Abstract
Although the polysialyltransferase ST8Sia IV is expressed in both primary and secondary human lymphoid organs, its product, polysialic acid (polySia), has been largely overlooked by immunologists. In contrast, polySia expression and function in the nervous system has been well characterized. In this context, polySia modulates cellular adhesion, migration, cytokine response, and contact-dependent differentiation. Provocatively, these same processes are vital components of immune development and function. We previously established that mouse multipotent hematopoietic progenitors use ST8Sia IV to express polySia on their cell surfaces. Here, we demonstrate that, relative to wild-type controls, ST8Sia IV−/− mice have a 30% reduction in total thymocytes and a concomitant deficiency in the earliest thymocyte precursors. T-cell progenitors originate in the bone marrow and are mobilized to the blood at regular intervals by unknown signals. We performed in vivo reconstitution experiments in which ST8Sia IV−/− progenitors competed with wild-type cells to repopulate depleted or deficient immune subsets. Progenitors lacking polySi exhibited a specific defect in T-cell development because of an inability to access the thymus. This phenotype probably reflects a decreased capacity of the ST8Sia IV−/− progenitors to escape from the bone marrow niche. Collectively, these results provide evidence that polySia is involved in hematopoietic development.
Keywords: bone marrow niche, hematopoiesis, mobilization, development
In the adult mouse, hematopoiesis takes place largely within the bone marrow. There, hematopoietic stem cells (HSCs) give rise to prolific multipotent progenitor cells from which all hematopoietic lineages are derived. Much of our knowledge of bone marrow progenitors comes from analyses performed on isolated cells from bone marrow aspirates. In these studies, progenitors (including HSCs) have been broadly defined by the absence of cell-surface lineage markers (Lin−) and the expression of Sca-1 and cKit (LSK) (1, 2). Although isolated LSKs are readily identified by multichannel flow cytometry, the number of antigens (and thus fluors) involved precludes their visualization in tissue sections. Recently, an alternative set of markers comprised of signaling lymphocytic activation molecule (SLAM) family receptors (CD150+, CD48−, CD41−) was described that allows localization of HSCs within tissues (3). This achievement is particularly significant because in the bone marrow HSCs exist in so-called niches, anatomical locations that provide space and supportive signals to the progenitor cells. By exploiting the new SLAM family markers, researchers have begun to explore the bone marrow niche and its relationship to HSCs.
Separate but perhaps related bone marrow niches have been identified—the endosteal and the vascular niches (4). In the former site, HSCs are found adjacent to osteoblasts and osteoclasts covering the endosteum. In addition to this anatomical association, the endosteal cells also have functional interactions with HSCs that are indicative of a bone marrow niche; for instance, mice with increased numbers of osteoblasts have increased numbers of bone marrow HSCs (5, 6). In the latter site, HSCs are associated with sinusoids, venules specialized for transport of macromolecules and cells between tissues and blood. It is thought that sinusoids mediate flux between circulating and bone marrow-bound HSCs (4). Cells in both niches express the chemokine CXCL12 (SDF-1α), the only factor known to induce chemotaxis of HSCs (7) and a critical regulator of HSC maintenance (8). The presence of CXCL12 in both niche locations suggests that the 2 sites may be functionally similar, although anatomically distinct.
For unknown reasons, HSCs undergo periodic cycles of mobilization from bone marrow niches into blood, followed by resettling in empty bone marrow niches. At steady-state levels there are 100–400 circulating long-term HSCs in the blood of a mouse (9). However, when exogenous progenitors are injected intravenously, 90% are cleared from the blood within 30 seconds, demonstrating the rapid flux of HSCs between the 2 compartments (9). An understanding of the mechanisms underlying this exchange would be useful for improving stem cell transplantations and other hematopoietic-related therapies. Empirically, the cytokines granulocyte colony-stimulating factor and stem cell factor have been shown to be efficacious in mobilizing progenitors. In the clinic, the former molecule is used increasingly to enrich hematopoietic progenitors in peripheral blood, which then is used in favor of bone marrow as a source of stem cells for transplants (10).
Granulocyte colony-stimulating factor acts in part by inducing neutrophil secretion of degradative enzymes that break down molecules retaining HSCs in the niche (11). Although it is not clear whether this cytokine also regulates the physiologic flux of HSCs into and out of the bone marrow, some endogenous participants in this process have been identified. An important player is the chemokine CXCL12, which attracts HSCs to the niche. Once HSCs have been recruited, numerous adhesion molecules are thought to be important in their retention within the niche. Annexin II, N-cadherin, CD44, CD164, vascular cell adhesion molecule-1, and intracellular adhesion molecule-1 are all thought to play a role in maintaining HSC/niche interactions (see refs. 12, 13 and references therein).
Although it has yet to be validated in vivo, the neural cell adhesion molecule (NCAM) also is poised to regulate associations between HSCs and their niche. NCAM is expressed on both bone marrow stroma and HSCs (14, 15) and participates in the maintenance of HSCs in culture (16). Importantly, NCAM is a target for 2 sialyltransferases, ST8Sia II and ST8Sia IV, both of which independently produce an unusual glycan, polysialic acid (polySia) (17). This large, negatively charged glycan is composed of α2,8-linked sialic acid residues. Through steric hindrance, polySia inhibits NCAM-mediated adhesion and prohibits close membrane apposition between neighboring cells (18). As a result, polySia expression globally down-regulates cell–cell contacts and promotes cell migration (19).
PolySia was first identified in the nervous system and to date has been studied almost exclusively in that context. However, we recently demonstrated polySia expression in the human and murine immune systems, specifically on human natural killer (NK) cells and murine multipotent hematopoietic progenitors and granulocytes (20). We determined that the polysialyltransferase responsible for “immuno-polySia” expression is sialyltransferase 8Sia IV (ST8Sia IV) and that NCAM is the scaffold for polySia on immune cells. Here, we investigated the functional consequences of ST8Sia IV deficiency on hematopoiesis. We found that ST8Sia IV−/− mice had a reduction in total thymocyte numbers, and we traced this defect to poor access of ST8Sia IV−/− progenitors to the thymus. Short-term and long-term competitive reconstitution assays suggested that lack of polySia predominantly affects progenitor mobilization from bone marrow. These results demonstrate, for the first time, a role for polySia in hematopoietic development.
Results
The Size of Double-Negative (DN)-1, DN2, DN3, and Double-Positive (DP) Subsets Is Decreased in ST8Sia IV−/− Thymi.
ST8Sia IV−/− mice were generated initially for the purpose of studying polySia in the nervous system (21). The mice were determined to be generally healthy animals with normal reproductive capacities and life spans. However, a thorough analysis of the immune compartment in these animals had not been performed before our studies. As an initial assessment of immunological development and homeostasis in ST8Sia IV−/− mice, we counted the numbers and relative percentages of leukocyte subsets in primary and secondary lymphoid organs. The cellularity of ST8Sia IV−/− bone marrow, spleen, lymph nodes (cervical and inguinal), liver, and peripheral blood was normal compared with age-matched (± 1 week) wild-type mice. Hematological analyses revealed essentially no differences between wild-type and ST8Sia IV−/− mice in terms of peripheral leukocyte, erythrocyte, or platelet counts, suggesting that the hematopoietic compartment is largely intact in the null animals (20). However, a prominent phenotype was revealed in the thymus, beginning with a reduction in total cell numbers. The average number of total thymocytes in ST8Sia IV−/− mice 3–9 weeks of age was reduced by about 30% relative to age-matched wild-type controls: 169.2 ± 43.9 million (n = 16) vs. 233.9 ± 53.5 million (n = 18; P = 0.0005).
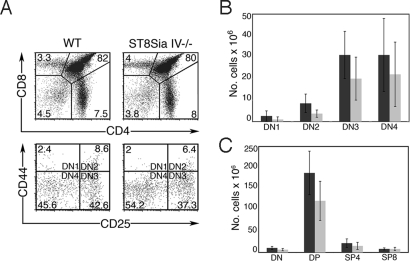
To dissect this phenotype further, thymocytes were analyzed for developmental markers by flow cytometry. T-cell precursors enter the thymus as CD4−CD8− DN progenitors and progress through an intermediate CD4+CD8+ DP stage before losing expression of 1 marker and becoming either CD4+ or CD8+ single-positive (SP) mature cells. DN subsets can be dissected further into very early developmental stages termed DN1–DN4, defined by the expression patterns of CD44 and CD25 (DN1, CD44+CD25−; DN2, CD44+CD25+; DN3, CD44−CD25+; DN4, CD44−CD25−) (22). ST8Sia IV−/− and wild-type mice had comparable percentages of total DN, DP, CD4+SP, and CD8+SP thymocytes (Table 1 and Fig. 1A, Top). However, ST8Sia IV−/− animals had reduced percentages of DN2 (P = 0.001) and DN3 (P = 0.004) subsets as compared with controls (Table 1 and Fig. 1A, Bottom). The ST8Sia IV−/− phenotype was even more apparent when the total number of cells in each of these populations was compared with wild type (Fig. 1 B and C). Significant differences were noted in the DN1 (P = 0.04), DN2 (P = 0.0004), DN3 (P = 0.006), total DN (P = 0.003), and DP (P = 0.0004) groups. Together, these data demonstrated abnormalities in the early stages of thymocyte development in ST8Sia IV−/− mice. Despite these developmental irregularities, the structure of the ST8Sia IV−/− thymus was grossly normal as observed by H&E-stained paraffin sections (data not shown), and we did not observe changes in peripheral T-cell populations.
Table 1.
Percentages of thymocyte developmental subsets found in wild-type and ST8Sia IV−/− mice
| DN | DP | SP4 | SP8 | DN1 | DN2 | DN3 | DN4 | |
|---|---|---|---|---|---|---|---|---|
| Wild type | 4 (1) | 82 (3) | 6 (4) | 3 (1) | 0.1 (0.1) | 0.5 (0.06) | 1.2 (0.3) | 1.2 (0.5) |
| ST8Sia IV−/− | 4 (2) | 80 (5) | 4 (2) | 4 (1) | 0.05 (0.05) | 0.4 (0.1) | 1 (0.2) | 1 (0.9) |
| P-value | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.001 | 0.004 | 0.2 |
Numbers represent the percentage (SD) of total thymocytes in each population. n = 18 wild-type and 16 ST8Sia IV−/− mice. DN, double negative; DP, double positive; SP, single positive.
Fig. 1.
ST8Sia IV−/− mice have decreased numbers of DN and DP subsets. Wild-type (WT) and ST8Sia IV−/− thymocytes were analyzed by flow cytometry. (A) Representative flow cytometry plots of thymocyte populations from individual WT and ST8Sia IV−/− mice. CD4 and CD8 expression defines the DN, DP, SP4, and SP8 populations (Top), whereas CD44 and CD25 expression identifies the DN1–4 subsets (Bottom). The numbers in the panels indicate the percentages comprising each population for the experiment shown. Refer to Table 1 for the mean percentage and SD of each subset. (B) Total cell numbers in DN, DP, SP4, and SP8 and (C) DN1–DN4 thymic populations were assessed in 18 WT (dark gray bars) and 16 ST8Sia IV−/−mice (light gray bars). Statistically significant decreases were noted in ST8Sia IV−/− animals in the DN1 (P = 0.04), DN2 (P = 0.0004), DN3 (P = 0.01), total DN (P = 0.003), and DP (P = 0.0004) subsets.
ST8Sia IV−/− Mice Have Empty Thymic Progenitor Niches.
A reduction in the number of DN2 and DN3 thymocytes has been correlated with decreased occupancy of thymic progenitor niches (1, 23). Empty niches represent available developmental space that can be repopulated by exogenous progenitors. We tested ST8Sia IV−/− mice for empty progenitor niches by injecting age-matched non-irradiated wild-type and ST8Sia IV−/− recipient mice with congenic Thy1.1+ bone marrow. After 3 weeks, thymocytes were analyzed for the presence of Thy1.1+ donor cells (Fig. 2). A significant contribution of Thy1.1+ cells was observed in ST8Sia IV−/− thymi (11.4% ± 5.4%, n = 7). By contrast, wild-type recipients, in accord with previous studies (1), exhibited minimal chimerism (0.47% ± 0.48%, n = 6;P = 0.0005).
Fig. 2.
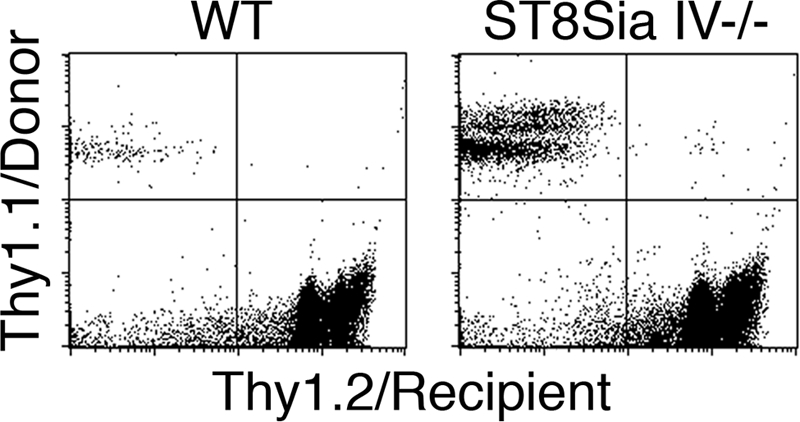
ST8Sia IV−/− mice have empty progenitor niches. Thy1.2+ WT and ST8Sia IV−/− recipients were injected with exogenous donor bone marrow cells, and 3 weeks later thymocytes were analyzed for transferred cells. As expected, WT recipients showed minimal engraftment (Left; 0.47% ± 0.48%, n = 6). In contrast, ST8Sia IV−/− thymocytes consistently contained a significant contribution from donor cells (Right; 11.4% ± 5.4%, n = 7; P = 0.0005). A representative example from 1 WT and 1 ST8Sia IV−/− recipient is shown.
Fewer Early T-Lineage Progenitors Are Observed in the ST8Sia IV−/− Thymus.
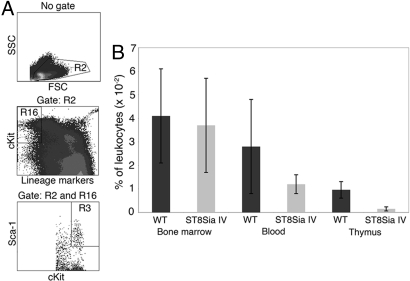
The empty thymic progenitor niches in ST8Sia IV−/− mice suggested a corresponding reduction in early T-lineage progenitors (ETPs), the cells that seed the thymus and occupy these spaces (1, 23). To test this notion, we performed flow cytometric analyses on age-matched samples of wild-type and ST8Sia IV−/− bone marrow, blood, and thymocytes (Fig. 3). HSCs and multipotent progenitors, such as ETPs, are defined broadly as lineage negative (Lin−), cKit+, Sca-1+ cells (LSKs) (2). The percentages of LSKs in bone marrow were comparable in wild-type and ST8Sia IV−/− mice (0.041 ± 0.02% vs. 0.037 ± 0.02%, respectively). The percentage of LSKs in blood was slightly reduced in ST8Sia IV−/− mice (0.028% ± 0.02% in wild-type vs. 0.012% ± 0.004% in null animals), although this difference was not statistically significant. Remarkably, there was a 6-fold decrease (P < 0.000003) in the percentage of LSKs in the ST8Sia IV−/− thymus as compared with wild-type (0.001% ± 0.0008% vs. 0.009% ± 0.003%, respectively).
Fig. 3.
The percentage of early T-lineage progenitors is decreased in the ST8Sia IV−/− thymus. (A) Bone marrow, blood, and thymocytes from age-matched WT and ST8Sia IV−/− (ST8Sia IV) mice were analyzed by flow cytometry for LSKs in the bone marrow, blood, and thymus.(B) The prevalence of LSKs in each organ was assessed as the percentage of total leukocytes. A 6-fold decrease in the percentage of LSKs was observed in ST8Sia−/− thymocytes relative to controls (P < 0.000003).
Long-Term in Vivo Competition Assays Reveal a Severe ST8Sia IV−/− Defect in Thymocyte Development.
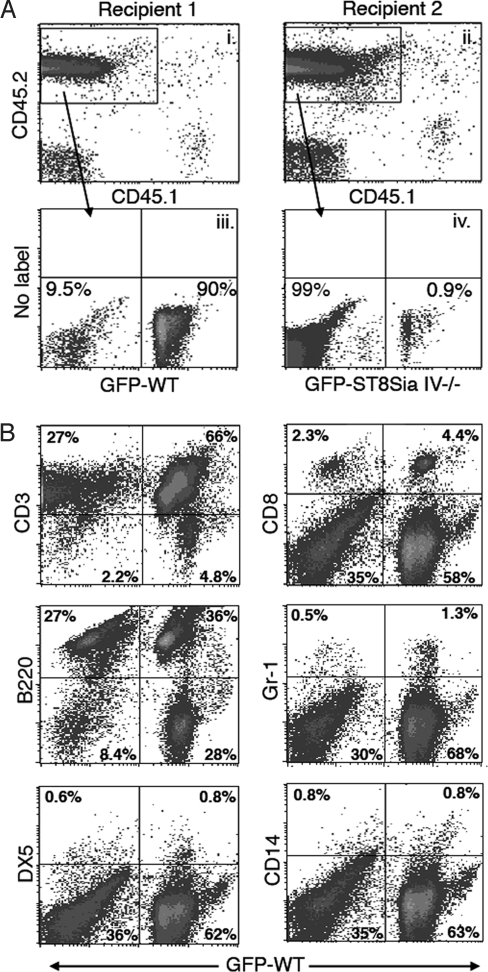
As a first assessment of the T-cell developmental capacity of ST8Sia IV−/− precursors, we tested their ability to reconstitute the thymus of an irradiated recipient mouse. Equal numbers of wild-type or ST8Sia IV−/− bone marrow cells were injected separately into wild-type or ST8Sia IV−/− recipients. In an initial experiment, the number of thymocytes was similar in null and wild-type progenitors, and the genotype of the recipient did not affect the number of thymocytes recovered (see Table S1). Because these data did not shed light on the observed ST8Sia IV−/− thymus phenotype, we moved on to competition experiments, which more thoroughly test the efficiency of thymic colonization by introduced progenitors. Irradiated wild-type recipient mice were reconstituted with a 1:1 ratio of wild-type and ST8Sia IV−/− bone marrow. To identify the source of engrafted lineages, we used CD45.1 congenic recipients and 2 combinations of donor cells: wild-type and ST8Sia IV−/−:GFP+ or wild-type:GFP+ and ST8Sia IV−/−. Recipient mice were killed 4 months after transfer, and thymocytes and splenocytes were analyzed by flow cytometry (Fig. 4). The results were striking: overall, wild-type donors gave rise to 94% ± 4% of total thymocytes, and ST8Sia IV−/− progenitors only contributed to 1.7% ± 1.7% of thymocytes (Fig. 4A and data not shown). By contrast, in the spleen, wild-type precursors yielded 43% ± 19% and ST8Sia IV−/− progenitors yielded 20% ± 5% of total splenocytes (data not shown). Analysis of specific splenocyte lineages (Fig. 4B) demonstrated that both wild-type and ST8Sia IV−/− progenitors contributed significantly to all leukocyte subsets, including B cells (identified as B220+), NK cells (DX5+), monocytes (CD14+), granulocytes (Gr-1+), and T cells (CD3+, CD8+). Overall, both donor strains produced about equal proportions of B cells, NK cells, and monocytes, but wild-type precursors gave rise to about 2-fold more granulocytes (Gr-1+) and about 5-fold more peripheral T cells (CD3 and CD8+) than did their ST8Sia IV−/− counterparts (data not shown). These data suggest that although ST8Sia IV−/− progenitors can contribute to both myeloid and lymphoid lineages, they exhibit an autonomous defect in early T-cell development.
Fig. 4.
ST8Sia IV−/− mice have a T-cell developmental defect. Irradiated CD45.1+ recipient mice were injected with equal numbers of congenic CD45.2+ wild-type and CD45.2+ ST8Sia IV−/− bone marrow cells. In each experiment, 1 of the donor animals (either wild type or ST8Sia IV−/−) also expressed GFP, as a marker of cell origin. Four months after injection, thymocytes (A) and splenocytes (B) were analyzed by flow cytometry. (A) Data from 2 representative recipient mice that received bone marrow transfers containing either wild-type:GFP (Recipient 1) or ST8SiaIV−/−:GFP (Recipient 2) are shown. (i and ii) Thymocyte CD45.1/CD45.2 expression was determined, and donor-derived (CD45.2+) cells were gated for further analyses. (iii) GFP+ cells, derived from wild-type precursors in this experiment, comprised 90% of all CD45.2+ thymocytes, whereas ST8Sia IV−/− descendants (CD45.2+/GFP−) accounted for 9.5%. (iv) GFP+ cells, derived from ST8Sia IV−/− precursors in this experiment, comprised less than 1% of all CD45.2 thymocytes, whereas wild-type descendants (CD45.2+/GFP−) accounted for 99%. (B) The representative splenocyte subset data shown were obtained from Recipient 1 (Panel A), which received donor bone marrow containing wild-type GFP+ progenitors. Splenocytes were analyzed for CD45.2/CD45.1 expression (data not shown), and CD45.2+ cells were gated for further analyses. A panel of antibodies against hematopoietic lineage markers was used to determine the contribution of wild-type and ST8Sia IV−/− donors to various leukocyte populations; co-expression of these antigens and GFP was assessed. Numbers represent the percentage of cells in each quadrant. Note that whereas the thymus of Recipient 1 was largely repopulated by GFP+ wild-type-derived cells (Panel A), the spleen of this animal contained significant numbers of GFP− ST8Sia IV−/−-derived cells (compare right and left quadrants of flow plots). Overall, combined data from all recipient mice revealed that both donors made similar contributions to the B-cell (B220), NK-cell (DX5), and monocyte (CD14) populations; however, wild-type progenitors yielded about twice as many neutrophils (Gr-1) and about 5 times as many T cells (CD8) as did ST8Sia IV−/− precursors (data not shown).
ST8Sia IV−/− Progenitors Compete Well with Wild-Type Controls in Short-Term in Vivo Competition Studies.
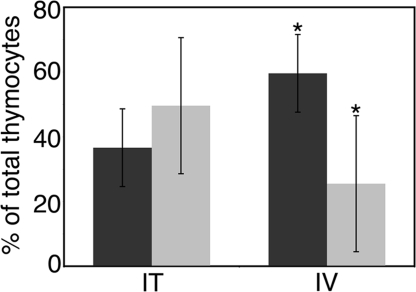
Finally, we asked whether the observed ST8Sia IV−/− phenotype reflected either poor progenitor survival/proliferation within the thymus or inefficient progenitor trafficking to this organ. To test these possibilities, we chose IL-7Rα−/− mice as recipients. These animals have abundant empty progenitor niches because their endogenous T-cell precursors undergo apoptosis due to an inability to respond to the survival factor IL-7. Importantly, their thymic stroma readily supports the development of transferred precursors (23). As in previous experiments, we used a combination of GFP expression and allelic variation to identify the origin of donor-derived cells. A 1:1 mixture of ST8Sia IV−/−:GFP+ and wild-type Thy1.1+ bone marrow was used. Half the recipient animals received the progenitors via a direct injection into the thymus (n = 6). In the remaining recipients, progenitors were introduced intravenously via the tail vein (n = 6). After 3 weeks, thymocytes were analyzed by flow cytometry to determine donor origin. ST8Sia IV−/− progeny were defined as Thy1.2+, Thy1.1−, GFP+, whereas cells derived from wild-type donors were identified as Thy1.2−, Thy1.1+, GFP−. When injected directly into the thymus, ST8Sia IV−/−-derived cells were recovered in equal numbers as wild-type-derived cells (Fig. 5). Conversely, when injected intravenously and forced to home autonomously to the thymus, the ST8Sia IV−/− progenitors did not perform as well as wild-type cells. The mean percentage of tail vein-injected ST8Sia IV−/−-derived thymocytes was decreased significantly (≈2-fold) compared with controls (P < 0.02).
Fig. 5.
ST8Sia IV−/− progenitors do not traffic effectively to the thymus. Bone marrow from Thy1.1 congenic WT (dark gray bars) and GFP-expressing ST8Sia IV−/− mice (light gray bars) was injected 1:1 either intravenously (IV) or intrathymically (IT) into congenic Thy1.2 IL-7Rα−/− recipient mice. Three weeks after injection, thymocytes were analyzed by flow cytometry to determine donor origin. When injected intravenously, ST8Sia IV−/− progenitors did not repopulate the thymus as efficiently as did WT cells (*, P < 0.02).
Discussion
Although the impact of polySia on neuronal development has long been appreciated, its expression and function outside the nervous system remains essentially unexplored. Numerous events regulated by polySia, including cell adhesion, migration, and cytokine response are critical activities in the life of an immune cell. Thus, we postulated that polySia, known to be expressed in lymphoid tissues (20), has a role in hematopoiesis. Here, we show that mice deficient in the polySia-generating sialyltransferase ST8Sia IV had T-cell developmental defects. Functional experiments suggested that this effect was attributable to inefficient thymic colonization by ST8Sia IV−/− progenitors.
Upon first inspection, ST8Sia IV−/− mice appeared healthy, with no overt defects and normal numbers of peripheral leukocyte subsets. However, many developmental phenotypes are masked by powerful homeostatic mechanisms that normalize peripheral cell numbers even in the face of developmental abnormalities (24, 25). Therefore, the decreased number of cells we observed in the ST8Sia IV−/− thymus, a primary lymphoid organ, was not inconsistent with the normal numbers of T cells found in the blood and secondary lymphoid organs of these mice. In fact, mice deficient in P-selectin glycoprotein ligand-1, a glycoprotein involved in LSK homing to progenitor niches, have no overt thymic defect and contain normal peripheral T-cell numbers (1). Further investigation of the ST8Sia IV−/− thymic phenotype revealed reductions in a number of specific developmental subsets, including the early DN2 and DN3 stages, suggesting the possibility of empty progenitor niches. Adoptive transfer studies confirmed that ST8Sia IV−/− mice had available thymic progenitor space that supported the development of competent precursors; this conclusion was supported by flow cytometry experiments showing a significant reduction in the percentage of thymic progenitors in ST8Sia IV−/− mice as compared with controls.
In accord with previous studies showing cyclical release of T-cell progenitors from bone marrow, our measurements of LSK percentages in wild-type and ST8Sia IV−/− bone marrow displayed substantial variation (Fig. 3). It is likely that part of this variation reflected the fact that we made no attempt to synchronize the mice for their progenitor production. Rather, we analyzed LSKs from unconditioned mice over a wide age range (3–12 weeks), thus sampling throughout the ≈4-week cycle involved in the generation and release of T-cell progenitors (26). Similarly, quantitation of LSKs in wild-type blood displayed large variation. Interestingly, the percentage of LSKs varied much less in ST8Sia IV−/− blood than in wild-type blood, suggesting that the release of LSKs into the blood in the null animals may not follow the same cyclical dynamics as in the wild-type mice. This result hints at a role for polySia in the mobilization of LSKs from bone marrow.
Weperformed long-term (4-month) competitive reconstitution experiments to compare the developmental potential of the null progenitors with controls. Notably, the thymus was repopulated almost exclusively by cells of wild-type origin, whereas the spleen contained more balanced contributions from both wild-type and ST8Sia IV−/− donors. Analysis of splenocyte subsets from recipient mice showed that null progenitors gave rise to equal numbers of B cells, NK cells, and monocytes but yielded≈ 5-fold fewer T cells in the spleen as compared with controls. We also noted that ST8Sia IV−/− progenitors produced about 2-fold fewer granulocytes than did their wild-type counterparts, an observation that was consistent with our previous studies demonstrating that these cells express polySia (20). Collectively, these data demonstrated that ST8Sia IV−/− progenitors lost the competition against wild-type cells to repopulate the thymus and generate T cells.
We addressed the possibility that the reduced T-cell developmental potential of ST8Sia IV−/− progenitors reflected a defect in their ability to populate thymic niches. IL-7Rα−/− mice provided an ideal system for this study, because their abundant empty thymic progenitor niches can support development of competent precursors without the requirement for conditioning by irradiation. We placed equal numbers of wild-type and ST8Sia IV−/− donor bone marrow in short-term (3-week) competition for thymic repopulation and used GFP and congenic Thy1.1/Thy1.2 expression to track the origin of resulting thymocytes. To experimentally decouple progenitor trafficking to the thymus from progenitor survival and differentiation within this organ, we used 2 methods to introduce donor bone marrow to the recipient animal— intravenous injection at the tail vein and direct intrathymic injection. As shown in Fig. 5, intrathymically injected ST8Sia IV−/− progenitors competed equally with wild-type cells. This result suggested that the ST8Sia IV−/− precursors survived and proliferated normally within the IL-7Rα−/− thymus. However, when forced to home autonomously through the bloodstream to the thymus, the null progenitors were half as efficient as wild-type cells.
Notably, although the long-term (Fig. 4) and short-term (Fig. 5) competition experiments showed similar trends, the relative contributions of wild-type and ST8Sia IV−/− cells to the total thymocyte populations were very different. Long-term competition resulted in an almost 50-fold advantage of wild-type over null cells, whereas short-term tests showed on average a 2-fold change between the 2 genotypes. We postulate that these disparities reflect the different requirements of these systems. Considering that LSKs exist in a state of flux between the circulation and the niche, the exogenous thymocytes detected in the short-term assay should derive both from progenitors that immediately settled in the thymus upon injection and progenitors that temporarily exited the circulation, remobilized at a later time, and trafficked to the thymus. In contrast, the kinetics of T-cell development dictate that exogenous thymocytes observed in the long-term experiment be derived from donor long-term HSCs that successfully colonized host bone marrow niches. Thus, although the short-term assay reflected both direct progenitor trafficking to the thymus and progenitor mobilization from the bone marrow, the long-term assay required bone marrow mobilization exclusively. The conspicuous difference in the proportion of ST8Sia IV−/−-derived thymocytes in the short-term and long-term assays therefore suggested that polySia had a minimal effect on progenitor trafficking but a significant effect on progenitor mobilization.
Based on these findings, we propose that polySia modulates the adhesive properties of LSKs to impact the mobilization of these cells from the bone marrow. Previous observations are consistent with this hypothesis. First, both polySia and its scaffold NCAM are present on multipotent bone marrow progenitors. We previously demonstrated that a bone marrow population expressing low levels of polySia can give rise to all hematopoietic lineages, including T cells (20). Similarly, NCAM expression has been observed on both bone marrow progenitors and stromal support cells, and this adhesion molecule participates in the maintenance of HSCs in culture (14, 16). Second, polySia has a well-documented role governing adhesion and migration through both global and NCAM-specific effects (19, 27). Third, an analogous function for polySia already has been established in the nervous system, where this glycan motif disrupts NCAM-mediated interactions between neuronal progenitors and their niche, thus promoting differentiation (28). It is possible that NCAM is similarly critical for maintaining HSCs in bone marrow. We favor a model in which NCAM adhesion helps maintain HSCs in the bone marrow niche, and progenitors poised to vacate the niche alleviate these interactions by up-regulating polySia. Settling in thymic progenitor niches may involve a reversal of these steps. Intriguingly, NCAMhigh thymic epithelial cell lines strongly support pro-T-cell colonization and proliferation in vitro, whereas NCAMlow/neg cells do not (29). However, because the thymic niche remains undefined, nothing is yet known about NCAM or polySia expression in that location.
In addition to its known role in modulating NCAM function, polySia also may interact with factors that remain to be identified. In this regard we envision that the structural similarities between polySia and the glycosaminoglycans (GAGs) found in the extracellular matrix reflect functional similarities. For instance, the non-sulfated and relatively uniform GAG hyaluronan (HA) has several specific binding partners, including the adhesion receptors CD44 and receptor for HA-mediated motility (30). Through interactions with these and other molecules, HA mediates critical cellular processes such as adhesion, migration, and proliferation. Similar receptors interacting with polySia could contribute to the known effects of this glycan on migration (31). An alternate scenario is suggested by the well-known role played by the GAG heparan sulfate in establishing chemokine gradients within tissues (32, 33). In a comparable fashion, polySia may modulate cell migration and trafficking through interactions with cytokines or chemokines. Supporting this theory is the recent study by Kanato et al (34), demonstrating direct binding of polySia to brain-derived neurotrophic factor. Finally, ST8Sia IV self-sialylates to create di-sialic acid with an α2,8-linkage, and it is possible that the enzyme generates this disaccharide on other protein substrates as well (35). In fact, similar di-sialic acids were identified on common mouse serum glycoproteins (36). Some of the T-cell developmental defects described herein may be mediated by the absence of these di-sialic acids in ST8Sia IV−/− mice.
In conclusion, this work expands the role played by polySia to include regulation of hematopoiesis. Beyond immune cells, polySia and NCAM have been found on human cancers including small-cell lung carcinomas (37, 38) and multiple myeloma (38). Metastasized cells from both these tumors find refuge in the bone marrow, where they receive both contact-dependant and soluble survival signals that confer de novo drug resistance (39). It is possible that polySia regulates mobilization and dissemination of these cells by the same mechanisms at play in the immune system.
Materials and Methods
Details on mice, antibodies, flow cytometry, and detection of early T-lineage progenitors are given in the SI Text.
Progenitor Niche Assays.
Bone marrow was harvested from donor Thy1.1 mice (5–16 weeks old, age-matched within 1 week) and equal numbers of cells (8–25 × 106 depending on the experiment) were adoptively transferred to non-irradiated, age-matched (±1 week) wild-type C57BL/6 (Thy1.2) or ST8Sia IV−/− (Thy1.2) recipients (4–15 weeks old). After 3 weeks, thymocytes were analyzed by flow cytometry for the presence of the Thy1.1 antigen. The experiment was repeated 4 times with similar results (n = 7 each for wild-type and ST8Sia IV−/− recipients).
Competitive Reconstitution of Irradiated Recipients.
Wild-type congenic CD45.1 recipient mice (6–8 weeks old) were irradiated by exposure to a cesium source (900 rad). Donor bone marrow was prepared from (CD45.2) wild-type:GFP+ and (CD45.2) ST8Sia IV−/− mice (4–5 months old). Bone marrow from each donor was combined 1:1, and equal numbers of cells were injected intravenously into 2–5 irradiated recipients (1.2–6.0 × 106 cells/mouse, depending on the experiment). Recipient animals were maintained on antibiotics for 2 weeks. After 4 months, thymocytes and splenocytes were analyzed by flow cytometry. In concurrent studies, we used wild-type and ST8Sia IV−/−:GFP+ strains as donor mice, following the same protocol. The experiment was repeated 5 times (n = 13 recipient mice) with similar results.
Competitive Reconstitution of IL-7Rα−/− Recipients.
Bone marrow was prepared from age-matched (± 2 weeks) congenic Thy1.1+ wild-type and Thy1.2+ ST8Sia IV−/−:GFP+ donor mice. An equal number of cells (7.0–20 × 106/mouse, depending on the experiment) was injected either intrathymically or intravenously into non-irradiated congenic IL-7Rα−/− (Thy1.2) recipients (6–8 weeks old).
Intrathymic injections were performed on anesthetized animals by manually removing the hair over the sternal notch, sterilizing the area, and then manipulating a Hamilton syringe (10-μL volume) through the skin, under the sternal notch, and into the thymus. The process was repeated once so that a total of 20 μL was delivered intrathymically per animal. For intravenous injections, the cell density was decreased 10-fold, and 200 μL of cells were injected via the tail vein. After 3–4 weeks, thymocytes were prepared, counted by hemocytometer, and analyzed by flow cytometry to determine donor origin. The experiment was repeated 3 times with similar results (n = 6 each for intrathymic and intravenous delivery).
Statistics.
Unless otherwise stated, average numbers represent the mean (1 SD). Statistical significance was calculated using an unpaired 2-tailed Student's t test. Exact p-values are reported; P < 0.05 was considered significant.
Supplementary Material
Acknowledgments.
The research was made possible by grant RS1–00365from the California Institute for Regenerative Medicine (CIRM). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of CIRM or any other agency of the State of California.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905188106/DCSupplemental.
References
- 1.Rossi FM, et al. Recruitment of adult thymic progenitors is regulated by P-selectin and its ligand PSGL-1. Nat Immunol. 2005;6(6):626–634. doi: 10.1038/ni1203. [DOI] [PubMed] [Google Scholar]
- 2.Schwarz BA, Bhandoola A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol. 2004;5(9):953–960. doi: 10.1038/ni1101. [DOI] [PubMed] [Google Scholar]
- 3.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 4.Kiel MJ, Morrison SJ. Maintaining hematopoietic stem cells in the vascular niche. Immunity. 2006;25(6):862–864. doi: 10.1016/j.immuni.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 7.Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195(9):1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294(5548):1933–1936. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 10.Thomas J, Liu F, Link DC. Mechanisms of mobilization of hematopoietic progenitors with granulocyte colony-stimulating factor. Current Opinion in Hematology. 2002;9(3):183–189. doi: 10.1097/00062752-200205000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Levesque JP, et al. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol (Charlottesville, Va) 2002;30(5):440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 12.Haug JS, et al. N-cadherin expression level distinguishes reserved versus primed states of hematopoietic stem cells. Cell Stem Cell. 2008;2(4):367–379. doi: 10.1016/j.stem.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Jung Y, et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110(1):82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas PS, et al. Demonstration of neural cell adhesion molecules on stromal cells that support lymphopoiesis. Leukemia. 1988;2(3):171–175. [PubMed] [Google Scholar]
- 15.Wang X, et al. The characteristics of hematopoietic stem cells from autoimmune-prone mice and the role of neural cell adhesion molecules in abnormal proliferation of these cells in MRL/lpr mice. Haematologica. 2007;92(3):300–307. doi: 10.3324/haematol.10603. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, et al. Neural cell adhesion molecule contributes to hemopoiesis-supporting capacity of stromal cell lines. Stem Cells. 2005;23(9):1389–1399. doi: 10.1634/stemcells.2004-0343. [DOI] [PubMed] [Google Scholar]
- 17.Colley KJ. Structural basis for the polysialylation of the neural cell adhesion molecule. Neurochem Res. 2008 doi: 10.1007/978-1-4419-1170-4_7. in press. [DOI] [PubMed] [Google Scholar]
- 18.Johnson CP, Fujimoto I, Rutishauser U, Leckband DE. Direct evidence that neural cell adhesion molecule (NCAM) polysialylation increases intermembrane repulsion and abrogates adhesion. J Biol Chem. 2005;280(1):137–145. doi: 10.1074/jbc.M410216200. [DOI] [PubMed] [Google Scholar]
- 19.Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nature Reviews Neuroscience. 2008;9(1):26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- 20.Drake PM, et al. Polysialic acid, a glycan with highly restricted expression, is found on human and murine leukocytes and modulates immune responses. J Immunol. 2008;181(10):6850–6858. doi: 10.4049/jimmunol.181.10.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckhardt M, et al. Mice deficient in the polysialyltransferase ST8SiaIV/PST-1 allow discrimination of the roles of neural cell adhesion molecule protein and polysialic acid in neural development and synaptic plasticity. J Neurosci. 2000;20(14):5234–5244. doi: 10.1523/JNEUROSCI.20-14-05234.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150(10):4244–4252. [PubMed] [Google Scholar]
- 23.Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J Immunol. 2004;173(3):1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- 24.Almeida AR, Borghans JA, Freitas AA. T cell homeostasis: Thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors. J Exp Med. 2001;194(5):591–599. doi: 10.1084/jem.194.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jameson SC. T cell homeostasis: Keeping useful T cells alive and live T cells useful. Semin Immunol. 2005;17(3):231–237. doi: 10.1016/j.smim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193(3):365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinhold B, et al. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280(52):42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- 28.Gascon E, Vutskits L, Kiss JZ. The role of PSA-NCAM in adult neurogenesis. Neurochem Res. 2008 doi: 10.1007/978-1-4419-1170-4_8. in press. [DOI] [PubMed] [Google Scholar]
- 29.Imaizumi A, Goldschneider I, Yoshida T. Reproducible procedures for establishing mouse thymic stromal cell lines. Cell Immunol. 1993;150(1):81–89. doi: 10.1006/cimm.1993.1180. [DOI] [PubMed] [Google Scholar]
- 30.Entwistle J, Hall CL, Turley EA. HA receptors: Regulators of signalling to the cytoskeleton. J Cell Biochem. 1996;61(4):569–577. doi: 10.1002/(sici)1097-4644(19960616)61:4<569::aid-jcb10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, Vutskits L, Calaora V, Durbec P, Kiss JZ. A role for the polysialic acid-neural cell adhesion molecule in PDGF-induced chemotaxis of oligodendrocyte precursor cells. J Cell Sci. 2004;117(Pt 1):93–103. doi: 10.1242/jcs.00827. [DOI] [PubMed] [Google Scholar]
- 32.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 33.Kuschert GS, et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry. 1999;38(39):12959–12968. doi: 10.1021/bi990711d. [DOI] [PubMed] [Google Scholar]
- 34.Kanato Y, Kitajima K, Sato C. Direct binding of polysialic acid to a brain-derived neurotrophic factor depends on the degree of polymerization. Glycobiology. 2008;18(12):1044–1053. doi: 10.1093/glycob/cwn084. [DOI] [PubMed] [Google Scholar]
- 35.Kitazume-Kawaguchi S, Kabata S, Arita M. Differential biosynthesis of polysialic or disialic acid Structure by ST8Sia II and ST8Sia IV. J Biol Chem. 2001;276(19):15696–15703. doi: 10.1074/jbc.M010371200. [DOI] [PubMed] [Google Scholar]
- 36.Yasukawa Z, Sato C, Sano K, Ogawa H, Kitajima K. Identification of disialic acid-containing glycoproteins in mouse serum: A novel modification of immunoglobulin light chains, vitronectin, and plasminogen. Glycobiology. 2006;16(7):651–665. doi: 10.1093/glycob/cwj112. [DOI] [PubMed] [Google Scholar]
- 37.Miyahara R, et al. Expression of neural cell adhesion molecules (polysialylated form of neural cell adhesion molecule and L1-cell adhesion molecule) on resected small cell lung cancer specimens: In relation to proliferation state. J Surg Oncol. 2001;77(1):49–54. doi: 10.1002/jso.1065. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki M, et al. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology. 2005;15(9):887–894. doi: 10.1093/glycob/cwi071. [DOI] [PubMed] [Google Scholar]
- 39.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14(9):2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.