Abstract
Some dinosaurs reached masses that were ≈8 times those of the largest, ecologically equivalent terrestrial mammals. The factors most responsible for setting the maximal body size of vertebrates are resource quality and quantity, as modified by the mobility of the consumer, and the vertebrate's rate of energy expenditure. If the food intake of the largest herbivorous mammals defines the maximal rate at which plant resources can be consumed in terrestrial environments and if that limit applied to dinosaurs, then the large size of sauropods occurred because they expended energy in the field at rates extrapolated from those of varanid lizards, which are ≈22% of the rates in mammals and 3.6 times the rates of other lizards of equal size. Of 2 species having the same energy income, the species that uses the most energy for mass-independent maintenance of necessity has a smaller size. The larger mass found in some marine mammals reflects a greater resource abundance in marine environments. The presumptively low energy expenditures of dinosaurs potentially permitted Mesozoic communities to support dinosaur biomasses that were up to 5 times those found in mammalian herbivores in Africa today. The maximal size of predatory theropods was ≈8 tons, which if it reflected the maximal capacity to consume vertebrates in terrestrial environments, corresponds in predatory mammals to a maximal mass less than a ton, which is what is observed. Some coelurosaurs may have evolved endothermy in association with the evolution of feathered insulation and a small mass.
Keywords: ectothermy, endothermy, energy expenditure, varanid lizards
Whether dinosaurs were endotherms or ectotherms has been controversial, as has been whether their thermal biology had any relevance to their attainment of extraordinarily large masses. Some authors (1–4) maintained that dinosaurs must have been endotherms because of their size, upright posture, bone structure, growth rates, presumed level of activity, and the high-latitude distributions of some species. Others (5–9) suggested that dinosaurs had a thermally constant body temperature as a result of large masses and small surface-to-volume ratios, but they probably had much lower levels of energy expenditure than would be expected of mammals (or birds) of the same mass, which was indicated by intermediate growth rates, narrow nasal passages, and unmodified, bellows-like septate lungs, which implies low ventilation rates. What most of these analyses have neglected is that the consumed resources ultimately control the energy expenditure and body size of organisms. Here, I propose that the maximal size of vertebrates is determined by resource abundance and how it is used by species.
A Model.
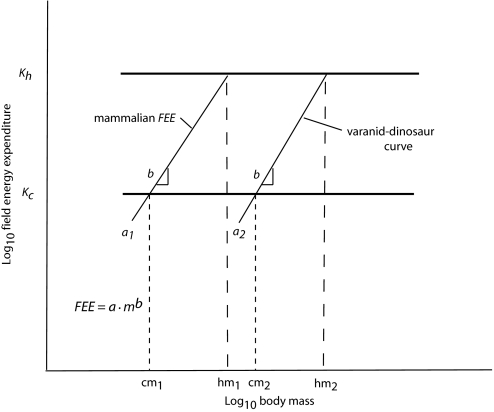
The maximal size of vertebrates is limited principally by the abundance and quality of the resources used to sustain their activities. The maximal daily field expenditure (K, kJ/d) of an individual varies with a variety of factors, including its mass, mobility, and the foods consumed. For example, the maximal expenditures (Kh) and body masses of herbivores are greater than the maximal expenditures (Kc) and body masses of carnivores (Fig. 1) because the resource base for herbivores is greater (10). The field energy expenditure (FEE) of an individual equals a·mb, where a is a coefficient that determines the level at which energy is expended, m is its mass, and b is the power of mass (11, 12). Over a large range in mass, b is fixed between 0.67 and 0.75 (12), so if the maximal individual FEE equals K, a tradeoff occurs between a and m; if a increases, m must decrease, and as a decreases, m may, or may not, increase, depending on the circumstances in the environment and the characteristics of the species, including its food habits (Fig. 1). That is, given a restraint on total energy expenditure, an individual with a lower mass-independent expenditure (represented by a) can attain a larger mass than one with a higher expenditure (6, 13), at least as long as the low-expenditure individual has sufficient mobility to find resources adequate to support its mass and expenditures. However, at 1 extreme along a continuum, the most sluggish of species would not be able to sustain the largest masses potentially permitted by resources because they could not find a sufficient resource base in a limited area to support a large mass, which therefore would reduce K and maximal m. Large species could maintain a constant body temperature over a range in energy expenditures facilitated by thermal inertia and a small surface-to-volume ratio. Evidence of the validity of this analysis would be that communities accommodated larger population biomasses of consumers with low mass-independent expenditures than they would of ecologically similar consumers with high mass-independent expenditures (6). The questions that remains are: Will this approach quantitatively account for the huge masses that were found in many herbivorous and carnivorous dinosaurs, the even larger masses of some marine mammals, and the intermediate masses of large reptiles? A preliminary answer (13) was that it would not, but the analysis reported here suggests that it will.
Fig. 1.
A general model of the relationship among log10 field energy expenditure, log10 body mass, and the level at which expenditure (a) occurs. Maximal field expenditures are indicated for terrestrial herbivores (Kh) and carnivores (Kc). When a1 > a2, the herbivore mass hm2 > hm1, and the carnivore mass cm2 > cm1.
Results
Application of the Model.
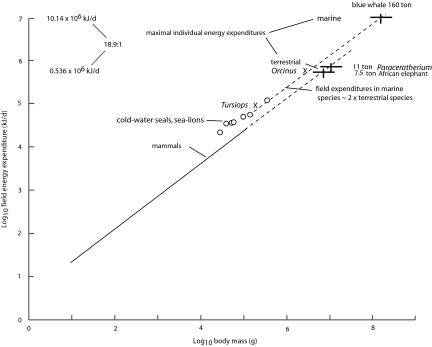
Extensive measurements of the FEEs of vertebrates have been made through the use of doubly labeled water (11). As expected, in mammals it increases with body mass (Fig. 2): FEE (kJ/d) = 4.82g0.734, where m is in grams. This relationship estimates that a 7.5-ton African elephant (Loxodonta africana), the largest living terrestrial herbivore, would have a FEE equal to 5.36 × 105 kJ/d and that an 11- to 15-ton Paraceratherium transouralicum, an extinct rhinoceros relative that is the largest known terrestrial mammalian herbivore (14), may at 11 tons have had a FEEs equal to 7.10 × 105 kJ/d. These values may indicate the maximal expenditures (Kh) of herbivorous vertebrates in a terrestrial environment, reflecting the abundance and quality of the available resources, and the ability of these species to move from 1 area to another to satisfy their nutritional requirements. Evidence indicates, however, that the mass-independent FEEs of marine mammals are approximately twice those of terrestrial species (Fig. 2), which may reflect a high cost of temperature regulation, at least in small species (15), and the abundance of resources in marine environments, which permits very large masses and high growth rates (16). This curve estimates that a blue whale weighing 160 tons would have a FEE equal to ≈10.14 × 106 kJ/d, which is 18.9 times the estimated expenditure of the African elephant. This value implies that baleen whales are more efficient consumers than terrestrial species, reflecting a lower cost of transport in water (17), a large, flexible feeding apparatus, and the high abundance and digestibility of plankton. In contrast, much of terrestrial plant mass consists of woody support tissue, most of which is unusable by vertebrates, and some foliage is vertically inaccessible to terrestrial species.
Fig. 2.
Log10 field energy expenditures of mammals as a function of log10 body mass. The solid curve is for 79 species as measured by doubly labeled water (11). Data on the energy expenditure of 9 species of cold-water marine mammals indicate that their field expenditures average approximately twice those of terrestrial species. The curve for terrestrial species is extrapolated (dashed line) to the mass of an African elephant and a Paraceratherium, and the curve for marine mammals is extrapolated to the mass of a blue whale.
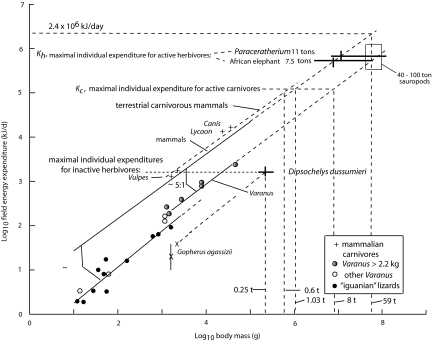
Most lizards in a terrestrial environment have FEEs that are only ≈6.2% of mammals of the same size (11) (Fig. 3) because they are ectothermic, i.e., do not regulate body temperature through the metabolism of foodstuffs. If dinosaur FEEs conformed to this lizard curve, they would have to weigh 330 tons to attain the elephant's estimated FEE, a mass that is much greater than the 40 to 80, and possibly 100 (18), tons found in the largest sauropods. Therefore, it is unlikely that dinosaurs had such low FEEs. Aggressively predatory lizards of the genus Varanus, including the largest living lizard, the Komodo dragon (V. komodoensis), which feeds on deer, pigs, and occasionally water buffalo and people (19), have FEEs that average 3.6 times those of most lizards (11) (Fig. 3), reflecting their high level of activity and high body temperatures (20). The relationship between FEE and body mass in 6 species of varanids, weighing 2.2 to 45.2 kg, is 1.07g0.735 (r2 = 0.964), which is 22% of mammalian FEEs of equal size (Fig. 3). If sauropods followed the varanid curve, the FEE of a 59-ton species would equal that of a 7.5-ton elephant and that of a 83-ton species would equal the FEE of a 11-ton Paraceratherium, i.e., when dinosaurs had masses between 7.5 and 7.9, nearly 8, times those of ecologically equivalent mammals.
Fig. 3.
Log10 field energy expenditures of lizards, desert tortoise, 6 species of varanid lizards, and 4 terrestrial carnivores as a function of log10 body mass (data from 11 and 26). As in Fig. 2, the mammal and varanid curves are extrapolated (dashed lines) to estimate field expenditures of an African elephant and Paraceratherium. FEEs expected for an Aldabran tortoise is estimated from a curve fitted to data from a desert tortoise. The maximal mass for a terrestrial mammalian carnivore is estimated from the FEE of an 8-ton theropod under the assumption that the theropod lies on the varanid curve.
The primary production of communities determines the maximal herbivore abundance and densities (21, 22), which of course reflects the rate at which herbivores consume resources. With herbivorous dinosaurs conforming to the varanid curve, terrestrial communities would be expected to accommodate larger population biomasses of dinosaurs than of mammals (6). For example, the highest mammalian biomass on the African plains varies between 17,500 and 20,000 kg/km2 (23). If herbivorous dinosaurs had FEEs that were only 22% of their mammalian equivalents and if Mesozoic plant communities were about as productive as East African communities today, the maximal dinosaur biomasses would be expected to fall between 80,000 and 90,000 kg/km2, similar to an estimate by Coe et al. (24).
As evidence that low FEEs lead to high consumer biomasses, Aldabran tortoises (Dipsochelys dussumieri) have been estimated (25) to maintain a biomass of 53,000 kg/km2 in the area of highest concentration, which is ≈3 times the biomass densities of African mammalian herbivores. The best estimate for its FEE can be derived from that of a 1.6-2.1 kg of North American desert tortoise (Gopherus agassizii), an inactive, ectothermic herbivore, which has a FEE that is ≈15% of that found in varanids (11, 26) (Fig. 3). The low FEE of tortoises is related to their “sluggish” lifestyle, which may be related to body composition, e.g., as reflected in small muscles masses and heavy protective armor, coupled with low rates of metabolism and low growth rates, an extremist approach to existence. Thus, desert tortoises are active for only 153 h/y, 1.7% of a year (27), a pattern seen in other tortoises (28, 29). An extrapolation of the tortoise's FEE suggests that a 250-kg male Aldabran tortoise might have a FEE equal to ≈1.43 × 103 kJ/d (Fig. 3), which may approach the maximal energy expenditure associated with its ponderous lifestyle. This intake could never support vertebrates the size of elephants or sauropods. The primary production on Aldabra may also be appreciably lower than that in East Africa. In contrast, African elephants are known to move long distances, especially in relation to the onset of rainfall (30), whereas most large dinosaurs appear to have been geographically restricted to comparatively small areas (31, 32), which also implies low energy expenditures in dinosaurs (compared with a mammalian standard), although large species in the Late Jurassic were less provincial (33).
This analysis of biomass densities assumes that the abundance and quality of the resources used by living elephants are similar to those that were available to herbivorous dinosaurs. The terrestrial vegetation that was available in the Mesozoic included an abundance and diversity of ferns, horsetails, Ginkgo, and conifers (5), which some analysts (34) have argued were of equal nutritional quality to plants presently available, thereby implying that the maximal Kh was similar to that found at present. If correct, this assumption does not permit sauropods to conform to the mammalian FEE curve because a 59-ton sauropod would have had an expenditure equal to ≈2.44 × 106 kJ/d, or 4.6 times that of a 7.5-ton African elephant (Fig. 3)! Daily feeding time in terrestrial mammalian megaherbivores increases with body size (35), a 3-ton African elephant spending ≈16 of 24-h feeding (36). Consequently, the mass-independent food intake of a 40 to 100-ton dinosaur must have been lower than found in mammals. If, however, the quality of the vegetation used by herbivorous dinosaurs in the Mesozoic was of a poorer quality than available today (5, 23), e.g., grasses had not yet evolved, then the maximal FEEs of dinosaurs would have been lower because of a decrease in Kh.
One way that herbivorous dinosaurs might have had a higher Kh is if they swallowed their food without mastication (4), whereas herbivorous mammals chew their food, which limits food intake. However, the rapid swallowing of coarse food is unlikely to increase Kh, because the limiting factor on food consumption then would be the rate of fermentation in the gut, which is reduced by swallowing unmasticated fibrous food, the time required for fermentation increasing with food intake and body mass (35). Thus, the huge abdominal masses of sauropods were undoubtedly large fermentation vats that may not have completely compensated for the absence of buccal processing of food. So, it is unlikely that Kh was appreciably higher for dinosaurs than for mammals, either because of greater food abundance or because of a higher efficiency in processing food, and thus could not account for their larger masses.
This analysis also applies to carnivores (Fig. 1). If one assumes that an 8-ton theropod (37) has a FEE that fell on the varanid curve, the estimated Kc for terrestrial carnivores would be ≈1.25 × 105 kJ/d, which is equivalent in mammals to 1.03 tons (Fig. 3). Terrestrial mammalian carnivores, however, have FEEs (10) that are 50% greater than the general mammalian curve (Fig. 3), which would further limit mammalian mass. If the theropod estimate of Kc is applied to the mammalian curve that has a carnivore “correction” of 1.50, a = 4.82 × 1.50 = 7.23, then the estimated maximal size of a terrestrial mammalian carnivore is 594 kg. This analysis implies that the largest terrestrial mammalian carnivores should fall between 0.6 and 1.0 ton, which encompasses the largest species known to have existed, including the creodont Megistotherium osteothlastes (880 kg) (38) and the short-faced bear (Arctodus simus, 0.7 to 1.0 ton) (39), a species that was committed to carnivory (40). Theoretical considerations (41) also limit terrestrial mammalian carnivores to masses <1 ton, although male polar bears (Ursus maritimus) may get up to 1 ton, but in a marine environment, where Kc is higher, and the largest vertebrate-eating specialist is the killer whale (Orcinus orca) at 9 tons.
Potential Difficulties.
A potential conflict with this analysis is that some estimates of growth rates in large theropod and sauropod dinosaurs (4, 42) were equal to those of mammals and precocial birds. Growth rates are 10-fold higher in endotherms than in ectotherms (16), and their value in mammal's increases with rate of metabolism (43). Yet, juvenile altricial birds are brooded by their parents and have lower rates of metabolism and higher growth rates than thermoregulating, juvenile precocial birds (44), which undercuts the concept that high growth rates always require high rates of metabolism. Other estimates of dinosaur growth rates (45, 46) are intermediate to those found in reptiles and precocial birds, which is compatible with the analysis of the energetics of dinosaurs proposed here and with the conclusion that “…large dinosaurs would grow quickly merely by virtue of inertial homoiothermy…” (47). But high growth rates, should they have existed in dinosaurs, may have resulted from the ability of species with low maintenance costs to channel more energy into growth and development than is found in endotherms (48, 49), which appears to be the case in altricial birds (50).
Another potential problem for this analysis is found in the occurrence of dinosaurs on the North Slope of Alaska (51). The Cretaceous flora at this locality was dominated by deciduous vegetation with an absence of evergreen species, which implies a cool-to-cold climate. These conditions have led to the argument that (these?) dinosaurs had an energy expenditure intermediate to that typical of reptiles and mammals (3, 52), namely at the “tenrec” level, which equals 70% of the mammal curve in the 8 largest tenrecs measured (53). Could dinosaurs have withstood presumptively cool-to-cold ambient temperatures with rates of metabolism that were at the varanid level, or could these dinosaurs have evaded seasonally low temperatures through migration (51)? Migration appears to have been unlikely (45), which must have been the case in island New Zealand (54), although migratory herds may have led to the production of the enormous bone beds of hadrosaurs and ceratopsians in western North America (55). A biophysical analysis (56) indicated that dinosaurs >2 tons would have been able to maintain Tb > 30 °C by behavioral temperature regulation at latitudes up to 55 °N, even under the assumption that they had rates of metabolism equal to those of crocodiles, which, given their propensity for sit-and-wait predation, presumably are below those of varanids. A difficulty here is the inability to define exactly the Cretaceous climate on the north slope. However, the atmospheric CO2 in the Cretaceous may well have been severalfold greater than at present with the lack of a polar ice-cap “…and a spread of low latitude marine and terrestrial organisms to higher latitudes” (57). So, the occurrence in dinosaurs at high latitudes may not have required high rates of metabolism.
Finally, measurements of oxygen isotopes contained in the bone phosphates of dinosaurs indicated only small differences in the temperature of various body parts, most notably in the 6-ton Tyrannosaurus (58) and the 8-ton Gigantosaurus (59), which implies that these dinosaurs were homeothermic, which is undoubtedly correct. There is also evidence (60) that some small juvenile and adult dinosaurs were more homeothermic than a Cretaceous varanid. Yet, this varanid was only ≈1 m long, which if it were a V. komodoensis would only weigh ≈1.5 kg (19), and small, living varanids have lower FEEs for their size than larger individuals (Fig. 3) (11). Furthermore, the suggestion (59) that the avian metabolism-mass curve can be used to predict dinosaur rates is unacceptable because its low power, compared with the mammalian curve, results principally from the accumulation of flightless birds at large masses (11, 61); flightless birds essentially have mammalian rates of metabolism (61). Besides, a mammalian, or even worse an avian, FEE in dinosaurs >10 tons would produce enough heat to threaten a heat stroke (62), unless they had some elaborate heat dissipating structures, such as elephants have with their ears.
Conclusions
I conclude that large herbivorous and carnivorous dinosaurs were homeothermic as a result of their very large masses (62), but they were not characterized by rates of metabolism that would be expected in mammals or flighted birds, which suggests that intermediate body temperatures that varied with body mass probably characterized sauropods and theropods (62, 63). The distinction in energetics between ectothermy and endothermy is clearest at masses <50 g (61), whereas at huge masses, this difference may be only marginally distinguishable. The presence of rates of metabolism in dinosaurs intermediate to those of most living reptiles and living birds and mammals (5, 6) is supported by a consideration of areas occupied (6, 45, 64), population sizes (6, 64), theropod coexistence (45, 64, 65), and an analysis of bone oxygen isotopes (58–60), which probably led to population biomass densities appreciably greater than found today in East African mammals (6, 21, 64). The conclusion (59) that dinosaurs had FEEs that were 5 times those of lizards (31% of mammals) and attained mammalian values at 8 times the mass of mammals is similar to the conclusion here that these ratios were 3.6 (22% of mammals) and 8 times, respectively. An appreciable physiological diversity appears to have been present in dinosaurs reflecting their anatomical, ecological, and behavioral diversities (5), especially given that some coelurosaurs progressively evolved a small mass [as small as 1-4 kg (66)], had feathered insulation (67), and possibly were evolving endothermy (56), the only dinosaurs to which this term might apply. If so, it is especially noteworthy that endothermy in dinosaurs may have evolved in association with a reduction in mass, as was apparently the case in the evolution of endothermy in the phylogeny of mammals (68).
Acknowledgments.
The author thanks Martin Sander and Marcus Clauss for the invitation to participate in a sauropod body size workshop in Bonn, thus prompting this work; David Dilcher, Jamie Gillooly, Harvey Lillywhite, Vassiliki B. Smocovitis, and David Steadman (all from the University of Florida); Marcus Clauss (Universität Zürich); Martin Sander (Universität Bonn); Meike Köhler (Universidad Autonoma de Barcelona); Doug Glazier (Juniata College); James Farlow (Indiana-Purdue University at Fort Wayne); and Stephan Pickering (Santa Cruz, CA) for their thoughtful suggestions on earlier versions of this manuscript; and Tom Lehman (Texas Tech University), and James Brown (University of New Mexico), who acted on behalf of the Editorial Board of PNAS.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Bakker RT. Dinosaur heresy-dinosaur renaissance. In: Thomas RDK, Olson EC, editors. A Cold Look at the Warm-Blooded Dinosaurs. Boulder, CO: Westview Press; 1980. pp. 351–462. [Google Scholar]
- 2.Ostrom JH. The evidence for endothermy in dinosaurs. In: Thomas RDK, Olson EC, editors. A Cold Look at the Warm-Blooded Dinosaurs. Boulder, CO: Westview Press; 1980. pp. 5–54. [Google Scholar]
- 3.Paul GS. Physiological, migratorial, climatological, geophysical, survival, and evolutionary implications of Cretaceous polar dinosaurs. J Paleont. 1988;62:640–652. [Google Scholar]
- 4.Sander PM, Clauss M. Sauropod gigantism. Science. 2008;322:200–201. doi: 10.1126/science.1160904. [DOI] [PubMed] [Google Scholar]
- 5.Weaver JC. The improbable endotherms: The energetics of the sauropod dinosaur. Brachiosaurus Paleobiology. 1983;9:173–182. [Google Scholar]
- 6.Farlow JO, Dodson P, Chinsamy A. Dinosaur biology. Ann Rev Ecol Syst. 1995;26:445–471. [Google Scholar]
- 7.Ruben JA, et al. The metabolic status of some late Cretaceous dinosaurs. Science. 1996;273:1204–1207. [Google Scholar]
- 8.Ruben JA, Jones TD, Geist NR, Hillenius WJ. Lung structure and ventilation in theropod dinosaurs and early birds. Science. 1997;278:1267–1270. [Google Scholar]
- 9.Seebacher F, Grigg GC, Beard LA. Crocodiles as dinosaurs: Behavioural thermoregulation in very large ectotherms leads to high and stable body temperatures. J Exp Biol. 1999;202:77–86. doi: 10.1242/jeb.202.1.77. [DOI] [PubMed] [Google Scholar]
- 10.Ricklefs RE. The Economy of Nature. New York, NY: Freeman; 1996. [Google Scholar]
- 11.Nagy KA, Girard IA, Brown TK. Energetics of free-ranging mammals, reptiles, and birds. Ann Rev Nutr. 1999;19:247–277. doi: 10.1146/annurev.nutr.19.1.247. [DOI] [PubMed] [Google Scholar]
- 12.Nagy KA. Review. Field metabolic rate and body size. J Exp Biol. 2005;208:1621–1625. doi: 10.1242/jeb.01553. [DOI] [PubMed] [Google Scholar]
- 13.Burness GP, Diamond J, Flannery T. Dinosaurs, dragons, and dwarfs: The evolution of maximal body size. Proc Natl Acad Sci USA. 2001;98:14518–14523. doi: 10.1073/pnas.251548698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortelius M, Kappelman J. The largest land mammal ever imagined. Zool J Linn Soc. 1993;107:85–101. [Google Scholar]
- 15.Ochoa-Acuña HG, McNab BK, Miller EH. Seasonal energetics of northern phocid seals. Comp Biochem Physiol A. 2009;152:341–350. doi: 10.1016/j.cbpa.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Case TJ. One the evolution and adaptive significance of postnatal growth rates in the terrestrial vertebrates. Quart Rev Biol. 1978;53:243–282. doi: 10.1086/410622. [DOI] [PubMed] [Google Scholar]
- 17.Fish FE. Aquatic locomotion. In: Tomasi TE, Horton TH, editors. Mammalian Energetics. Ithaca, NY: Cornell Univ Press; 1992. pp. 34–63. [Google Scholar]
- 18.Appenzeller T. Paleontology-Argentine dinos vie for heavyweight title. Science. 1994;266:1805. doi: 10.1126/science.266.5192.1805. [DOI] [PubMed] [Google Scholar]
- 19.Auffenberg W. The Behavioral Ecology of the Komodo Monitor. Gainesville, FL: University Press Florida; 1981. [Google Scholar]
- 20.McNab BK, Auffenberg W. The effect of large body size on the temperature regulation of the Komodo dragon, Varanus komodoensis. Comp Biochem Physiol A. 1976;55:345–350. doi: 10.1016/0300-9629(76)90058-x. [DOI] [PubMed] [Google Scholar]
- 21.East R. Rainfall, soil nutrient status, and biomass of large African savannah mammals. Afr J Ecol. 1984;22:245–270. [Google Scholar]
- 22.Pettorelli N, Bro-Jørgenssen J, Durant SM, Blackburn T, Carbone C. Energy availability and density estimates in African ungulates. Am Nat. 2009;173:698–704. doi: 10.1086/597379. [DOI] [PubMed] [Google Scholar]
- 23.Coe MJ, Cumming DH, Phillipson J. Biomass and production of large African herbivores in relation to rainfall and primary production. Oecologia. 1976;22:341–354. doi: 10.1007/BF00345312. [DOI] [PubMed] [Google Scholar]
- 24.Coe MJ, Dilger DL, Farlow JO, Janzen DM, Russell DA. Dinosaurs and land plants. In: Frus EM, Chaloner WG, Crane PR, editors. The Origins of Angiosperms and Their Biological Consequences. Cambridge, UK: Cambridge Univ Press; 1987. pp. 225–258. [Google Scholar]
- 25.Bourn D, Coe M. The size, structure, and distribution of the giant tortoise population of Aldabra. Phil Trans Roy Soc B. 1978;282:139–175. [Google Scholar]
- 26.Henen BT. Seasonal and annual energy budgets of female desert tortoises (Gopherus agassizii) Ecology. 1997;78:283–296. [Google Scholar]
- 27.Nagy KA, Medica PA. Physiological ecology of desert tortoises in southern Nevada. Herptelogica. 1986;42:73–82. [Google Scholar]
- 28.Lagarde F, et al. Foraging behaviour and diet of an ectothermic herbivore: Testudo horsfieldi. Ecography. 2003;26:236–242. [Google Scholar]
- 29.Lagarde F, et al. Slowness and acceleration: A new method to quantify the activity budget of chelonians. Anim Behav. 2008;75:319–329. [Google Scholar]
- 30.Cushman SA, Chase M, Griffin C. Elephants in space and time. Oikos. 2005;109:331–341. [Google Scholar]
- 31.Lehman TH. Late Campanian dinosaur biogeography in the western interior of North America. Dinofest Intern Proc. 1997:223–240. [Google Scholar]
- 32.Fricke HC, Rogers RR, Gates TA. Hadrosaurid migration: Inferences based on stable isotope comparisons among Late Cretaceous dinosaur localities. Paleobiology. 2009;35:270–288. [Google Scholar]
- 33.Foster JR. Jurassic West. Bloomington, IN: Indiana Univ Press; 2007. [Google Scholar]
- 34.Hummel J, Gee CT, Südekum K-H, Sander PM, Nogge G, Clauss M. In vitro digestibility of fern and gymnosperm foliage: Implications for sauropod feeding ecology and diet selection. Proc R Soc B. 2008;275:1015–1021. doi: 10.1098/rspb.2007.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen-Smith N. Megaherbivores—The Influence of Very Large Body Size on Ecology. Cambridge, UK: Cambridge Univ Press; 1988. [Google Scholar]
- 36.Wyatt JR, Eltringham SK. The daily activity of the elephant in the Rwenzori National Park. E Afr Wildl J. 1974;12:273–289. [Google Scholar]
- 37.Coria RA, Salgado L. A new giant carnivorous dinosaur from the Cretaceous of Patagonia. Nature. 1995;377:224–226. [Google Scholar]
- 38.Savage RJG. Megistotherium, new genus gigantic hyaenodont from Miocene of Gegel Zelten Lybia. Bull Brit Mus (Nat Hist) Geol. 1973;22:485–511. [Google Scholar]
- 39.Christensen P. What size were Arctodus simus and Ursus spelaeus (Carnivora: Ursidae)? Ann Zool Fennici. 1999;36:93–102. [Google Scholar]
- 40.Matheus PE. Diet and co-ecology of Pleistocene short-faced bears and brown bears in eastern Beringia. Quarter Res. 1995;44:447–453. [Google Scholar]
- 41.Carbone C, Teacher A, Rowcliffe JM. The costs of carnivory. PLoS Bio. 2007;5:363–368. doi: 10.1371/journal.pbio.0050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erickson GM, Rogers KC, Yeroy SA. Dinosaurian growth patterns and rapid avian growth rates. Nature. 2001;412:429–433. doi: 10.1038/35086558. [DOI] [PubMed] [Google Scholar]
- 43.McNab BK. Food habits, energetics, and the population biology of mammals. Am Nat. 1980;116:106–124. [Google Scholar]
- 44.Ricklefs RE. Patterns of growth in birds. 2. Growth-rate and mode of development. Ibis. 1973;115:177–201. [Google Scholar]
- 45.Lehman TM. Growth and population age structure in the horned dinosaur Chasmosaurkus. In: Charpenter K, editor. Horns and Beaks: Ceratopsian and Ornithopod Dinosaurs. Bloomington, IN: Indiana Univ Press; 2007. pp. 259–317. [Google Scholar]
- 46.Lehman TM, Woodward HN. Modeling growth rates for sauropod dinosaurs. Paleobiology. 2008;34:264–281. [Google Scholar]
- 47.Padian K, de Ricqlès AJ, Horner JR. Dinosaurian growth rates and bird origins. Nature. 2001;412:405–408. doi: 10.1038/35086500. [DOI] [PubMed] [Google Scholar]
- 48.Pough FH. Advantages of ectothermy for tetrapods. Am Nat. 1980;115:92–112. [Google Scholar]
- 49.Lavigne DH. Similarity in energy budgets of animal populations. J Anim Ecol. 1982;51:195–206. [Google Scholar]
- 50.Bryant DM, Gardiner A. Energetics of growth in house martins (Delicon urbica) J Zool, Lond. 1979;189:275–304. [Google Scholar]
- 51.Parrish JM, Parrish JT, Hutchison JH, Spicer RA. Late Cretaceous vertebrate fossils from north slope of Alaska and implications for dinosaur ecology. Palaios. 1987;2:377–389. [Google Scholar]
- 52.Paul GS. Physiology and migration of north slope dinosaurs. In: Thurston DK, Fujita K, editors. Proc Intl Conf Arctic Margins. Anchorage, Alaska: US Dept Interior; 1994. pp. 405–408. [Google Scholar]
- 53.McNab BK. An analysis of the factors that influence the level and scaling of mammalian BMR. Comp Biochem Physiol A. 2008;151:5–28. doi: 10.1016/j.cbpa.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 54.Molnar RE, Wiffen J. A Late Cretaceous polar dinosaur fauna from New Zealand. Cret Res. 1994;15:689–706. [Google Scholar]
- 55.Bell PR, Snively E. Polar dinosaurs on parade: A review of dinosaur migration. Alcheringa. 2008;32:271–284. [Google Scholar]
- 56.Seebacher F. Dinosaur body temperatures: The occurrence of endothermy and ectothermy. Paleobiology. 2003;29:105–122. [Google Scholar]
- 57.Berner RA, Lasaga AC, Garrels RM. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am J Sci. 1983;283:641–683. [Google Scholar]
- 58.Barrick RE, Showers WJ. Thermophysiology of Tyrannosaurus rex: Evidence from oxygen isotopes. Science. 1994;265:222–224. doi: 10.1126/science.265.5169.222. [DOI] [PubMed] [Google Scholar]
- 59.Barrick RE, Showers WJ. Thermophysiology and biology of Gigantosaurus: Comparison with Tyrannosaurus. Paleo Electr. 1999 http://palaeo-electronica.org/1999–2/gigan/issue2–99.htm.
- 60.Barrick RE, Showers WJ, Fischer AG. Comparison of thermoregulation of four ornithischian two dinosaurs and a varanid lizard from the Cretaceous Two Medicine Formation: Evidence from oxygen isotopes. Palaios. 1996;11:295–305. [Google Scholar]
- 61.McNab BK. Ecological factors affect the level and scaling of avian BMR. Comp Biochem Physiol A. 2009;152:22–45. doi: 10.1016/j.cbpa.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 62.O'Conner MP, Dodson P. Biophysical constraints on the thermal ecology of dinosaurs. Paleobiology. 1999;25:341–368. [Google Scholar]
- 63.Gillooly JF, Allen AP, Charnov EL. Dinosaur fossils predict body temperatures. PLoS Biol. 2006;4:1467–1469. doi: 10.1371/journal.pbio.0040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Farlow JO. On the rareness of big, fierce animals: Speculations about the body sizes, population densities, and geographic ranges of predatory mammals and large carnivorous dinosaurs. Am J Sci. 1993;293A:167–199. [Google Scholar]
- 65.Farlow JO, Pianka ER. Body size overlap, habitat partitioning and living space requirements of terrestrial vertebrate predators: Implications for the paleoecology of large theropod dinosaurs. Hist Biol. 2002;16:21–40. [Google Scholar]
- 66.Callison G. Small problems: Biological implications of tiny dinosaurs. In: Czerkas SJ, Olson EC, editors. Dinosaurs Past and Present. Vol. I. Seattle, WA: University Washington Press; 1987. pp. 70–80. [Google Scholar]
- 67.Norell MA, Xu X. Feathered dinosaurs. Ann Rev Earth Plant Sci. 2005;33:277–299. [Google Scholar]
- 68.McNab BK. The evolution of endothermy in the phylogeny of mammals. Am Nat. 1978;112:1–21. [Google Scholar]