During the course of evolution, all vertebrates have been exposed to multiple waves of cross-species infection by retroviruses. Some of these viruses have succeeded in infecting germ cells and are now inherited in integrated, proviral form (1). These so-called endogenous retroviruses have undergone further amplification and now make up a greater fraction of our DNA than do normal coding sequences (2). For a considerable while it was an open question whether these proviruses provided significant benefits to their hosts or simply represented junk or selfish DNA. It is now clear that several such elements contribute to protection against retroviral infection (3). Moreover, a number of lines of indirect evidence have pointed to a possible role in placental development (4). Now comes compelling evidence that one or more proviruses are “paying their way” with a specific contribution to normal physiology. In this issue of PNAS, Dupressoir et al. (5) show that mutant mice in which the syncytin-A gene has been knocked out die in utero, apparently as a result of the failure of trophoblast cells to fuse and form one of the two syncytiotrophoblast layers present in the placenta of mice. These structures are present on the exterior of the placenta, at the fetal–maternal interface, and are thought to play a key role in exchanging nutrients and waste between mother and fetus. Syncytin-A is derived from a retroviral env gene (6).
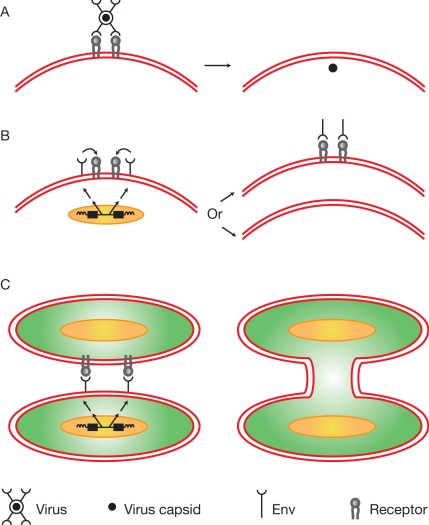
The receptor binding and membrane fusion activities that play key roles in the retrovirus life cycle (7) are provided by the two protein products of the env gene, SU (surface) and TM (transmembrane). Synthesized as one polypeptide precursor that is proteolytically cleaved during transport to the cell surface, they remain physically linked even after virus production. Binding of SU to its cognate receptor can have a number of consequences (Fig. 1). In the viral life cycle, membrane fusion activity allows viruses to enter target cells (8) (Fig. 1A). Alternatively, Env produced by infected cells can down-regulate or saturate free receptor on the cell surface (Fig. 1B), resulting in resistance to infection (3). If encoded by an endogenous retrovirus, such resistance will be inherited in Mendelian fashion. Examples of resistance to retroviruses arising in this manner can be found in a number of species, including chickens, mice, and cats (3); they represent some of the first examples of “useful” endogenous retroviruses. Finally, there are multiple examples of retrovirus infection leading to cell membrane fusion and the formation of multinucleated cells (9, 10) (Fig. 1C); the importance of this phenomenon for retrovirus-induced pathogenicity remains to be determined. In any event, Env-mediated membrane fusion is a key feature of the retroviral life cycle, and it is this activity that now appears to have been appropriated for use in placental formation.
Fig. 1.
Consequences of Env–receptor interactions. (A) Retrovirus infection. After receptor binding, Env-mediated fusion between virus and cell membranes occurs, resulting in the uptake of viral cores into the cell cytoplasm. Precise details of the triggering of fusion, other factors involved, and the site of membrane fusion vary from virus to virus. (B) Restriction of virus entry. The product of a retrovirus-derived env gene interacts with receptor protein leading to the absence of free receptor on the cell surface thereby preventing infection. Where in the cell the receptor–Env interaction takes place and the mechanism of resistance, e,g. blocking or down-regulation from the cell surface, is likely to vary on a case-by-case basis. (C) Cell fusion. Env-mediated membrane fusion between two cells results in the formation of multinucleate cells.
Most endogenous proviruses have been present in the germ line for many millions of years and have suffered the inevitable mutational decay of genomic passengers not subject to positive selection (11). Thus, of the tens of thousands of proviral elements in the human genome, few, if any, can replicate as viruses although many are transcribed in a differentiation-specific manner. Searches for functional proviral genes have therefore focused on viral sequences that (i) are conserved in evolution and encode ORFs and/or (ii) show interesting patterns of expression. One such gene is the env gene encoded by the single-copy ERV-3 locus (12), a provirus that is present in all apes and Old World monkeys and expressed primarily in the placenta. However, a detailed survey of SNPs in the human population revealed the presence of a homozygous stop codon within the env gene of ERV-3 in 1% of the population (13), a finding that would be inconsistent with an essential role in placenta formation. Attention turned to a gene dubbed syncytin (14). It corresponds to the env gene of one particular member of the HERV-W family of proviruses (15). It is conserved in evolution and highly expressed in placenta, specifically in the syncytiotrophoblast. When introduced into cultured cells, it can mediate fusion, an activity that can be blocked by addition of antisera directed against syncytin to the media. Subsequently, a second conserved env gene (syncytin-2), highly conserved in evolution and encoded by an endogenous provirus of the HERV-FRD group, also with fusion activity, was identified by a genomewide screen for env genes with ORFs (only 16 were identified) followed by functional testing (16). Detailed SNP analysis yielded no data inconsistent with a functional role for syncytin-1 or syncytin-2 (17).
These findings are highly suggestive of a role for one or both syncytins in placental morphogenesis but do not represent definitive proof. Because HERV-W and HERV-FRD proviruses are present only in primates, gene knockout approaches to test the functions of syncytin-1 and syncytin-2 are not feasible. However, the discovery in mice of an additional pair of env genes, syncytin-A and syncytin-B, at chromosomal locations 5qG2 and 14qD1, with properties analogous to the human genes, opens up this possibility (6). Syncytin-A and syncytin-B are present in all Muridae and show placenta-specific expression and in vitro fusogenic activity. Dupressoir et al. (5) describe the properties of mice carrying a null mutation in the syncytin-A gene. Homozygous embryos die in utero between 11.5 and 13.5 days post coitum. Cellular and subcellular examination of placentae from the null mice revealed disruption of the syncytiotrophoblast-containing regions associated with a surplus of trophoblasts, suggesting their failure to fuse and providing strong evidence that the retrovirally derived syncytin-A plays a key role in placentation. A description of the properties of the syncytin-B knockout is not yet available but will be of considerable interest given observed differences in the fusion specificity of syncytin-A and syncytin-B (6). Further, the syncytins may prove valuable tools for studying placental development.
Syncytins may prove valuable tools for studying placental development.
It should be stressed that the pair of murine syncytins are unrelated in sequence or chromosomal location to the primate genes and were acquired independently from one another. The human loci resemble intact proviruses much more closely than do the murine genes that retain little more than the env genes of the integrating viruses (6, 15, 16). Nevertheless the results with the syncytin-A knockout would appear to strengthen the case for the involvement of syncytins in human placentation. Furthermore evidence using an in vivo morpholino loss of function approach also argues for a role of one or more endogenous retrovirus-encoded env genes, clearly different from the syncytins, in the regulation of periimplantation placental growth and differentiation in sheep (18). It therefore appears that retroviral env genes have been co-opted to play a part in placental development on a number of independent occasions. Comparative studies reveal significant structural diversity among the placentae of different orders of mammals (19, 20); one might therefore speculate that the specific roles played by these viral genes may differ, perhaps depending on the cellular expression patterns of Envs and their specific receptors. It is possible that proviral inheritance will provide a better predictor of the diversities of syncytial morphologies than taxonomy alone.
Footnotes
The author declares no conflict of interest.
See companion article on page 12127.
References
- 1.Boeke JD, Stoye JP. Retrotransposons, endogenous retroviruses, and the evolution of retroelements. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 343–435. [PubMed] [Google Scholar]
- 2.Jern P, Coffin JM. Effects of retroviruses on host genome function. Annu Rev Genet. 2008;42:709–732. doi: 10.1146/annurev.genet.42.110807.091501. [DOI] [PubMed] [Google Scholar]
- 3.Best S, Le Tissier PR, Stoye JP. Endogenous retroviruses and the evolution of resistance to retroviral infection. Trends Microbiol. 1997;4:313–318. doi: 10.1016/S0966-842X(97)01086-X. [DOI] [PubMed] [Google Scholar]
- 4.Harris JR. Placental endogenous retrovirus (ERV): Structural, functional, and evolutionary significance. BioEssays. 1998;20:307–316. doi: 10.1002/(SICI)1521-1878(199804)20:4<307::AID-BIES7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 5.Dupressoir A, et al. Syncytin-A knockout mice demonstrate the critical role in placentation of a fusogenic, endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci USA. 2009;106:12127–12132. doi: 10.1073/pnas.0902925106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupressoir A, et al. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc Natl Acad Sci USA. 2005;102:725–730. doi: 10.1073/pnas.0406509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanstrom R, Wills JW. Synthesis, assembly, and processing of viral proteins. In: Coffin JM, Hughes SH, Varmus HE, editors. Retroviruses. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 263–334. [PubMed] [Google Scholar]
- 8.Melikyan GB. Common principles and intermediates of viral protein-mediated fusion: The HIV-1 paradigm. Retrovirology. 2008;5:111. doi: 10.1186/1742-4690-5-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sodroski J, Goh WC, Rosen C, Campbell K, Haseltine WA. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature. 1986;322:470–474. doi: 10.1038/322470a0. [DOI] [PubMed] [Google Scholar]
- 10.Rowe WP, Pugh WE, Hartley JW. Plaque assay for murine leukemia viruses. Virology. 1970;42:1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- 11.Stoye JP. Endogenous retroviruses: Still active after all this time? Curr Biol. 2001;11:R914–R916. doi: 10.1016/s0960-9822(01)00553-x. [DOI] [PubMed] [Google Scholar]
- 12.Boyd MT, Bax CM, Bax BE, Bloxam DL, Weiss RA. The human endogenous retrovirus ERV-3 is up-regulated in differentiating placental trophoblast cells. Virology. 1993;196:905–909. doi: 10.1006/viro.1993.1556. [DOI] [PubMed] [Google Scholar]
- 13.de Parseval N, Heidmann T. Physiological knockout of the envelope gene of the single-copy ERV-3 human endogenous retrovirus in a fraction of the Caucasian population. J Virol. 1998;72:3442–3445. doi: 10.1128/jvi.72.4.3442-3445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mi S, et al. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 15.Blond JL, et al. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J Virol. 2000;74:3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blaise S, de Parseval N, Benit L, Heidmann T. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc Natl Acad Sci USA. 2003;10:13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Parseval N, et al. Comprehensive search for intra- and interspecific sequence polymorphisms among coding envelope genes of retroviral origin found in the human genome: Genes and pseudogenes. BMC Genomics. 2005;6:117. doi: 10.1186/1471-2164-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunlap KA, et al. Endogenous retroviruses regulate periimplantation placental growth and differentiation. Proc Natl Acad Sci USA. 2006;103:14390–14395. doi: 10.1073/pnas.0603836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carter AM, Enders AC. Comparative aspects of trophoblast development and placentation. Reprod Biol Endocrinol. 2004;2:46. doi: 10.1186/1477-7827-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leiser R, Kaufmann P. Placental structure: In a comparative aspect. Exp Clin Endocrinol. 1994;102:122–134. doi: 10.1055/s-0029-1211275. [DOI] [PubMed] [Google Scholar]