide C16 column. J Chromatogr B. 2005;816:67–72. doi: 10.1016/j.jchromb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 39.Ronconi RA, Wong SNP. Estimates of changes in seabird numbers in the Grand Manan Archipelago, New Brunswick, Canada. Waterbirds. 2003;26:462–472. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.