Abstract
Syndecan (Sdc) is a conserved transmembrane heparan sulfate proteoglycan (HSPG) bearing additional chondroitin sulfate (CS) modifications on its extracellular domain. In vertebrates, this extracellular domain of Sdc is shed and acts as a soluble effector of cellular communication events, and its cytoplasmic domain participates in intracellular signaling needed to maintain epithelial integrity. In Drosophila, Sdc has been shown to be necessary for Slit signaling-dependent axon and myotube guidance during CNS development and muscle pattern formation. We report that Sdc acts in a cell-autonomous manner in Slit-receiving cells and that its membrane-anchored extracellular domain is sufficient to mediate Slit signaling. Sdc activity can be replaced by the human homolog hsdc2. However, the HSPG Dally-like protein (Dlp), which lacks CS modifications at its extracellular domain, can only partially substitute for Sdc function, and its activity is not restricted to the Slit target cells. Our results suggest that Sdc and Dlp act in a cooperative but nonredundant fashion in axon and myotube guidance. We propose that Dlp, which lacks CS modifications, participates in the transfer of Slit from its site of expression to the target cells, where CS-modified Sdc concentrates and presents the ligand.
Keywords: Drosophila, heparan sulfate, Slit signal transduction, axon guidance
Heparan sulfate proteoglycans (HSPGs) are secreted or cell-associated ECM proteins that are modified with specific linear heparan sulfate (HS) glycosaminoglycan polymers (1, 2). Studies of mutants with impaired HS synthesis and of HSPGs themselves have revealed their essential role in the transport and reception of secreted factors, including Wingless, Hedgehog, Decapentaplegic, Fibroblast Growth Factor (3), and Slit (4, 5). Drosophila contains 4 HSPGs: Perlecan (6), Division abnormally delayed (Dally), Dally-like protein (Dlp; 7, 8), and Syndecan (Sdc; 4, 5). The requirement for Sdc has been established for vertebrates, showing that the HSPG acts as an independent signaling receptor and has a number of functional features assigned to its cytoplasmic and extracellular domains, respectively. Its cytoplasmic domain functions in intracellular signal transduction and plays a role in the maintenance of epithelial integrity by linking the ECM to the actin cytoskeleton (9–12). Furthermore, the transmembrane domain of Sdc not only serves to localize Sdc at the cell membrane but to mediate protein-protein interactions (13). Finally, the extracellular domain of vertebrate Sdc is proteolytically shed (14–17) and acts as an extracellular effector in cell communication events (15, 16).
In Drosophila, Sdc was shown to regulate Slit signaling (4, 5). Slit, a secreted ligand produced in ventral midline cells, acts as a repellent in both axon and myotube guidance during embryogenesis, 2 processes that are mediated by Robo receptors in the target cells (18). Loss of Slit signaling causes axons and muscle fibers to cross the ventral midline of the embryo (18), a mutant phenotype that is also observed in the absence of Sdc activity (4, 5) [supporting information (SI) Fig. S1]. Here, we show that Sdc is specifically required in the target cells. The membrane-anchored chondroitin sulfate (CS)-modified extracellular domain of Drosophila Sdc is both necessary and sufficient to mediate proper Slit signaling.
Results and Discussion
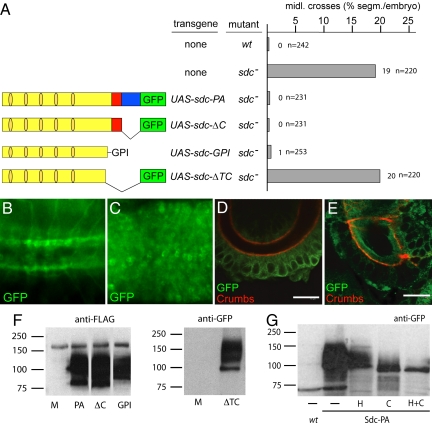
To understand Sdc function both in molecular and functional terms, to identify its cellular requirement, and to elucidate the mechanism by which Sdc mediates Slit/Robo signaling, we asked which portions of the Sdc protein are required for that signaling process and how Sdc function compares with that in vertebrates. Reduced Slit activity is evident in ventral midline crossovers of axon fascicles and muscles never observed in WT embryos (18, 19) (Fig. S1). To test various portions of Sdc to determine whether they are required for Slit signaling, we generated Sdc deletion mutants and expressed them in specific subsets of cells in sdc mutant embryos (5). For these experiments, we used the Gal4/UAS expression system (20) and conditions used in previous experiments (5) showing that expression of WT Sdc [Sdc-PA] can fully rescue the sdc mutant phenotype (Fig. 1A).
Fig. 1.
Anchorage of the Sdc extracellular domain is a prerequisite for function. (A) Percentage of segments with ectopic ventral midline crossover of ipsilateral axon fascicles stained with anti-FASII antibody in wt, sdc, and sdc homozygous mutants rescued with UAS-sdc-PA, UAS-sdc-ΔC, UAS-sdc-GPI, and UAS-sdc-ΔTC driven by elavG4. n, number of segments analyzed. (Left) Schematic representation of transgenes. Light yellow indicates sdc extracellular domain, dark yellow indicates HS attachment sites, red indicates sdc transmembrane domain, and blue indicates sdc cytoplasmic domain. Sdc-PA and Sdc-ΔC contain FLAG and GFP tags, whereas Sdc-GPI and Sdc-ΔTC contain a FLAG tag and a GFP tag, respectively. For details, see Methods. Ventral view of 3 segments (Left, anterior) showing GFP expression in first instar larvae in response to UAS-sdc-PA (B) and UAS-sdc-ΔTC (C) expression using elavG4. Sdc-PA is localized along CNS axon trajectories, whereas Sdc-ΔTC is not localized. Cross sections of stage 16 hindgut showing Sdc-PA (D) and Sdc-ΔTC (E) expression in response to daG4. Anti-Crumbs antibody labels the apical membrane of the hindgut bordering the lumen. Note that Sdc-PA is attached to cell membranes (D), whereas Sdc-ΔTC is secreted into the lumen (E). (Scale bar: 10 μm.) (F) Western blot of Kc167 cell extracts expressing Sdc-PA, Sdc-ΔC, or Sdc-GPI and the supernatant of cells expressing Sdc-ΔTC to confirm their expression and modification. M, mock transfected cells. Sdc-PA, Sdc-ΔC, and Sdc-GPI were detected with anti-FLAG antibody, and Sdc-ΔTC was detected with anti-GFP antibody. (G) Sdc-PA is modified by HS and CS. Extracts of embryos expressing Sdc-PA (using tubPG4) were either mock treated (—) or treated with heparinase (H), chondroitinase (C), or a combination of heparinase and chondroitinase (H+C). Proteins were detected with anti-GFP antibody. Treatment with chondroitinase dramatically reduced molecular weight, but only treatment with both enzymes released unmodified Sdc.
We asked whether, like in vertebrates, the Drosophila Sdc cytoplasmic domain is required for intracellular Slit signal transduction. To test for this feature, we generated sdc-ΔC, which contains GFP in place of the Sdc cytoplasmic domain. On Gal4/UAS-driven expression in neurons, where Sdc is normally expressed (4, 5), sdc-ΔC rescued the sdc mutant axon phenotype (Fig. 1A). Thus, the cytoplasmic domain is not essential for Sdc function in Slit signaling. To test further whether the transmembrane domain of Sdc mediates essential protein-protein interactions (13), we tested an sdc variant containing a heterologous GPI anchor (sdc-GPI) in place of both the Sdc transmembrane and cytoplasmic domains (Fig. 1A), allowing the extracellular domain of Sdc to associate with the target cell membrane via the GPI anchor. Fig. 1A shows that sdc-GPI expression rescues the sdc mutant phenotype. Taken together, these findings demonstrate that the membrane-anchored extracellular domain of Sdc is sufficient to mediate proper Slit signaling in target cells.
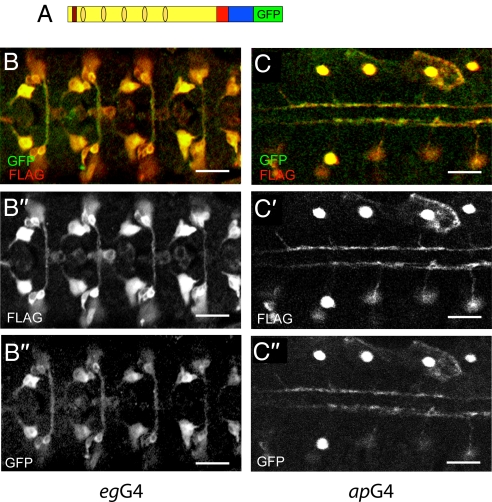
We next asked whether shedding of the extracellular domain is required to generate a physiologically active form of Sdc in Drosophila (14–16). To mimic shedding, we used a secreted extracellular domain of Sdc (Sdc-ΔTC; Fig. 1A). Fig. 1 B–D shows that Sdc-ΔTC is indeed secreted when expressed in the CNS (Fig. 1 B and C), in the embryonic hindgut (Fig. 1 D and E), in the tracheal system (Fig. S2), and in tissue culture cells (Fig. 1F). However, no rescue was observed in response to Sdc-ΔTC expression in the CNS or any other place of the embryo (Fig. 1A). The fact that the membrane-anchored extracellular domain of Sdc exhibited rescue activity, whereas the secreted extracellular domain did not could be attributable to deficient glycosaminoglycan modifications of the protein that were shown to be critical for ligand binding (21). To test this possibility, we performed modification-specific enzyme degradation assays with heparinase and/or chondroitinase, showing that WT Sdc was modified by HS and CS (Fig. 1G) and that Sdc-ΔC, Sdc-GPI, and the secreted Sdc-ΔTC were modified by glycosaminoglycans (Fig. 1F). Thus, an absence of glycosaminoglycan modifications of the Sdc extracellular domain cannot be the reason why Sdc-ΔTC is functionally inactive. We also found that expression of Sdc-ΔTC in WT embryos had no dominant negative effect on Slit signaling, suggesting that it does not interfere with Slit activity when released from the cell membrane. The simplest explanation for this finding is that Slit binding by Sdc requires one or several components that are present in the ECM of the target cells. In fact, a complex composed of Sdc, Slit, and Robo has been reported recently (4). Thus, it is possible that Slit only interacts with Sdc in conjunction with, for example, Slit receptors. These results support the conclusion that shedding of Sdc is not a prerequisite for its function in vivo. To demonstrate the lack of significance of shedding in vivo further, we marked the N-terminus of the Sdc extracellular domain with a 2X-FLAG tag and the C-terminus of the cytoplasmic domain of Sdc with GFP and then expressed the double-marked protein in embryonic neurons. We found that the two markers, 2X-FLAG and GFP, always colocalize wherever Sdc is expressed (Fig. 2), indicating that there is no notable shedding of Drosophila Sdc. The lack of shedding is consistent with the reported surface staining by antibodies directed against the Sdc extracellular domain (17). Taken together, the in vivo results support the conclusion that Sdc-dependent Slit signaling requires only the extracellular portion of the protein to be attached to the target cell membrane.
Fig. 2.
Sdc extracellular domain is not shed in vivo. (A) Schematic representation of the double-tagged sdc-PA: FLAG-sdc-GFP. The FLAG tag is shown in red, and the others are as described in Fig. 1. UAS-FLAG-sdc-GFP expression using egG4 (B) and apG4 (C). The amino and carboxy termini of Sdc were detected with anti-FLAG and anti-GFP antibodies, respectively. Note colocalization of the N- (B′ and C′) and C- (B″ and C″) termini. (Scale bar: 20 μm.)
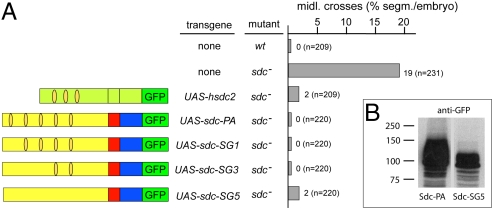
To determine whether the protein sequence of the extracellular domain of Sdc, the HS and/or CS modifications, are of functional relevance, we performed rescue experiments with human sdc2 (hsdc2), which carries both HS and CS modifications on its otherwise highly divergent extracellular domain (22) (Fig. S3). In addition, we performed the same kind of rescue experiments with Dlp, a different membrane-anchored HSPG. Dlp lacks CS modifications but contains more (i.e., 9 canonical HS attachment sites compared with 5 for Sdc). These sites are defined by a serine residue followed by glycine (1). Gal4/UAS-driven hsdc2 expression in neurons resulted in a complete rescue of the sdc mutant axon phenotype, as had been observed with Drosophila Sdc (Fig. 3A). This finding suggests that the apparently nonconserved amino-acid sequence of the extracellular domain is not essential for Slit signaling, and thus emphasizes the potential importance of HS and CS modifications. In contrast, the corresponding expression of Dlp, which lacks CS modifications, could only partially rescue the sdc mutant phenotype (4) (Table 1), supporting the argument that the CS modifications of Sdc are required for Slit signaling. We addressed this point more directly by expressing Sdc mutants bearing gradually decreasing numbers of canonical HS attachment sites (21) (Fig. 3A). Expression of these Sdc mutant proteins, including one that lacked all canonical HS modifications (Sdc-SG5; Fig. 3A), in neurons resulted in a complete or nearly complete rescue of the axon phenotype. The Sdc-SG5 mutant protein shows a dramatic reduction of sugar modifications (Fig. 3B) and consists mostly of remaining CS modifications as revealed by modification-specific enzyme assays with heparinase and chondroitinase (Fig. S4). Low-level HS modifications may still occur at noncanonical HS modification sites (Fig. S4). The complete rescue in response to Sdc-SG5 and hSdc2 expression in contrast to the comparatively weak rescue activity in response to Dlp further supports the conclusion that Sdc-dependent Slit signaling requires either CS modifications or a combination of CS and HS modifications and is not based on HS modifications of the HSPG only. Furthermore, it seems unlikely that the protein core of the extracellular domain plays a role, because the corresponding domain of hsdc2 contains a highly divergent amino acid sequence (Fig. S3) that can fully compensate for Sdc function in the fly.
Fig. 3.
CS modifications contribute to Sdc activity. (A) Percentage of segments with ventral midline crossover of ipsilateral axon fascicles stained with anti-FASII antibody in wt, sdc, and sdc homozygous mutants rescued with UAS-hsdc2, UAS-sdc-PA, UAS-sdc-SG1, UAS-sdc-SG3, and UAS-sdc-SG5. n, number of segments analyzed. (Left) Schematic representation of transgenes. Light green indicates human sdc2, and the others are as described in Fig. 1. (B) Western blot to compare Sdc-PA and Sdc-SG5. Sdc-PA and Sdc-SG5 were expressed in embryos (using tubPG4) and visualized by anti-GFP antibody in embryo extracts. The mutation of all HS attachment sites results in a dramatically reduced apparent molecular weight of Sdc-SG5 compared with Sdc-PA.
Table 1.
Cooperation of Sdc and Dlp in Slit signalling
| Driver | Mutant | Axon midline crossing, % segment/embryo |
|---|---|---|
| None | wt | 0 (n = 209) |
| None | sdc− | 19 (n = 198) |
| None | ttv− | 0 (n = 220) |
| None | dlp− | 0 (n = 220) |
| None | sdc−;ttv− | 39 (n = 198) |
| None | sdc−;dlp− | 50 (n = 176) |
| None | wt | 0 (n = 220) |
| None | sdc− | 19 (n = 176) |
| simG4:UAS-sdc | sdc− | 19 (n = 198) |
| repoG4:UAS-sdc | sdc− | 16 (n = 231) |
| elavG4:UAS-sdc | sdc− | 0 (n = 209) |
| simG4:UAS-dlp | sdc− | 11 (n = 231) |
| repoG4:UAS-dlp | sdc− | 5 (n = 220) |
| elavG4:UAS-dlp | sdc− | 9 (n = 198) |
The percentages of segments with ventral midline crossover of ipsilateral axon fascicles stained with anti-FASII antibody are listed. n, number of segments analyzed. Analyzed embryos were wt, sdc, ttv, dlp, sdc;ttv, and sdc;dlp homozygous mutants as well as sdc homozygous mutants rescued with UAS-sdc-PA or UAS-dlp expressed in Slit-secreting midline cells (simG4), intermediate glia tissue (repoG4), or axonal target tissue (elavG4).
The different rescue abilities of Sdc and Dlp suggest that they play different roles in the Slit signaling process. In fact, dlp is expressed in the CNS when axons grow toward their target (4). To test whether Dlp acts in an Sdc-like fashion, we examined whether the lack of Dlp and the absence of HS biosynthetic enzyme activity, as in tout-velu (ttv) mutants (23), affects axon and muscle guidance. A lack of Dlp or Ttv activity had no effect on axon and muscle patterns. However, in double-mutant combinations such as sdc;ttv and sdc;dlp, the sdc mutant phenotype was strongly enhanced, with a 2-fold increase in ventral midline crossovers of axon fascicles (2.0- and 2.6-fold, respectively; Table 1). Because sdc is essential for Slit signaling (4, 5), its genetic interaction with ttv and dlp suggests that the products of the 2 genes are also involved in Slit signaling, acting either in conjunction with or parallel to Sdc.
Finally, we asked whether Dlp can replace Sdc function and which cells of the embryo require Sdc, Dlp, or both of these HSPGs for proper Slit signaling. We performed rescue experiments in which Sdc or Dlp was expressed ubiquitously or in distinct sets of cells using the Gal4/UAS system (20). Ubiquitous expression was achieved by a UAS-driven transgene in response to da-Gal4 (5) in the Slit-secreting ventral midline cells by sim-Gal4, in cells between midline and Slit target cells by repo-Gal4, and in the Slit target neurons in response to elav-Gal4. In addition, Sdc was expressed in myotubes in response to mef2-Gal4 and in the cells between the Slit-expressing and Slit-responding muscle cells by elav-Gal4. Ubiquitous expression of WT Sdc (Sdc-PA) restored both axon and muscle patterns (5). Sdc expression in the Slit-secreting cells had no rescue effect on either axons or muscles (Table 1 and Fig. S5), indicating that Sdc is not required for Slit production and/or Slit secretion. Furthermore, Sdc expression in cells connecting the Slit-secreting and Slit-responding target cells also failed to rescue the sdc mutant phenotype (Table 1 and Fig. S5). Thus, Sdc is not required for the transport of secreted Slit to the Slit-responding cells. In contrast, Sdc expression in the Slit target cells [i.e., neurons (Table 1) and muscles (Fig. S5)] resulted in a complete rescue, indicating that Sdc is required in these cells only and that Sdc acts in a cell-autonomous manner. Corresponding expression of Dlp caused only a partial rescue of the sdc mutant phenotype, irrespective of its site of expression (Table 1). Thus, Dlp can only partially compensate for the loss of Sdc activity, irrespective of its site of expression, in Slit-secreting cells, the target cells, or the cells in between. Hence, the genetic interaction between Sdc and Dlp is based on independent functions of the 2 HSPGs in Slit signaling. Sdc functions exclusively and in a cell-autonomous manner in Slit-receiving cells, where it is coexpressed with Robo (4, 5). This finding suggests that Sdc plays a role in the concentration or presentation of Slit to the Robo receptors and that this specific activity depends on CS modifications of the extracellular domain. Dlp, which lacks such CS modifications and acts in a non-cell autonomous manner, could participate in the transport of Slit, a function that was already established in conjunction with a different signaling molecule, Hedgehog, in wing imaginal discs (7, 8). Alternatively or in addition, Dlp might be required for the concentration or presentation of Slit from neighboring cells in trans.
Our results provide evidence that only the extracellular domain of Sdc, in association with the target cell membrane, is both necessary and sufficient to promote Slit signaling. The human homolog hSdc2 and the Sdc-SG5 mutant exert complete Sdc WT activity when expressed in sdc lack-of-function mutants, whereas Dlp does not. HSdc2 carries both CS and HS modifications on its otherwise highly diverged extracellular domain, Sdc-SG5 is modified by CS but has all canonical HS modification sites deleted, and Dlp is modified by HS but lacks CS modifications. Thus, it is reasonable to conclude that the CS modifications of the Sdc extracellular domain are specifically necessary to concentrate the ligand Slit at the receiving cells and to receive and/or present the ligand to the Robo receptors. The observed non-cell autonomous requirement for Dlp, combined with the results of the genetic interaction studies on sdc and dlp mutants, prompts us to propose that Dlp participates in the transport of Slit from its site of expression in the ventral midline cells toward the receiving cells. The different cellular requirements for Sdc and Dlp as revealed by the cell-specific expression studies suggest a model in which the 2 HSPGs participate in different aspects of Slit signaling (i.e., the transport and the proper reception of Slit, respectively).
The functional properties established for vertebrate Sdc, such as intracellular signaling, linking the ECM to the actin cytoskeleton (11, 12), and the proteolytic release of the extracellular domain that promotes cell communication events (15, 16) are clearly not essential for Drosophila Sdc to promote Slit signaling. The results unambiguously establish that only the extracellular domain of Sdc participates in the Slit signaling process, provided that it is attached to the Slit target cell membrane. At this location, Sdc can serve as a tether, or coreceptor, to facilitate Slit binding to the Robo receptors (18). This conclusion is consistent with the observation that shedded Sdc has no dominant-negative effect when overexpressed and that Sdc and Robo receptors are coexpressed in both axons and myotubes and are capable of physical interaction (4, 5). This cooperation of 2 differently modified HSPGs in the transport and reception of ligands could explain how a small number of HSPGs can shape multiple ligand/receptor interactions as suggested by the analysis of mutants that fail to synthesize HS (2, 3).
Materials and Methods
Molecular Biology.
All transgenes were cloned in pUAST. Drosophila sdc domains were amplified from cDNA LD08230, hsdc2 from cDNA MGC:14,928, and dlp GPI anchor sequence from a Drosophila cDNA library. Protein analysis tools (available at: www.expasy.org) were used to predict the Sdc signal peptide (1–29 aa), extracellular (1–339 aa), transmembrane (340–364 aa), and cytoplasmic (365–399 aa) domains and Dlp GPI anchor sequence (terminal 66aa common to dlpRA and dlpRB). Five putative HS attachment sites (serine glycine motifs) were identified in dSdc (Ser-62, Ser-79, Ser-81, Ser-109, and Ser-194), 3 were identified in hSdc2 (Ser-41, Ser-55, and Ser-57), and 9 were identified in Dlp (Ser-147, Ser-380, Ser-463, Ser-504, Ser-625, Ser-629, Ser-630, Ser-643, Ser-686). Sequential mutation of dSdc HS attachment sites was performed to generate sdc-SG1 (Ser62Ala), sdc-SG3 (Ser62Ala, Ser79Ala, and Ser81Ala) and sdc-SG5 (Ser62Ala, Ser79Ala, Ser81Ala, Ser109Ala, and Ser194Ala). sdc-PA (1–399 aa), sdc-ΔC (1–364 aa), sdc-ΔTC (1–339 aa), hsdc2, sdc-SG1, sdc-SG3, and sdc-SG5 carried a C-terminal GFP in frame with their ORF. A 2X-FLAG tag with an internal SpeI restriction site was inserted after the sdc signal peptide sequence, after Glu-29, to create FLAG-sdc-GFP and FLAG-sdcΔC-GFP. FLAG-tagged sdc extracellular domain was fused in frame with the Dlp-GPI anchor sequence to generate sdc-GPI. All plasmids used for generation of transgenic flies were sequenced before injection. Control experiments employing Western blot analysis with protein extracts of transfected cells and of embryos expressing WT Sdc and Sdc mutant proteins, respectively, revealed similar protein expression levels (within a 2-fold range; Fig. 1F).
Fly Strains.
The sdc mutant used in this study, sdc23, had been generated previously (5). w1118, elavG4, simG4, repoG4, daG4, tubulinPG4, and ttv00681b were obtained from the Bloomington Stock Centre. The apG4 and egG4 fly stocks were kindly provided by B. Dickson (Institute of Molecular Pathology, Vienna), dlpA187 and UAS-dlp were provided by X. Lin (Children's Hospital Medical Center, Cincinnati), and mef2G4 was provided by M. Taylor (Cardiff University, UK). Transgenic flies were generated by P-element–mediated germline transformation.
Immunohistochemistry.
Whole-mount antibody staining was performed as described previously (5). β-galactosidase (1:1,000; Promega), rabbit anti-β-galactosidase (1:1,000; Cappel), mouse (anti-Fasciclin II) 1D4 [1:5; Developmental Studies Hybridoma Bank (DSHB)], mouse 2A12 (1:5; DSHB), mouse (anti-Crumbs) Cq4 (1:1,000; DSHB), mouse anti-FLAG M2 (1:1,000; Sigma-Aldrich), rabbit anti-GFP (1:1,000; Synaptic Systems), and rabbit anti-MHC (1:2,000; kindly provided by D. Kiehart, Duke University, Durham, NC ) were used as primary antibodies. Goat anti-mouse IgG and anti-rabbit IgG (coupled to Alexa 488 or 568, 1:400; Molecular Probes) and donkey anti-mouse IgM (coupled to Cy3, 1:400; Jackson Labs) were used as secondary antibodies. Stained embryos were analyzed on either a Zeiss epifluorescence microscope or Leica TCS SP2 confocal laser scanning microscope.
Cell Culture.
Drosophila Kc167 cells were cultured in Gibco's Drosophila medium supplemented with 10% FCS (vol/vol) and streptomycin (100 μg/mL). To determine protein expression and modification, cells were transfected (Effectene reagent; Qiagen) with the respective UAS-transgene and actinG4 plasmids. Cell lysates were analyzed; to test secretion, the cell supernatant was concentrated 10 times (Vivaspin columns; Sartorius) and analyzed by Western blotting.
Enzymatic Assay.
Embryos squashed in the respective enzyme buffers following the supplier's instructions were incubated for 3 h at 37 °C with heparinase III (1.75 U/mL), Chondroitinase ABC (1.5 U/mL) (Sigma-Aldrich), or a combination of the 2 and analyzed by Western blotting.
Supplementary Material
Acknowledgments.
We thank our colleagues in the laboratory for a variety of contributions and for critical discussions, Tomma Eisbein for technical assistance, and U. Jahns-Meyer for generating fly transformants. We are grateful to D. Blaurock for editing the manuscript and to Barry Dickson, Xinhua Lin, and Michael Taylor for providing fly stocks. This work was supported by the Deutsche Forschungsgemeinschaft and the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901148106/DCSupplemental.
References
- 1.Bernfield M, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 2.Kramer KL, Yost HJ. Heparan sulfate core proteins in cell-cell signaling. Annu Rev Genet. 2003;37:461–484. doi: 10.1146/annurev.genet.37.061103.090226. [DOI] [PubMed] [Google Scholar]
- 3.Häcker U, Nybakken K, Perrimon N. Heparan sulphate proteoglycans: The sweet side of development. Nat Rev Mol Cell Biol. 2005;6:530–541. doi: 10.1038/nrm1681. [DOI] [PubMed] [Google Scholar]
- 4.Johnson KG, et al. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol. 2004;14:499–504. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Steigemann P, Molitor A, Fellert S, Jäckle H, Vorbrüggen G. Heparan sulfate proteoglycan syndecan promotes axonal and myotube guidance by slit/robo signaling. Curr Biol. 2004;14:225–230. doi: 10.1016/j.cub.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Voigt A, Pflanz R, Schäfer U, Jäckle H. Perlecan participates in proliferation activation of quiescent Drosophila neuroblasts. Dev Dyn. 2002;224:403–412. doi: 10.1002/dvdy.10120. [DOI] [PubMed] [Google Scholar]
- 7.Franch-Marro X, et al. Glypicans shunt the Wingless signal between local signalling and further transport. Development. 2005;132:659–666. doi: 10.1242/dev.01639. [DOI] [PubMed] [Google Scholar]
- 8.Han C, Belenkaya TY, Wang B, Lin X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development. 2004;131:601–611. doi: 10.1242/dev.00958. [DOI] [PubMed] [Google Scholar]
- 9.Adams JC, Kureishy N, Taylor AL. A role for syndecan-1 in coupling fascin spike formation by thrombospondin-1. J Cell Biol. 2001;152:1169–1182. doi: 10.1083/jcb.152.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greene DK, Tumova S, Couchman JR, Woods A. Syndecan-4 associates with alpha-actinin. J Biol Chem. 2003;278:7617–7623. doi: 10.1074/jbc.M207123200. [DOI] [PubMed] [Google Scholar]
- 11.Ethell IM, et al. EphB/syndecan-2 signaling in dendritic spine morphogenesis. Neuron. 2001;31:1001–1013. doi: 10.1016/s0896-6273(01)00440-8. [DOI] [PubMed] [Google Scholar]
- 12.Oh ES, Woods A, Lim ST, Theibert AW, Couchman JR. Syndecan-4 proteoglycan cytoplasmic domain and phosphatidylinositol 4,5-bisphosphate coordinately regulate protein kinase C activity. J Biol Chem. 1998;273:10624–10629. doi: 10.1074/jbc.273.17.10624. [DOI] [PubMed] [Google Scholar]
- 13.Choi S, et al. Transmembrane domain-induced oligomerization is crucial for the functions of syndecan-2 and syndecan-4. J Biol Chem. 2005;280:42573–42579. doi: 10.1074/jbc.M509238200. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kato M, et al. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nat Med. 1998;4:691–697. doi: 10.1038/nm0698-691. [DOI] [PubMed] [Google Scholar]
- 16.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 17.Spring J, Paine-Saunders SE, Hynes RO, Bernfield M. Drosophila syndecan: Conservation of a cell-surface heparan sulfate proteoglycan. Proc Natl Acad Sci USA. 1994;91:3334–3338. doi: 10.1073/pnas.91.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidd T, et al. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- 19.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 20.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 21.Esko JD, Selleck SB. Order out of chaos: Assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem. 2002;71:435–471. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 22.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellaiche Y, The I, Perrimon N. Tout-velu is a Drosophila homologue of the putative tumour suppressor EXT-1 and is needed for Hh diffusion. Nature. 1998;394:85–88. doi: 10.1038/27932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.