Abstract
Binding of the peptide hormone vasopressin to its type-2 receptor (V2R) in kidney triggers a cAMP-mediated translocation of Aquaporin-2 water channels to the apical membrane, resulting in water reabsorption and thereby preventing dehydration. Mutations in the V2R gene lead to Nephrogenic Diabetes Insipidus (NDI), a disorder in which this process is disturbed, because the encoded, often intrinsically functional mutant V2 receptors are misfolded and retained in the endoplasmic reticulum (ER). Since plasma membrane expression is thought to be essential for V2R activation, cell permeable V2R antagonists have been used to induce maturation and rescue cell surface expression of V2R mutants, after which they need to be displaced by vasopressin for activation. Here, however, we show that 3 novel nonpeptide V2R agonists, but not vasopressin, activate NDI-causing V2R mutants at their intracellular location, without changing their maturation and at a sufficient level to induce the translocation of aquaporin-2 to the apical membrane. Moreover, in contrast to plasma membrane V2R, degradation of intracellular V2R mutants is not increased by their activation. Our data reveal that G protein-coupled receptors (GPCRs) normally active at the plasma membrane can be activated intracellularly and that intracellular activation does not induce their degradation; the data also indicate that nonpeptide agonists constitute highly promising therapeutics for diseases caused by misfolded GPCRs in general, and NDI in particular.
Keywords: GPCR, pharmacological chaperones, signaling, ER retention, osmoregulation
In mammals, G protein-coupled receptors (GPCRs) form the largest family of plasma membrane receptors that transduce information from hundreds of extracellular signals to intracellular messengers. Not surprisingly, mutations in GPCR genes are the cause of many genetic diseases, including retinitis pigmentosa (1), hypogonadotropic hypogonadism (2), obesity (3), hypothyroidism (4), and X-linked nephrogenic diabetes insipidus (NDI) (5). Disease-causing mutations in GPCR genes can be divided in 5 classes, based on their cell-biological fates (6). Class II comprises more than 40% of gene mutations and causes so-called “conformational diseases,” which lead to misfolded receptors that are recognized and retained by the quality control system of the endoplasmic reticulum (ER). Often, these proteins are targeted for degradation by the proteasome (7).
The vasopressin type-2 receptor (V2R) is a prototype GPCR in such studies. In states of hypernatremia or hypovolemia, arginine-vasopressin (AVP) binding to the V2R of renal collecting duct principal cells induces a cAMP-signaling cascade, resulting in translocation of aquaporin-2 (AQP2) water channels to the apical membrane and water reabsorption from pro-urine (antidiuresis). Patients with V2R gene mutations suffer from X-linked congenital NDI, a disease in which the antidiuretic response to AVP is lacking, leading to polyuria and polydipsia, with the risk of severe dehydration, especially in infancy. In cell-culture studies, nearly all missense V2R mutants in NDI appear misfolded, ER-retained, and unstable. However, many of these V2R mutants appear intrinsically-functional, because they exert a vasopressin-induced cAMP response when, due to over expression, some V2R mutants are expressed in the plasma membrane (8). Therefore, the inability of intracellularly-retained V2R mutants to escape the ER quality control and traffic to the basolateral membrane is fundamental to the disease.
Like many other GPCRs, V2R is thought to function only when expressed in the plasma membrane. Therefore, to work toward a putative therapeutic treatment of NDI, nonpeptide V1R and V2R antagonists have been used, which can interact with and stabilize ER-retained V2R mutants and to rescue their cell surface expression. There, they could be activated following incubation with and displacement by high concentrations of dDAVP (9–12).
Interestingly, however, the pharmaceutical industry has recently developed nonpeptide V2R-specific agonists because these drugs can be orally administered to treat incontinence and bed wetting. Since with oral administration, these drugs have to pass the intestinal cell layer to be taken up in our vascular system, we reasoned that they must be able to pass cell membranes. If so, they then may also facilitate the transport of V2R mutants in NDI to the cell surface, where they can directly activate the mutant receptors instead of having the need for displacement by vasopressin as with antagonists.
Here, we tested how one recently developed and 2 novel V2R-specific nonpeptide agonists, but not the peptidic analogue of AVP (dDAVP), induce V2R mutants in NDI to generate cAMP at their intracellular site and thereby restore the normal physiological response, i.e., the translocation of AQP2 to the apical membrane. As such, our data reveal that nonpeptide agonists are highly promising novel therapeutics to treat diseases due to misfolded GPCRs in general, and NDI due to V2R mutations in particular.
Results
Activation of the V2R Pathway by NonPeptide Agonists.
The chemical structures of the recently developed nonpeptide agonist OPC51 (13) and 2 novel nonpeptide V2R agonists VA88 and VA89 are shown in supporting information (SI) Fig. S1A and their characteristics summarized in Table S1. In vivo, AVP binding to the V2R triggers a cAMP signaling cascade, which increases AQP2 expression and induces its redistribution from vesicles to the apical membrane. To characterize the activity of the nonpeptide V2R agonists, we used Madin-Darby Canine kidney (MDCK)-V2R cells, which show a subcellular localization and regulation of human V2R as anticipated to occur in vivo (14). Intracellular cAMP measurements revealed dose-response curves with similar maximal activities for dDAVP, VA88, and OPC51 (Fig. S1B, P > 0.05 at 1 μM agonist). However, the EC50 values for VA88 (11.9 ± 1.0 nM) and OPC51 (13.6 ± 0.7 nM) were approximately 10-fold higher than for dDAVP (1.1 ± 0.4 nM). Nontransfected MDCK cells did not respond to any of the agonists (not shown).
To test whether the compounds increase AQP2 expression, polarized collecting duct (mpkCCD) cells, which show AVP-induced expression of endogenous AQP2 (15), were incubated for 4 days with 0.1 or 10 nM of the agonists. Subsequent immunoblotting of the cell lysates revealed AQP2 expression with all 3 nonpeptide agonists and dDAVP at 10 nM, and with dDAVP and VA89 at 0.1 nM (Fig. S1C). Moreover, cell surface biotinylation experiments (Fig. S1D) and confocal laser scanning microscopy (CLSM) (Fig. S1E Left) revealed that AQP2, expressed upon treatment with dDAVP or the nonpeptide agonists, localized in the apical membrane. With removal of the ligands for 2 hours, AQP2 was internalized into vesicles (Fig. S1E Middle), whereas subsequent reapplication of dDAVP or the nonpeptide agonists re-established the localization of AQP2 in the apical membrane (Fig. S1E Right), indicating that all nonpeptide agonists can also redistribute AQP2 from vesicles to the apical membrane. These data reveal that all 3 nonpeptide agonists activate human V2R with similar efficacy, although with lower potency, compared to dDAVP and that they regulate AQP2 expression and trafficking similar to dDAVP.
Many V2R Mutants in NDI Are Intrinsically Functional.
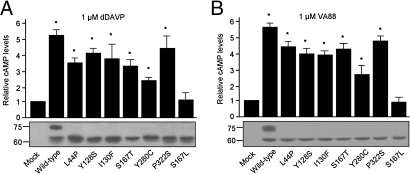
With transient over-expression of V2R mutants in cells, a fraction of the ER-retained V2R mutants localizes to the plasma membrane, which can then be used to determine the intrinsic functionality (i.e., ability to bind agonist and induce a cAMP response) of ER-retained V2R mutants. To test this for V2R mutants in NDI, GFP-tagged forms of WT-V2R, 6 V2R mutants associated with NDI (L44P, Y128S, I130F, S167T, Y280C, P322S), and the nonfunctional mutant V2R-S167L (8) were over-expressed in COS cells and stimulated with 1 μM dDAVP or 1 μM VA88, VA89, or OPC51. With all compounds, cAMP levels were significantly increased for WT-V2R (5–6-fold; P < 0.01) and all V2R mutants (3–4-fold; P < 0.01), except V2R-S167L (shown for dDAVP [Fig. 1A] and VA88 [Fig. 1B]). The variations in response between V2R mutants are likely intrinsic to the mutant proteins, because immunoblotting revealed similar expression levels for WT-V2R and its mutants (Fig. 1 A and B). These data show that 6 out of 7 V2R mutants in NDI are intrinsically functional.
Fig. 1.
Activation of wild-type and mutant V2R by peptidic and nonpeptidic agonists. (A) Intrinsic activity of V2R mutants in NDI. COS-M6 cells transiently expressing WT-V2R-GFP or its mutants were treated for 10 min. with 1 μM dDAVP or 1 μM VA88, (B) washed, lysed, and cAMP levels measured as described above. Untreated samples were set to 1 and significantly-increased cAMP levels (P < 0.05; student's t test) compared to mock-transfected cells are indicated by an asterisk. In parallel, cell equivalents were analyzed by immunoblotting. Protein masses are indicated in kDa.
Subcellular Localization of V2R Mutants in Polarized MDCK Cells.
Next, we determined the localization of V2R mutants in NDI. In vivo, the V2R is present in the basolateral membrane of polarized collecting duct cells and expressed at low levels. As these conditions are nicely mimicked in stably transfected MDCK-V2R cells ((14) and Fig. S2A), the V2R mutants were also expressed in MDCK cells. In contrast to WT-V2R, the V2R mutants -L44P, -I130F, -S167T, -Y128S, -Y280C, and -P322S are misfolded and mainly retained in the ER, as they colocalize to a great extent with the ER-marker Protein Disulfide Isomerase (PDI; Fig. S2A).
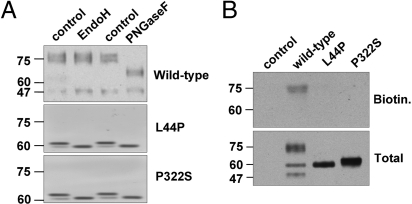
Glycosylated membrane proteins en route to the plasma membrane change their high-mannose glycosylation, added to them in the ER lumen, for complex glycosylation as they pass the medial-trans Golgi compartment (16). While high-mannose glycosylated V2R are 60 to 62 kDa, mature receptors are 75 kDa and resistant to endoglycosidase H (endo H), while they remain sensitive to protein N-glycosidase F (PNGase F). WT-V2R in MDCK cells is expressed as a mature 75 kDa protein, because it is insensitive to endoH and reduced to its O-glycosylated form of 65 kDa with PNGase F digestion (Fig. 2A). In contrast, the V2R mutants are expressed as 62 kDa immature proteins that are quantitatively equally sensitive to digestion with EndoH and PNGase F (Fig. 2A), indicative of localization in the early secretory pathway.
Fig. 2.
Subcellular localization of WT-V2R and V2R mutants in NDI. (A) MDCK WT-V2R, V2R-L44P, or -P322S protein samples were digested with Endoglycosydase H or Protein N-glycosydase F. Subsequently, protein samples and their respective undigested controls were analyzed by immunoblotting. (B) MDCK cells expressing WT-V2R, V2R-L44P, or -P322S were subjected to cell surface biotinylation and equalized total lysate and cell surface samples were analyzed by immunoblotting.
To investigate further whether V2R mutants were essentially absent from the plasma membrane, cells expressing WT-V2R or its L44P and P322S mutants were subjected to cell surface biotinylation. As shown in Fig. 2B, only mature WT-V2R was detected at the cell surface, whereas no immature receptors were detected for either WT or the mutants.
To analyze the subcellular localization of the V2R mutants in more detail, EM analysis was performed on empty MDCK cells or cells expressing WT-V2R, V2R-P322S, -L44P, or -Y128S (Fig. S2B) and signals were quantified for WT-V2R and V2R-P322S (Table 1). Because the ER in MDCK cells is fragmented and therefore not easy to discern, MDCK cells expressing WT-V2R or V2R-P322S were double labeled for the ER-marker PDI and V2R (Fig. S2C). Consistent with LM studies (14), WT-V2R is mainly present in the plasma membrane and endosomes/lysosomes. In contrast, V2R-P322S localizes predominantly to the endoplasmic reticulum and Golgi region. However, low levels of mutant receptors were also found in endosomes and in the plasma membrane (Table 1). These data reveal that in MDCK cells, V2R mutants associated with NDI are expressed in an immature form and are mainly retained in the ER and pre-Golgi compartments, although a small fraction of V2R mutant is found in post-Golgi compartments, including the plasma membrane.
Table 1.
Electron microscopic analysis of cells expressing GFP-tagged WT-V2R or V2R-P322S
| WT V2R | V2R-P322S | |
|---|---|---|
| ER | 5 ± 1.2 | 36 ± 3.5 |
| Golgi region | 2 ± 0.3 | 21 ± 1.8 |
| Golgi stack | 2 ± 0.4 | 9 ± 1.4 |
| PM | 53 ± 1.8 | 10 ± 1.1 |
| Endosomes | 31 ± 2.7 | 17 ± 2.3 |
| Lysosomes | 6 ± 1.8 | 2 ± 0.7 |
| Undefined | 1 ± 0.2 | 6 ± 0.7 |
Numbers indicate the percentages of total labeling for the indicated compartments. Two different grids were scanned resulting in 3,123 gold particles for WT V2R and 2,251 for V2R-P322S. ER, endoplasmic reticulum; PM, plasma membrane.
Nonpeptide Agonists, but Not dDAVP, Rescue V2R Mutants' Functioning.
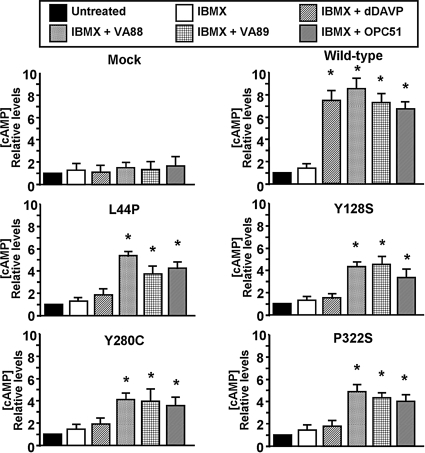
Because WT-V2R and its mutants had clearly different subcellular localizations and the mutants were essentially absent from the plasma membrane, we determined the ability of the nonpeptide agonists to generate a cAMP response in our V2R mutant-expressing MDCK cell lines. Incubation with the Emax concentrations of dDAVP (100 nM) or the nonpeptide agonists (1 μM; Fig. 1B) for only 10 min induced an approx. 7–9-fold over basal cAMP response in MDCK-V2R cells, whereas no effect was observed in mock-transfected MDCK cells (Fig. 3). Administration of 100 nM dDAVP did not induce a significant cAMP response in cells expressing the V2R mutants. However, incubation with 1 μM VA88, VA89, or OPC51, significantly increased cAMP in MDCK cells expressing V2R-L44P, -Y128S, -Y280C, -P322S, (Fig. 3; P < 0.01), -I130F, and -S167T (data not shown). Similar cAMP data were obtained when the cells were incubated with the agonists while cell surface V2Rs were continuously blocked with a peptidic V2R antagonist (not shown). These data indicated that nonpeptide agonists, but not dDAVP, can activate intracellularly-retained V2R mutants in NDI.
Fig. 3.
Functionality of retained V2R mutants upon treatment with nonpeptide agonists. Confluent WT-V2R, V2R-L44P, -Y128S, -Y280C, or -P322S cells were left untreated or treated for 10 min with IBMX alone or in combination with 100 nM dDAVP, 1 μM VA88, VA89, or OPC51. Subsequently, cells were lysed and cAMP levels were measured. Untreated samples were set to 1, and relative changes are shown. Triplicate samples were measured, and experiments were performed at least in 3-fold. Asterisks indicate significantly increased cAMP levels (P < 0.05) compared to IBMX alone.
Nonpeptide Agonists Do Not Affect the Localization or Maturation of V2R Mutants.
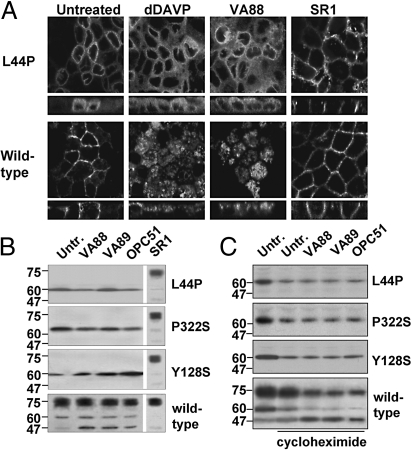
Nonpeptide antagonists induce the maturation and trafficking of V2R mutants from the ER to the basolateral membrane (10–12, 17). Moreover, activation of WT-V2R by AVP induces its internalization to lysosomes (14). Because the nonpeptide agonists activate the ER-retained V2R mutants, we investigated their effects on the localization and maturation of these mutants. For this, WT and mutant V2R-expressing MDCK cells were treated for 16 h with 100 nM dDAVP, or 1 μM of the nonpeptide agonists or the CPAn SR1. Subsequent CLSM analysis revealed that none of the 3 nonpeptide agonists nor dDAVP changed the ER localization of V2R-L44P or -Y128S (Fig. 4A and Fig. S3). In contrast and as reported (11), SR1 clearly increased the basolateral membrane localization of these mutants. However, WT-V2R was internalized from the basolateral membrane by dDAVP and the nonpeptide agonists, but not by SR1 (Fig. 4A Bottom and Fig. S3), which shows that the unchanged localization of the V2R mutants with the nonpeptide agonists was not due to the absence of agonist activity of the compounds.
Fig. 4.
Agonist action on the localization, maturation and stability of V2R mutants. (A) Localization. Confluent MDCK WT-V2R or V2R-L44P cells were left untreated or treated with 100 nM of the peptide agonist dDAVP, 1 μM the nonpeptide agonists VA88, or the nonpeptide antagonist SR1 for 16 h followed by CLSM analysis. (B) V2R Maturation and stability. MDCK WT-V2R, V2R-L44P, -Y128S, or -P322S cells were left untreated, treated with the nonpeptide agonists VA88, VA89 or OPC51, or treated with the nonpeptide antagonist SR1 for 16 h. Subsequently, receptor proteins were analyzed by immunoblotting. (C) Stability. MDCK WT-V2R, V2R-L44P, -Y128S or -P322S cells were left untreated, treated with 50 μM cycloheximide alone, or supplemented with 1 μM VA88, VA89 or OCP51 for 6 h. Subsequently, receptor proteins were analyzed by immunoblotting.
Because trafficking of V2R-GFP to the Golgi complex and beyond, but also of V2R mutants when incubated with SR1, increases its mass from 62 to 75 kDa (11), cells treated as above were also subjected to immunoblot analyses. Consistent with our CLSM data, none of the nonpeptide agonists induced maturation of the V2R mutants, as only the immature 62 kDa form was detected. In contrast, incubation with SR1 shifted the mass of the V2R mutants from 62 to 75 kDa (Fig. 4B; shown for V2R-L44P, -Y128S, and -P322S). For WT-V2R, treatment with VA88, VA89, or OPC51 did not significantly affect the level of maturation. In line with the agonistic activity, however, they caused an increase of a 47 kDa degradation product for V2R-GFP (Fig. 4B), which was also observed in LLC-PK1-V2R cells treated with dDAVP (18). Together, these data indicate that activation by nonpeptide agonists does not change the localization or maturation of the V2R mutants.
Nonpeptide Agonist Effects on V2R Expression and Stability.
Nonpeptide antagonists stabilize the expression of intracellularly-retained V2R mutants in NDI and promote their maturation (10, 11), whereas activation of WT-V2R by agonists induces its lysosomal degradation (14, 18). As the nonpeptide agonists activate the V2R mutants, but do not induce their maturation, we investigated their effects on V2R mutant expression and stability. Semiquantification of the signals in Fig. 4B revealed that 16 h treatment with any of the 3 nonpeptide agonists did not significantly affect the expression levels of the 62 and 75 kDa forms of WT-V2R, but did increase the signal of its 47 kDa degradation product (P < 0.01). Moreover, treatment with the agonists did not significantly change the V2R-L44P or V2R-P322S expression levels (Fig. 4B), nor those of V2R-I130F, -S167T, and Y280C. Only for V2R-Y128S, all 3 compounds significantly (P < 0.03) increased the expression of this mutant (Fig. 4B).
The unchanged expression levels observed in Fig. 4B are a balance between V2R synthesis and degradation, which may both be increased with the agonists. As a lack of V2R mutant degradation with nonpeptide agonists activation may be clinically beneficial, we tested whether the agonists increase degradation of the ER-retained mutants. Cells were treated with the nonpeptide agonists for 6 h in the absence or presence of the protein synthesis inhibitor cycloheximide. Subsequent immunoblotting revealed that with cycloheximide, immature WT-V2R expression markedly decreased, mature V2R expression slightly decreased, and expression of the 47 kDa degradation product remained unchanged (Fig. 4C), indicating general degradation. Cotreatment with the agonists VA88, VA89, or OPC51, however, further decreased the 62 and 75 kDa signals and increased the 47 kDa signal. Together with their localization in late endosomes/lysosomes (Fig. 4A), this indicates that the nonpeptide agonists induce the lysosomal degradation of WT V2R.
Analysis of the V2R mutants showed that also their general expression was reduced by cycloheximide. Coincubation with any of the agonists, however, did not lead to a further decrease of their expression, nor did the 47 kDa signal appear (Fig. 4C). This indicated that, although the nonpeptide agonists activate the retained V2R mutants, they do not induce their degradation.
Activation of Retained V2R Mutants by Nonpeptide Agonists Induces the Translocation of AQP2 to the Apical Membrane.
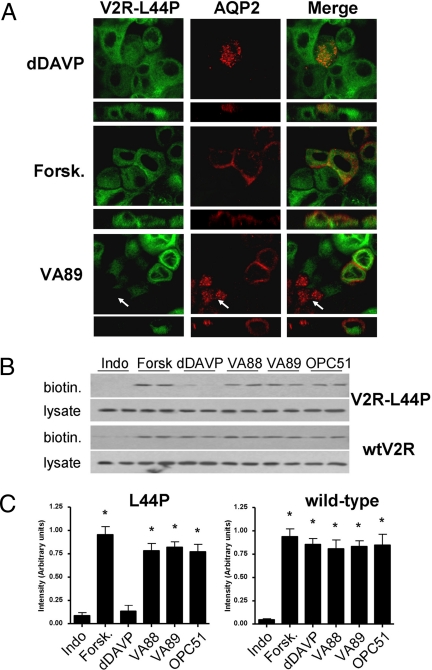
To relieve NDI in patients, the cAMP response generated from V2R mutants upon activation by nonpeptide agonists should be sufficient to activate PKA and allow re-distribution of AQP2 to the plasma membrane. To test this in vitro, MDCK-V2R-L44P cells were infected with recombinant AQP2 lentiviruses, treated with agonists for 2 h, and analyzed by immunocytochemistry. In line with the NDI phenotype, AQP2 localizes to intracellular vesicles in MDCK-V2R-L44P cells treated with 100 nM dDAVP (Fig. 5A Upper). In contrast, coincubation with the adenylate cyclase activator forskolin, or 1 μM of the nonpeptide agonists VA89 (Fig. 5A), VA88, or OPC51 (Fig. S4) induced redistribution of AQP2 from vesicles to the apical and, to some extent, basolateral region of the cells. Note the absence of AQP2 redistribution in VA89-treated cells lacking V2R-L44P (indicated by arrows).
Fig. 5.
Agonist induced translocation of AQP2. MDCKII-V2R-L44P cells infected with an AQP2-lentivirus were incubated overnight with 50 μM indomethacin. (A) Then, cells were supplemented with 100 nM dDAVP, 10 μM forskolin (Forsk.), or 1 μM VA89 for 2 h. Next, the cells were subjected to immunocytochemistry using rabbit anti-AQP2 antibodies. (B) In parallel, MDCKII-V2R-L44P and WT-V2R cells were pretreated as under (A) and subjected to cell surface biotinylation. The 15 μg samples of total cell lysates and equalized cell surface fractions (biotin) were analyzed by immunoblotting using anti-AQP2 antibodies. (C) The cell-surface data from (B) were semiquantified using densitometry. Samples indicated with an asterisk are significantly different from the control (P < 0.05, student's t test).
To determine whether V2R-L44P activation actually resulted in AQP2 redistribution into the apical membrane, MDCK-V2R-L44P and MDCK-V2R control cells were treated as above and subjected to apical cell surface biotinylation. Consistent with the immunocytochemical data, AQP2 immunoblotting of the apical cell surface proteins from MDCK-V2R-L44P cells revealed a significant increase in apical membrane AQP2 following treatment with forskolin or any of the nonpeptide agonists, but not with dDAVP (Fig. 5 B and C). In contrast, in MDCK-V2R cells, a significantly increased apical membrane expression of AQP2 was observed with all agonists, including dDAVP. These differences were not due to variations in AQP2 expression levels, because these were similar (Fig. 5B, lysates). These data indicate that cAMP generated by the intracellular activation of V2R-L44P is sufficient to induce redistribution of AQP2 from vesicles to the apical membrane.
Discussion
Nonpeptide Agonists Activate Intracellularly-Retained Mutant V2R Proteins in NDI.
One known and 2 novel nonpeptide V2R agonists showed all of the characteristics of V2R agonists, because they activated V2R to generate cAMP, caused internalization of V2R-GFP following binding, and induced AQP2 synthesis and redistribution from vesicles to the apical membrane (Fig. S1, Fig. 4 and 5).
The general concept that activation of GPCRs activated by cell-permeable ligands needs to occur at the plasma membrane is challenged by our data with these nonpeptide V2R agonists: While 6 out of 7 V2R mutants tested were intrinsically functional receptors (Fig. 1 A and B), MDCK cells stably expressing these 6 functional V2R mutants were unresponsive to the cell-impermeable dDAVP (Fig. 3). Moreover, all V2R mutants tested mainly localize to the ER-Golgi complex (Fig. S2; Table 1). Apparently, the low levels of V2R mutant detected in the plasma membrane (Table 1) are too low to induce a detectable response. In contrast to dDAVP, however, all 3 nonpeptide agonists induced a significant cAMP response with MDCK cells expressing the 6 functional V2R mutants, (Fig. 3), even when blocking the plasma membrane V2R. Because dDAVP and nonpeptide agonists concentrations were used at equimolar concentrations, the difference in effect is not due to higher activity of the nonpeptide agonists, but most likely due to a higher propensity of the nonpeptide agonists to penetrate the cell membrane. As such, our data reveal that a GPCR that normally functions at the cell membrane can generate its corresponding signaling cascade from an intracellular location. Moreover, nonpeptide agonists can activate intrinsically-functional but disease-causing V2R mutants from these intracellular sites.
While the functional data above are clear, the lack of signaling from plasma membrane V2R-P322S is surprising. The most likely explanation is the low number of mutant receptors in the plasma membrane. Indeed, while 10% localizes in the plasma membrane, the total abundance of V2R-P322S in our EM analysis was considerably less than of WT-V2R (Table 1), which can be attributed to their reduced stability (8). Alternatively, plasma membrane V2R-P322S may signal less efficient than WT-V2R.
Recent observations that the β2-adrenergic receptor assembles with Gαs and adenylate cyclase in the ER (19, 20) and that the naturally cell permeable agonist estrogen induces signaling from the ER-localized GPR30 (21, 22) indicate that nonpeptide agonists may activate the V2R mutants to generate cAMP while located in the ER. However, considering the expression of some V2R mutants beyond the ER-Golgi complex, this remains to be established.
Intracellular Activation of V2R Mutants in NDI Does Not Change Their Localization or Stability.
Even after prolonged incubation, none of the nonpeptide agonists induce any appreciable shift in localization of the V2R mutants, nor change their state of maturation (Fig. 4). In contrast, the nonpeptide V2R antagonist SR1 clearly increased V2R mutant expression at the cell surface and induced maturation (Fig. 4)(10–12, 17). The absence of translocation of V2R mutants to the cell surface is further supported by the fact that cAMP responses are obtained within 10 min following agonists addition, whereas it takes V2R antagonists 4–8 h to insert V2R mutants in the plasma membrane (11, 23). Our data are different from those of Petaja-Repo et al. (24), who found that nonpeptide agonists were able to stabilize the expression and maturation of ER-retained wild-type δ-opioid receptor (DOR). This indicates that nonpeptide agonist-induced maturation and rescue of cell surface expression is receptor-, mutation- and/or agonist-specific. Since in our study all V2R mutants tested remained unaffected in their localization and maturation, a difference in receptor is most the most likely explanation.
At present, it is unknown whether intracellular receptors are desensitized similar to their cell surface counterparts upon activation. While agonist-induced lysosomal targeting and degradation reveals that WT-V2R is down-regulated in MDCK cells, nonpeptide agonists did not increase degradation of the intracellularly-retained V2R mutants in NDI (Fig. 4). It remains to be established which step of the normal desensitization steps is abrogated with the mutant V2R proteins and whether this is generally observed for intracellular GPCRs.
High Mannose-Glycosylated V2R Mutants Are Retained in, but Not Confined to, the ER-Early-Golgi Compartment.
A paradigm is that integral membrane proteins on their way to the plasma membrane exchange their high-mannose (ER) glycosylation for complex-glycosylated moieties when passing through the Golgi complex. However, although all intracellularly-retained V2R mutants in NDI localize to a great extent in the early secretory pathway (Table 1), are only high-mannose glycosylated, and are equally sensitive to endoH and PNGaseF digestion (Fig. 2A), our EM analysis revealed that a considerable pool of V2R-P322S localized to organelles beyond this stage. In transient expression studies (e.g., Fig. 1), non- or high-mannose glycosylated membrane proteins beyond the Golgi complex are commonly found, which is attributed to the inability of the ER quality control system to retain the high number of membrane proteins synthesized. This, however, does not explain the observation in our stably-transfected MDCK cells, because WTV2R is fully matured (Fig. 2) and expressed at a higher level than the V2R mutants.
Interestingly, of several other membrane proteins, including the cystic fibrosis transmembrane conductance regulator (CFTR), protein phosphatase CD45, and the epithelial sodium channel (ENaC), post-Golgi expression of immature forms have been found for endogenously expressed proteins or in stably-transfected cells (25–27). Possibly, low levels of proteins bypass the Golgi and may directly be delivered to post-Golgi compartments, or all proteins traffic to the plasma membrane via the conventional pathway, but a population of these proteins are resistant to posttranslational processing (26). In line with this, misfolding of the V2R mutants may affect proper exchange of sugar moieties in the Golgi complex, or a small fraction may bypass this organelle in our transfected MDCK cells. Our data do show, however, that expression of only the immature glycosylated form of a mutant membrane protein is an indicator of retention in the ER or early Golgi compartment, but does not necessarily exclude that a small fraction localizes beyond the Golgi complex. While it remains unknown whether GPCR mutants traffic beyond the Golgi complex in humans, trafficking of immature membrane proteins beyond the Golgi complex has been observed for endogenously expressed membrane proteins and may thus also apply to V2R mutants in vivo.
Potential Implications of the Use of V2R Nonpeptide Agonists for NDI Patients.
The lack of V2R activation in NDI patients with V2R mutations causes reduces AQP2 expression levels and abrogates AQP2 translocation to the apical membrane in renal collecting duct principal cells (28). Our data indicate that the nonpeptide V2R agonists constitute highly promising therapeutics for the treatment of NDI resulting from intracellularly-retained V2R mutants. First, the nonpeptide agonists are unlikely to cause major side effects, because they are highly selective for V2R (29). Nevertheless, monitoring of the V2R-specific extra-renal release of coagulation factors will be essential in patients. Secondly, these agonists induce a cAMP response mediated by ER-retained V2R mutants (Fig. 3) and are able to induce the cAMP-dependent expression of endogenous AQP2 (Fig. 1C). Thirdly, the cAMP response generated by the intracellularly activated V2R mutants appears sufficiently strong to mediate the critical step in renal water reabsorption, the trafficking of AQP2 proteins from vesicles to the plasma membrane (Fig. 5).
While administration of the V1R antagonist SR49059 to patients was the first in vivo proof of principle that nonpeptide antagonists can relieve NDI in patients (17), the direct activation of functional ER-retained V2R mutants observed here indicates that treatment with nonpeptide V2R agonists is likely superior over nonpeptide antagonists because rescue of cell surface expression of the V2R mutants and subsequent displacement of the antagonists by endogenous AVP is circumvented. Considering the low stability of ER-retained folding mutants (8), the lack of increased V2R mutant degradation upon activation by nonpeptide agonists provides an additional advantage for their therapeutic use. Determination of their in vivo therapeutic value for NDI, however, awaits approval for their use and testing in patients, because mice lacking functional V2Rs die soon after birth (30) and OPC51 (803), VA (9990)88, and VA(9990)89-like compounds are still in early phase clinical trials. However, the observed decrease in urine output with OPC51803 in Brattleboro rats, which have functional V2Rs but lack AVP, already reveals the in vivo activity of nonpeptide V2R agonists on a normal functioning renal concentrating mechanism (29) and underscores the potential of nonpeptide V2R agonists as therapeutic agents for NDI patients due to retained, but intrinsically-functional, V2R mutants.
In conclusion, our data show that a GPCR, which normally functions in the plasma membrane, can generate a signaling cascade from an intracellular location to induce the receptor's physiological response. Moreover, the activation of intracellular V2R mutants by our nonpeptide agonists does not lead to increased degradation. As approximately 40% of GPCR mutants in diseases are misfolded and retained intracellularly, our data indicate that nonpeptide agonists are ideal therapeutics to treat patients suffering from one of the many “conformational diseases” due to misfolded and retained GPCRs in general, and NDI due to misfolded V2R mutants in particular.
Materials and Methods
Ligands.
The nonpeptide V2R agonists VA999088 and VA999089 were kindly provided by Dr. Haigh (Vantia Ltd.), OPC51803 was kindly provided by Dr. Komuro (Otsuka Pharmaceutical Co., Tokushima, Japan). The V2R nonpeptide antagonist SR121463B was provided by Dr. Serradeil-Le Gal (Sanofi Synthélabo Recherche, Toulouse, France). dDAVP and the peptidic antagonist [Adamantaneacetyl1, O-Et-D-Tyr2, Val4, Aminobutyryl6, Arg8,9]-Vasopressin were from Sigma. Their abbreviations and relevant characteristics are summarized in Table S1 and fully described in SI Materials and Methods.
Expression Constructs.
With site-directed mutagenesis (Stratagene), the NDI-related mutations Y128S, Y280C, and P322S were introduced into the hV2R cDNA and the mutation-containing fragments were subcloned into the corresponding sites of pEGFP-N1-V2R as described in SI Materials and Methods.
To generate a recombinant lentivirus encoding AQP2, the AQP2 cDNA was cloned into pLV-PGK (Tronolab) to yield pLV-PGK-AQP2. Viruses were generated in HEK293 cells and titrated as described in SI Materials and Methods.
Cell Culture and Biochemical Assays.
MDCK cell lines stably expressing GFP-tagged WT-V2R and mutants in NDI were generated as described (8, 14) and culture and transfection of COS cells was performed as described (8). cAMP measurements, cell surface biotinylation assays, Western blotting, and immunocytochemistry were performed as described (8, 14). These assays and the antibodies used are described in more detail in SI Materials and Methods section.
Supplementary Material
Acknowledgments.
We thank Koji Komuro (Otsuka Pharmaceutical Co., Tokushima, Japan) for kindly supplying OPC51. We thank Dr. Robert Haigh (Vantia Ltd., Chilworth, U.K.) for kindly supplying VA88 and VA89 and Dr. Claudine Serradeil-Le Gal (Sanofi Synthélabo Recherche, Toulouse, France) for kindly supplying SR1. We thank Rene Scriwanek and Marc van Peski for preparation of the electron micrographs. J.K. and P.M.T.D. are recipients of VICI grants 918.56.611 and 865.07.002 of the Netherlands Organization for Scientific Research. We acknowledge the Dutch Kidney Foundation (PC 104, C06.2164), RUNMC and Netherlands Organization for Scientific Research (865.07.002) and Coordination Theme 1 (Health) of the European Community's 7th Framework Program (HEALTH-F2–2007-201590, entitled EUNEFRON).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900130106/DCSupplemental.
References
- 1.Illing ME, Rajan RS, Bence NF, Kopito RR. A rhodopsin mutant linked to autosomal dominant retinitis pigmentosa is prone to aggregate and interacts with the ubiquitin proteasome system. J Biol Chem. 2002;277:34150–34160. doi: 10.1074/jbc.M204955200. [DOI] [PubMed] [Google Scholar]
- 2.De Roux N., et al. A family with hypogonadotropic hypogonadism and mutations in the gonadotropin-releasing hormone receptor. N Engl J Med. 1997;337:1597–1602. doi: 10.1056/NEJM199711273372205. [DOI] [PubMed] [Google Scholar]
- 3.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 4.Biebermann H, Gruters A, Schoneberg T, Gudermann T. Congenital hypothyroidism caused by mutations in the thyrotropin receptor gene. N Engl J Med. 1997;336:1390–1391. doi: 10.1056/NEJM199705083361914. [DOI] [PubMed] [Google Scholar]
- 5.Van den Ouweland AMW, et al. Mutations in the Vasopressin Type-2 Receptor Gene (Avpr2) Associated with Nephrogenic Diabetes-Insipidus. Nat Genet. 1992;2:99–102. doi: 10.1038/ng1092-99. [DOI] [PubMed] [Google Scholar]
- 6.Robben JH, Knoers N VAM, Deen PMT. Cell biological aspects of the vasopressin type-2 receptor and aquaporin 2 water channel in nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2006;291:F257–F270. doi: 10.1152/ajprenal.00491.2005. [DOI] [PubMed] [Google Scholar]
- 7.Vembar SS, Brodsky JL. One step at a time: Endoplasmic reticulum-associated degradation. Nat Rev Mol Cell Biol. 2008;9:944–957. doi: 10.1038/nrm2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robben JH, Knoers…., Deen PMT. Characterization of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus in a polarized cell model. Am J Physiol Renal Physiol. 2005;289:F265–F272. doi: 10.1152/ajprenal.00404.2004. [DOI] [PubMed] [Google Scholar]
- 9.Bernier V, et al. Functional Rescue of the Constitutively Internalized V2 Vasopressin Receptor Mutant R137H by the Pharmacological Chaperone Action of SR49059. Mol Endocrinol. 2004;18:2074–2084. doi: 10.1210/me.2004-0080. [DOI] [PubMed] [Google Scholar]
- 10.Morello JP, et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robben JH, Sze M, Knoers N, Deen PMT. Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: Relevance to therapy of Nephrogenic Diabetes Insipidus. Am J Physiol. 2007;292:F253–F260. doi: 10.1152/ajprenal.00247.2006. [DOI] [PubMed] [Google Scholar]
- 12.Tan CM, Nickols HH, Limbird LE. Appropriate polarization following pharmacological rescue of V2 vasopressin receptors encoded by X-linked nephrogenic diabetes insipidus alleles involves a conformation of the receptor that also attains mature glycosylation. J Biol Chem. 2003;278:35678–35686. doi: 10.1074/jbc.M301888200. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura S, et al. Characterization of a novel nonpeptide vasopressin V2-agonist, OPC-51803, in cells transfected human vasopressin receptor subtypes. Br J Pharmacol. 2000;129:1700–1706. doi: 10.1038/sj.bjp.0703221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robben JH, Knoers NVAM, Deen PMT. Regulation of the vasopressin V2 receptor by vasopressin in polarized renal collecting duct cells. Mol Biol Cell. 2004;15:5693–5699. doi: 10.1091/mbc.E04-04-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasler U, et al. Long-term regulation of aquaporin-2 expression in vasopressin- responsive renal collecting duct principal cells. J Biol Chem. 2002;277:10379–10386. doi: 10.1074/jbc.M111880200. [DOI] [PubMed] [Google Scholar]
- 16.Sadeghi HM, Innamorati G, Birnbaumer M. Maturation of receptor proteins in eukaryotic expression systems. J Recept Signal Transduct Res. 1997;17:433–445. doi: 10.3109/10799899709036619. [DOI] [PubMed] [Google Scholar]
- 17.Bernier V, et al. Pharmacologic chaperones as a potential treatment for X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2006;17:232–243. doi: 10.1681/ASN.2005080854. [DOI] [PubMed] [Google Scholar]
- 18.Bouley R, et al. Downregulation of the vasopressin type 2 receptor after vasopressin-induced internalization: Involvement of a lysosomal degradation pathway. Am J Physiol. 2005;288:C1390–C1401. doi: 10.1152/ajpcell.00353.2004. [DOI] [PubMed] [Google Scholar]
- 19.Dupre DJ, et al. Seven transmembrane receptor core signaling complexes are assembled prior to plasma membrane trafficking. J Biol Chem. 2006;281:34561–34573. doi: 10.1074/jbc.M605012200. [DOI] [PubMed] [Google Scholar]
- 20.Dupre DJ, Baragli A, Rebois RV, Ethier N, Hebert TE. Signalling complexes associated with adenylyl cyclase II are assembled during their biosynthesis. Cell Signal. 2007;19:481–489. doi: 10.1016/j.cellsig.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 22.Filardo EJ, Quinn JA, Frackelton AR, Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- 23.Wuller S, et al. Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biol Chem. 2004;279:47254–47263. doi: 10.1074/jbc.M408154200. [DOI] [PubMed] [Google Scholar]
- 24.Petaja-Repo UE, et al. Ligands act as pharmacological chaperones and increase the efficiency of delta opioid receptor maturation. EMBO J. 2002;21:1628–1637. doi: 10.1093/emboj/21.7.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Planes C, et al. Hypoxia and beta 2-agonists regulate cell surface expression of the epithelial sodium channel in native alveolar epithelial cells. J Biol Chem. 2002;277:47318–47324. doi: 10.1074/jbc.M209158200. [DOI] [PubMed] [Google Scholar]
- 26.Hughey RP, Bruns JB, Kinlough CL, Kleyman TR. Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem. 2004;279:48491–48494. doi: 10.1074/jbc.C400460200. [DOI] [PubMed] [Google Scholar]
- 27.Baldwin TA, Ostergaard HL. The protein-tyrosine phosphatase CD45 reaches the cell surface via golgi-dependent and -independent pathways. J Biol Chem. 2002;277:50333–50340. doi: 10.1074/jbc.M209075200. [DOI] [PubMed] [Google Scholar]
- 28.Kanno K, et al. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N Engl J Med. 1995;332:1540–1545. doi: 10.1056/NEJM199506083322303. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura S, et al. Antidiuretic effects of a nonpeptide vasopressin V2-receptor agonist, OPC-51803, administered orally to rats. J Pharmacol Exp Ther. 2000;295:1005–1011. [PubMed] [Google Scholar]
- 30.Yun J, et al. Generation and phenotype of mice harboring a nonsense mutation in the V2 vasopressin receptor gene. J Clin Invest. 2000;106:1361–1371. doi: 10.1172/JCI9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.