Abstract
Steroid hormones regulate many physiological processes in vertebrates, nematodes, and arthropods through binding to nuclear receptors (NR), a metazoan-specific family of ligand-activated transcription factors. The main steps controlling the diversification of this family are now well-understood. In contrast, the origin and evolution of steroid ligands remain mysterious, although this is crucial for understanding the emergence of modern endocrine systems. Using a comparative genomic approach, we analyzed complete metazoan genomes to provide a comprehensive view of the evolution of major enzymatic players implicated in steroidogenesis at the whole metazoan scale. Our analysis reveals that steroidogenesis has been independently elaborated in the 3 main bilaterian lineages, and that steroidogenic cytochrome P450 enzymes descended from those that detoxify xenobiotics.
Keywords: evolution, nuclear-receptor ligand, steroidogenesis
Multicellular organisms have complex endocrine systems, allowing responses to environmental stimuli, regulation of development, reproduction, and homeostasis. Nuclear receptors (NRs), a metazoan-specific family of ligand-activated transcription factors, play central roles in endocrine responses, as intermediates between signaling molecules and target genes (1). The NR family includes ligand-bound and orphan receptors, that is, receptors with no known ligand or for which there is no ligand pocket (2). Understanding NR evolution has been further improved by comparison of several completed genomes, particularly those of deuterostomes and ecdysozoans (3–6).
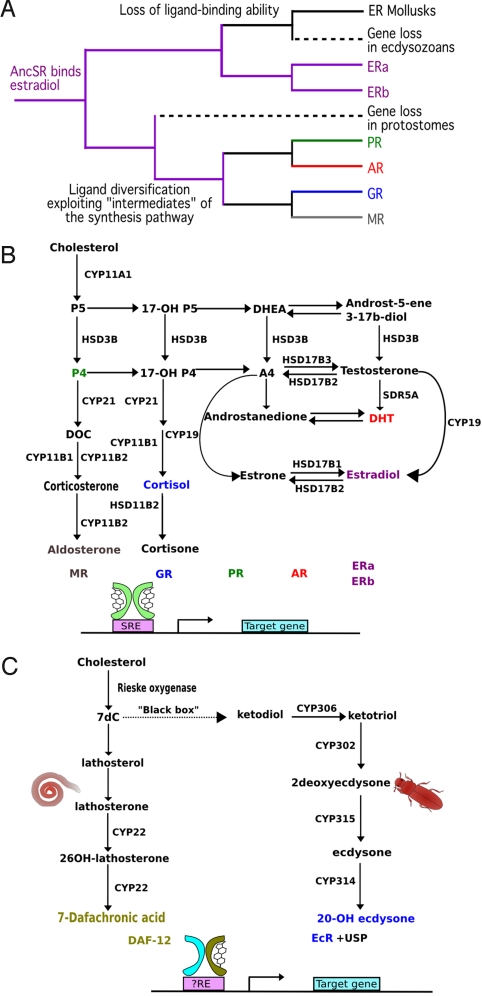
In contrast, evolution of NR ligands is still much debated. One hypothesis proposes that several independent gains and losses of ligand-binding ability in NRs occurred in protostomes and deuterostomes (7–9). A second hypothesis, pertaining to the NR3 subfamily (vertebrate steroid hormone receptors and estrogen-related receptor), proposes that before the divergence of protostomes and deuterostomes, there was an ancestral steroid receptor (AncSR) that was ligand-activated and that orphan receptors secondarily lost the ability to bind a ligand (10, 11). Phylogenetic analyses indicate that AncSR was able to bind estrogens (10, 11), which formed the basis for an intriguing “ligand exploitation model” (10, 12) for the evolution of vertebrate steroid receptors. In this model, estradiol (E2), a terminal product of the steroid biosynthetic pathway, was the first ligand for AncSR. Synthesis of E2 also requires the synthesis of steroid intermediates (Fig. 1). However, receptors for these intermediate steroids had not yet evolved. It was only after duplication of AncSR that NR3 receptors for these intermediate steroids evolved. The “ligand exploitation model” explains divergence in ligand specificity seen in steroid receptors, namely AR/NR3C4, GR/NR3C1, MR/NR3C2, PR/NR3C3, and ERs/NR3A (10, 12, Fig. 1A and B).
Fig. 1.
Study background. (A) The ligand exploitation hypothesis. The ancestral receptor, that is supposed to bind estradiol, should have been lost in ecdysozoans, have lost its ligand-binding ability in mollusks, and have undergone ligand diversification through gene duplications in vertebrates. (B) The human steroid signaling pathway. (C) The steroid signaling pathways in ecdysozoans.
The ligand exploitation model is based mainly on NR data. But it has implications for the evolution of ligand synthesis. For example, it implies that 17ß-estradiol (E2) was a ligand for an ER in Urbilateria, the common ancestor of protostomes and deuterostomes (10, 12, 13). Such a hypothesis can be tested by searching for the origins of the enzymes involved in the synthesis of vertebrate adrenal and sex steroids.
As to steroid hormones in metazoans, there are major structural differences among different classes of steroids synthesized in vertebrates, insects and nematodes (Fig. S1). In insects and nematodes, the active steroid hormones retain all or most of the C17 side chain of cholesterol, with selective hydroxylations providing specificity for a given NR (Fig. 1C) (14–16). In contrast, in vertebrates, such as humans, synthesis of the main active steroids [estradiol for ERs, dihydroxytestosterone (DHT) for AR, progesterone (P4) for PR, cortisol for GR, and aldosterone for MR] begins with cleavage of the C17 side chain at C20 by CYP11A1 to yield pregnenolone (P5) (Fig. 1B) (17). Further enzymatic modifications involving selective hydroxylations, oxido-reductions and isomerizations of P5 and its metabolites yield ligands for adrenal and sex steroid receptors (Fig. 1B).
Many searches for “human”-type steroid hormones such as E2 or P4, throughout metazoan groups have been prone to artefacts and/or misidentification. To date, biochemical evidence (immunological and/or chromatographic methods linked to mass spectrometry) for presence of vertebrate steroids in lophotrochozoans, ecdysozoans, and cnidarians have not been substantiated by molecular characterization of enzymes directly involved in their de novo biosynthesis (18, 19). Thus, the presence of human-type steroids in protostomes remains an open question.
With this in mind, we investigated origins of enzymes in the pathways leading to steroid hormones in vertebrates. Our phylogenetic analyses of all enzymes known to be implicated in vertebrate (Fig. 1B) or ecdysozoan (Fig. 1C) steroid biosynthesis [belonging to the cytochrome P450 (CYP, 20, 21), short-chain dehydrogenase/reductase (SDR, 22), 3-ß hydroxysteroid dehydrogenase (HSD3B, 23) and steroid 5-α reductase (SRD5A) families] suggest that steroidogenesis was independently elaborated in vertebrates and protostomes, partly through recruitment of xenobiotic-metabolising CYPs. This has important implications on our views about the ligand-binding abilities of AncSR. Our analyses also show that there are pitfalls in extrapolating about the role in steroidogenesis of human or tetrapod genes to homologs in protostomes and other distant metazoans.
Results
General Strategy.
To date, the best characterized steroidogenic enzymes belong to the CYP, SDR, HSD3B, and SRD5A families in human, mouse, Drosophila, and C. elegans. We screened recently completed metazoan genomes (Fig. S2) looking for orthologs of these steroidogenic enzymes. The retrieved sequences were used for phylogenetic reconstruction (using maximum likelihood coupled with bootstrapping) to determine their orthology with vertebrate sequences.
Orthology was defined on the basis of robust branches containing a consistent phylogenetic sampling (that is only protostome sequences for example) and/or high (>90%) bootstrap values. The large sequence variability present in some families such as CYPs precluded phylogenetic reconstruction, but our systematic survey revealed clear orthology in specific cases relevant to steroidogenesis evolution (24, 25).
Twenty-one complete genomes were screened, including 6 recently sequenced lophotrochozoan genomes, making it highly probable that not finding a given protein in a given zoological group (e.g., protostomes) indicates a real absence. Fig. 1A exemplifies our reasoning for estrogen receptors.
Our strategy successfully identified clear orthologs of known steroidogenic enzymes. For example, we identified in Daphnia, a crustacean, orthologs of enzymes that metabolize insect steroids (i.e., CYP302, CYP314, CYP315, and CYP306) known in Drosophila and other insects (Fig. 2A and Fig. 3 and Figs. S3 and S4).
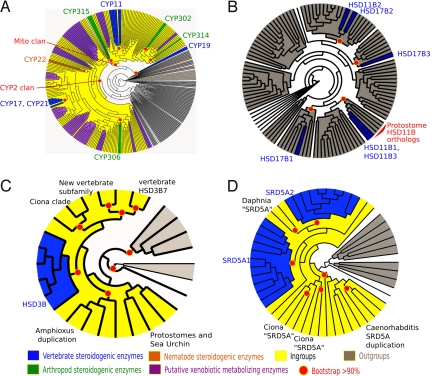
Fig. 2.
Simplified Maximum-likelihood phylogenies of the CYP, SDR, HSD3B, and SRD5A families in metazoans. (A) CYP family. (B) SDR family. (C) HSD3B family. (D) SRD5A family. Steroidogenic proteins are highlighted in different colors. This clearly illustrates that in most cases the steroidogenic enzymes are dispersed in the evolutionary trees, suggesting independent acquisition of their steroid specificity.
Fig. 3.
A simplified Maximum-Likelihood phylogeny of the mitochondrial clan. Vertebrate and arthropod steroidogenic enzymes are highlighted in blue and green, respectively, and the molecules they produce are indicated. These molecules are 20-OH Ecdysone for CYP314, Ecdysone for CYP315, 2deoxyecdysone for CYP302, pregnenolone for CYP11A, and cortisol for CYP11B. Colored residues in the chemical formulas are those that are modified by the catalytic reaction.
Fig. 2 provides a general overview of the phylogeny of the 4 protein families analyzed. For more complete versions of these phylogenies, and the relevant specific branches see Figs. S3–S7. These phylogenies, based on several sequenced genomes, are in good agreement with published studies (25).
Polyphyletic Origin for Steroidogenic CYPs.
The metazoan CYP family is currently divided into 11 clans (21, 22, Fig. 2A and Fig. S3), including the mito clan that clusters mitochondrial proteins in vertebrates and insects (Fig. 3 and Fig. S4). To date, all mitochondrial CYPs identified in vertebrates are involved in metabolism of endogenous compounds (e.g., CYP27A1 for bile acids) or hormone biosynthesis (CYP11A and CYP11B for steroid hormones), with CYP11A catalyzing cleavage of the cholesterol side chain at C20 (Fig. 1B). In contrast, arthropod mitochondrial CYPs include several xenobiotic-metabolizing proteins (e.g., CYP12) and enzymes catalyzing steroid biosynthesis (CYP302, CYP314, and CYP315) (Fig. 3). We observed that the vertebrate (CYP11A and CYP11B) and arthropod steroidogenic enzymes (CYP302, CYP314, and CYP315) do not form a monophyletic clade, and are rather dispersed at various places in the tree, often linked to non-steroidogenic proteins (Fig. 2A).
The most parsimonious scenario for these different activities within family- and lineage-specific duplications, is that these different steroidogenic activities arose independently in arthropod and vertebrate mitochondrial CYPs. This scenario implies that, if the substrate for an ancestral mito CYP was a steroid, then it probably was not a vertebrate steroid found in present-day organisms.
Similar conclusions can be drawn for other CYP clans. For example, in the CYP2 clan, steroidogenic activity seems to have appeared independently at least 3 times, in vertebrates, in insects, and in nematodes (Fig. 2A and Fig. S3). An important point is that many of the vertebrate members of this clan are known to be xenobiotic-metabolizing enzymes that are correlated with a high rate of lineage-specific duplications (24). Lineage-specific duplications are also abundant within lophotrochozoan members of this clan, thus indicating that these enzymes may be xenobiotic metabolisers.
SDR: Convergent Acquisition of the same Biochemical Activity.
Short-chain dehydrogenase/reductase (SDRs) enzymes display a wide substrate spectrum, ranging from steroids, retinoids, alcohols, sugars, and aromatic compounds to xenobiotics (22). In terms of steroidogenesis, this family contains proteins with 17ß-hydroxysteroid dehydrogenase (17ßHSD) activity as well as 11ßHSD activity (26, 27), characterized as steroidogenic enzymes in vertebrates (HSD17B1, −2, and −3; HSD11B1, −2, and −3). Previous reports (26, 28) noted that 17ßHSD and 11ßHSD activities arose independently many times in the SDR family. We confirm and extend this notion by finding that among the vertebrate steroidogenic proteins, only 1, HSD11B1, that is involved in the synthesis of cortisol from cortisone in vertebrates, has clear orthologues in lophotrochozoans (Fig. 2B). All of the other proteins, and especially those implicated in estrogen synthesis (HSD17B1, −2, and −3), arose from vertebrate-specific duplications and have no orthologues in protostomes.
The subfamily 3 of SDR (Fig. S5) illustrates this notion. It contains 1 human enzyme, HSD17B1 that clusters with a group containing the human RDH8, a photoreceptor-associated retinol dehydrogenase, as well as many vertebrate paralogs with uncharacterized activities. All of these vertebrate proteins cluster with proteins found in the cnidarian Nematostella whose activities are unknown. These data are consistent with the hypothesis that an ancestral HSD17B1 acquired the 17ßHSD biological function for synthesis of estradiol late during vertebrate evolution (8, 26).
HSD3B and SRD5A: Independent Lineage-Specific Duplications Within Chordates.
The HSD3B family contains 5 robust clades (Fig. 2C and Fig. S6) that are the products of lineage-specific duplications in deuterostomes (23). The protostome sequences are external to these groups. According to the topology of this tree, Ciona, amphioxus and protostome proteins may have a HSD3B activity, but it is not possible to infer whether the function of the Ciona and protostome proteins is to metabolize vertebrate steroid hormones, bile acids, or other molecules. Similarly, in the SRD5A family (Fig. 2D and Fig. S7), lineage-specific duplications also occurred in vertebrates, Ciona, Daphnia, and Caenorhabditis, whereas the gene was lost in insects. Thus, in these 2 gene families, lineage-specific elaboration of steroidogenic enzymes occurred in vertebrates.
Two Key Enzymes Necessary to Generate Vertebrate Steroids Are Specific to Vertebrates.
The first step of vertebrate steroid synthesis is the cleavage of the side chain present in cholesterol (17). This activity is catalyzed by CYP11A, which is, as discussed above, specific to vertebrates. This clearly shows that vertebrate-type steroids either may not be present outside vertebrates or, if present, are generated using enzymes of different phylogenetic origins. The latter case is an example of evolutionary convergence.
Interestingly, the very last step of estrogen synthesis, namely aromatization of testosterone or androstenedione, is catalyzed by CYP19, an aromatase, which arose in chordates. The phylogenetic analyses of CYP11A and CYP19 support our model that steroidogenic enzymes for adrenal and sex steroids arose in the deuterostome line, in which we also propose arose their cognate steroid receptors (7, 8).
Discussion
Independent Elaboration of Steroidogenesis in the 3 Main Bilaterian Lineages.
Except for vertebrate SRD5A and HSD11B1, for which orthologous genes were found in protostomes and/or cnidarians (even if their biochemical activity is not known), other enzymes known to be involved in steroidogenesis in arthropods, nematodes, or vertebrates have no clear orthologues outside their respective metazoan phyla. This indicates that the steroidogenic enzymes have evolved independently within each phylum, through lineage-specific duplications, and subsequent neofunctionalization. Such convergent evolution of synthesis pathways for complex molecules is not unique: examples include morphine synthesis in plants and animals (29) and gibberellin in plants and fungi (30).
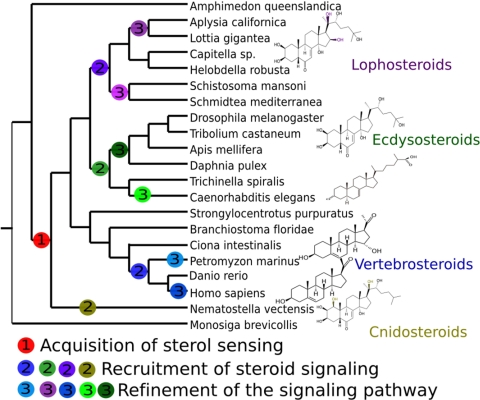
An important point is that the major active steroid hormones identified so far in vertebrates, arthropods, and nematodes have important differences in their structures (Figs. 3 and 4 and Fig. S1), which is consistent with our phylogenetic analyses of steroidogenic enzymes and argues for independent evolution of the steroidogenic pathway in these metazoan groups.
Fig. 4.
A hypothesis about the acquisition of steroidogenic pathways in metazoans. We propose that steroid sensing by NR was already present in the last common ancestor of all eumetazoans (step 1), but that steroid signaling was independently recruited many times from slightly different molecules (step 2), with subsequent refinement in some lineages (e.g., lamprey; step 3).
To clarify the fundamentally different characteristics of the steroid hormones across metazoan phyla and to highlight their independent evolutionary elaboration, one could apply a taxonomic based nomenclature, namely lophosteroids, ecdysosteroids, vertebrosteroids, and cnidosteroids (Fig. 4 and SI Text). Each of these compounds has a defined structural feature; for example, vertebrosteroids exhibit a characteristic cleavage of the long C17 side chain found in cholesterol. It is only when more biochemical and functional data become available in non-model taxa such as lophotrochozoans that a clear and unambiguous nomenclature can be defined.
Caution Is Needed in Assigning a Function Solely from Sequence Data.
The CYP and SDR family members are known to exhibit a huge variation of substrate specificity, even at the subfamily level. This indicates that one must exercise caution in attributing vertebrate-like steroidogenic activities to homologs in protostomes and cnidarians. For example, although it was convincingly shown that LET-767 is able to transform androgens into estrogens in mammalian cell cultures, as HSD17B3 does, and that this substrate-specificity can be altered by selective mutations (31), it does not necessarily follow that LET-767 and HSD17B3 have similar functions in vivo. Ecdysozoans have cholesterol-like steroids, in which there is a side-chain at C-17. Thus, there is no C17 alcohol or ketone for modification by a 17ß-HSD in nematode cells. Future characterization of the biological activity of LET-767 in C. elegans is necessary to provide insights into the evolution of substrate specificity in 17ß-HSD and its paralogs.
CYP19 Is a Chordate Aromatase.
The only non-vertebrate to contain a CYP19 ortholog is amphioxus, a chordate that is a close relative of vertebrates. Thus, our analysis (Fig. 2A) shows that, in contrast to recent claims (32), there is no support for the presence of an ortholog of vertebrate CYP19 in protostomes and cnidarians. This could be explained either by long-branch attraction in chordates CYP19 (which would be consistent with a functional shift) or by secondary loss of the CYP19 genes in protostomes and cnidarians. Since this is observed for other CYP families, for example CYP20, which seems to be orthologous to the sponge CYP38, with no counterparts in cnidarians and protostomes, we favor the hypothesis of secondary loss of an ancestral gene with no aromatizing activity. If an aromatization reaction really occurs in some lophotrochozoans (33), our analysis indicates that this reaction is carried out by a protein that is not a member of the chordate CYP19 family. This could be a CYP from the CYP1 and CYP3 clan (especially CYP3A4), which can aromatize indoline (34), or it may even be a protein from another family. Such an example of convergent evolution has already been described in the case of allene oxide synthase of a coral being able to metabolize a fatty acid peroxide in a way that was previously thought to be specific to CYPs (35). However, aromatization of steroids by these enzymes or a lophotrochozoan enzyme has not been reported.
Implications for the Presence of Vertebrate Steroids in Protostomes and Cnidarians.
Our phylogenetic analyses are relevant to studies in the comparative endocrinology field, which discuss the presence of vertebrate-type steroids and steroidogenic activities in non-model species, especially in protostomes or cnidarians (33, 36). In non-vertebrate species, the presence of steroids is usually monitored by radioimmunoassay (RIA) using antibodies generated against vertebrate hormones. Most importantly, vertebrate antibodies may cross-react with other steroids, including non-vertebrate steroids.
The limits of such approaches were clearly demonstrated in sea lamprey. Whereas classical RIA studies led to the identification of vertebrate-type steroids (37), more recent experiments, based on high performance liquid chromatography (HPLC) with 2 different solvents showed that the main circulating steroids are 15α-hydroxylated steroids and not vertebrate-type steroids as determined through RIA (38).
We show in this paper that genes orthologous to the vertebrate steroidogenic enzymes are not present in lophotrochozoans and cnidarians. Thus, there is no reason “a priori,” other than a residual anthropomorphism, to search specifically for the presence of vertebrate-type steroids in lophotrochozoans and cnidarians, and to imagine that those vertebrate steroids—if present—would be more likely to act as hormones, through vertebrate-like transduction pathways, than other steroids that are supposed to be present in non-vertebrate animals.
In our opinion, the first step in characterizing new steroidogenic pathways in non-model organisms should be identification of all steroids with sensitive methods such as GC-MS, and verification that these molecules are synthesized de novo from a defined sterol precursor. The physiological effects of these molecules should be tested, and the potential enzymes capable of catalyzing the different steps of the synthesis pathway identified. This is of course very challenging experimentally, but the only way to progress and to build knowledge on solid ground.
Xenobiotic-Metabolizing CYPs Are Ancestors of Steroidogenic Enzymes.
Many CYPs hydroxylate xenobiotics, which increases their solubility, facilitating excretion of the hydroxylated metabolite, and in optimal situation, leading to metabolites with reduced toxicity due to a lower affinity for enzymes and/or receptors (20, 21). On the other hand, CYPs can also lead to an increased affinity of lipophilic molecules for enzymes and/or receptors. Thus, hydroxylation of various lipophilic molecules, such as cholesterol, ergosterol, bile acids, retinoids, and vertebrate steroids, by CYPs can yield ligands that activate nuclear receptors (39–42). Indeed, selective expression of CYPs in specific tissues is an important mechanism for regulating the actions of vertebrate steroids and other ligands. Phylogenetic analysis of CYPs indicates that they are ancient and found in bacteria, yeast, and basal metazoans (20, 21, Fig. 2 and Figs. S3 and S4), preceding the evolution of steroid receptors in arthropods and deuterostomes. The broad substrate specificity and micromolar affinity of CYPs for xenobiotics allows a few CYPs to protect their host organism. Our phylogenetic analyses indicate that key steroidogenic enzymes, such as CYP11A, CYP11B, and CYP19 (aromatase) in vertebrates, CYP22 in nematodes, or CYP314, CYP315, CYP306, and CYP302 in arthropods, arose late in animal evolution and are most likely descended from CYPs that metabolize xenobiotics. These steroidogenic CYPs have evolved increasing their specificity for different steroids regarding hydroxylation, aromatization or cleavage of the C17 side chain. This specificity is an important mechanism for regulating steroid hormone action.
Like xenobiotic-metabolizing CYPs, some nuclear receptors are xenobiotic sensors, in that these transcription factors bind a wide range of molecules with micromolar affinity (42). An example of an ancient liganded receptor system is the NR1H/NR1I/NR1J group containing FXR, LXR, ECR, PXR, CAR, and VDR in vertebrates and also DHR96 in Drosophila and DAF12 in nematodes. Some of these receptors (FXR, PXR, CAR, and VDR) regulate CYPs and other transcription factors that detoxify xenobiotics. VDR, ECR, and LXR also are steroid-regulated transcription factors (43, 44). A characteristic of the nuclear receptors that respond to xenobiotics is their broad substrate specificity (43, 45), which is important in protection from the effects of xenobiotics. In contrast, chordate steroid receptors have nanomolar affinity for different adrenal and sex steroids, which is important in selective activation of endocrine pathways. Interestingly, 17ß -ethynylestradiol and the xenoestrogen, 4-nonylphenol, activate responses for detoxification of xenobiotics (46, 47), which suggests that the vertebrate ER activates some responses to xenobiotics.
AncSR Was Not a Hormone Receptor, but More Likely a Sensor.
Our phylogenetic analysis of steroidogenic enzymes favors the independent elaboration of different steroid synthesis pathways in metazoan groups. These data support the hypothesis that the responses of nuclear receptors in vertebrates and arthropods evolved independently (7). This model differs from the “ligand exploitation” model (10), in which the first active steroid would be estradiol, which would act through the AncSR in all bilaterians. Only later on, other “intermediate” steroids (androgens, corticosteroids, progestins, etc.) would have become ligands after gene duplication of the AncSR gave rise to new receptors that could exploit these intermediates (see Fig. 1A and B). This model indeed implies that the whole pathway governing estrogen production evolved in an ancestral bilaterian and that enzymes involved in estrogen synthesis (the ancestral ligand) are evolutionarily conserved in metazoans, and this is not what we observed.
To date, all of the binding data on ancestral SR were interpreted in the framework of vertebrate steroids being present in all metazoans and opposing an unliganded AncSR to an hormone-binding AncSR. Given the fact that vertebrate steroid hormones are not synthesized in other metazoans and that there are many possible crosstalks between the hormone synthesis and xenobiotic detoxification pathways, we propose that AncSR was able to bind estrogen with micromolar affinity but that it was not an hormone receptor, but rather a sensor, that was able to bind a broad range of various metabolites, such as sterol food derivatives and xenobiotics. Indeed, some current sensors, like PXR, are able to bind both xenobiotics and estradiol (48).
Materials and Methods
Protein sequences were retrieved in various public databases (Dataset S1), aligned with muscle (49), and alignments were checked by eye and edited with Seaview (50). Phylogenetic trees were made using PHYML (51), a fast and accurate maximum likelihood heuristic method, under the JTT substitution model (52), with 100 bootstrap replicates. The trees were first made with sequences of experimentally characterized proteins, for which a cDNA was cloned. Then the sampling was completed with EST-based or ab initio predictions to check the presence of the studied genes in non-model organisms. For additional details see SI Text.
Supplementary Material
Acknowledgments.
We thank Stéphanie Bertrand, Pascale Chevret, Ferdinand Marlétaz, and Loïc Ponger for technical advice; François Bonneton, Frédéric Brunet, Guillaume Lecointre, Mathilde Paris, Bruno Querat, Marc Robinson-Rechavi, and Michael Schubert for useful discussions; David Nelson for naming new CYP families; and the reviewers and editor for constructive comments. This work was supported by Ministère de l'Education Nationale, de la Recherche et de la Technologie, the Cascade Network of Excellence (FOOD-CT-2003–506319), Ecole Normale Supérieur e de Lyon, and Centre National de la Recherche Scientifique.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0812138106/DCSupplemental.
References
- 1.Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov. 2004;3:950–964. doi: 10.1038/nrd1551. [DOI] [PubMed] [Google Scholar]
- 2.Benoit G, et al. International Union of Pharmacology. LXVI. Orphan nuclear receptors. Pharmacol Rev. 2006;58:798–836. doi: 10.1124/pr.58.4.10. [DOI] [PubMed] [Google Scholar]
- 3.Laudet V, Hanni C, Coll J, Catzeflis F, Stehelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992;11:1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laudet V. Evolution of the nuclear receptor superfamily: Early diversification from an ancestral orphan receptor. J Mol Endocrinol. 1997;19:207–226. doi: 10.1677/jme.0.0190207. [DOI] [PubMed] [Google Scholar]
- 5.Escriva H, et al. Ligand binding was acquired during evolution of nuclear receptors. Proc Natl Acad Sci USA. 1997;94:6803–6808. doi: 10.1073/pnas.94.13.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertrand S, et al. Evolutionary genomics of nuclear receptors: From twenty-five ancestral genes to derived endocrine systems. Mol Biol Evol. 2004;21:1923–1937. doi: 10.1093/molbev/msh200. [DOI] [PubMed] [Google Scholar]
- 7.Escriva H, Delaunay F, Laudet V. Ligand binding and nuclear receptor evolution. Bioessays. 2000;22:717–727. doi: 10.1002/1521-1878(200008)22:8<717::AID-BIES5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 8.Baker ME. Evolution of adrenal and sex steroid action in vertebrates: A ligand-based mechanism for complexity. Bioessays. 2003;25:396–400. doi: 10.1002/bies.10252. [DOI] [PubMed] [Google Scholar]
- 9.Iwema T, et al. Structural and functional characterization of a novel type of ligand-independent rxr-usp receptor. EMBO J. 2007;26:3770–3782. doi: 10.1038/sj.emboj.7601810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thornton JW. Evolution of vertebrate steroid receptors from an ancestral estrogen receptor by ligand exploitation and serial genome expansions. Proc Natl Acad Sci USA. 2001;98:5671–5676. doi: 10.1073/pnas.091553298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton JW, Need E, Crews D. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science. 2003;301:1714–1717. doi: 10.1126/science.1086185. [DOI] [PubMed] [Google Scholar]
- 12.Bridgham JT, Carroll SM, Thornton JW. Evolution of hormone-receptor complexity by molecular exploitation. Science. 2006;312:97–101. doi: 10.1126/science.1123348. [DOI] [PubMed] [Google Scholar]
- 13.Bridgham JT, Brown JE, Rodríguez-Marí A, Catchen JM, Thornton JW. Evolution of a new function by degenerative mutation in cephalochordate steroid receptors. PLoS Genet. 2008;4:e1000191. doi: 10.1371/journal.pgen.1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Motola DL, et al. Identification of ligands for DAF-12 that govern dauer formation and reproduction in C. elegans. Cell. 2006;124:1209–1223. doi: 10.1016/j.cell.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 15.Gerisch B, et al. A bile acid-like steroid modulates Caenorhabditis elegans lifespan through nuclear receptor signaling. Proc Natl Acad Sci USA. 2007;104:5014–5019. doi: 10.1073/pnas.0700847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rewitz KF, Rybczynski R, Warren JT, Gilbert LI. The Halloween genes code for cytochrome P450 enzymes mediating synthesis of the insect molting hormone. Biochem Soc Trans. 2006;34:1256–1260. doi: 10.1042/BST0341256. [DOI] [PubMed] [Google Scholar]
- 17.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 18.Lafont R, Mathieu M. Steroids in aquatic invertebrates. Ecotoxicology. 2007;16:109–130. doi: 10.1007/s10646-006-0113-1. [DOI] [PubMed] [Google Scholar]
- 19.Markov GV, Paris M, Bertrand S, Laudet V. The evolution of the ligand/receptor couple: A long road from comparative endocrinology to comparative genomics. Mol Cell Endocrinol. 2008;293:5–16. doi: 10.1016/j.mce.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Nelson DR. Metazoan cytochrome P450 evolution. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1998;121:15–22. doi: 10.1016/s0742-8413(98)10027-0. [DOI] [PubMed] [Google Scholar]
- 21.Nelson DR. Cytochrome P450 nomenclature, 2004. Methods Mol Biol. 2006;320:1–10. doi: 10.1385/1-59259-998-2:1. [DOI] [PubMed] [Google Scholar]
- 22.Jornvall H, et al. Short-chain dehydrogenases/reductases (SDR) Biochemistry. 1995;34:6003–6013. doi: 10.1021/bi00018a001. [DOI] [PubMed] [Google Scholar]
- 23.Simard J, et al. Molecular biology of the 3beta-hydroxysteroid dehydrogenase/delta5-delta4 isomerase gene family. Endocr Rev. 2005;26:525–582. doi: 10.1210/er.2002-0050. [DOI] [PubMed] [Google Scholar]
- 24.Thomas JH. Rapid birth-death evolution specific to xenobiotic cytochrome P450 genes in vertebrates. PLoS Genet. 2007;3:e67. doi: 10.1371/journal.pgen.0030067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rewitz KF, Gilbert LI. Daphnia Halloween genes that encode cytochrome P450s mediating the synthesis of the arthropod molting hormone: evolutionary implications. BMC Evol Biol. 2008;8:60. doi: 10.1186/1471-2148-8-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker ME. Evolution of 17beta-hydroxysteroid dehydrogenases and their role in androgen, estrogen and retinoid action. Mol Cell Endocrinol. 2001;171:211–215. doi: 10.1016/s0303-7207(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 27.Baker ME. Evolutionary analysis of 11beta-hydroxysteroid dehydrogenase-type 1, -type 2, -type 3 and 17beta-hydroxysteroid dehydrogenase-type 2 in fish. FEBS Lett. 2004;574:167–170. doi: 10.1016/j.febslet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 28.Belyaeva OV, Kedishvili NY. Comparative genomic and phylogenetic analysis of short-chain dehydrogenases/reductases with dual retinol/sterol substrate specificity. Genomics. 2006;88:820–830. doi: 10.1016/j.ygeno.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Boettcher C, Fellermeier M, Boettcher C, Dräger B, Zenk MH. How human neuroblastoma cells make morphine. Proc Natl Acad Sci USA. 2005;102:8495–8500. doi: 10.1073/pnas.0503244102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawaide H. Biochemical and molecular analyses of gibberellin biosynthesis in fungi. Biosci Biotechnol Biochem. 2006;70:583–590. doi: 10.1271/bbb.70.583. [DOI] [PubMed] [Google Scholar]
- 31.Desnoyers S, et al. Caenorhabditis elegans LET-767 is able to metabolize androgens and estrogens and likely shares common ancestor with human types 3 and 12 17beta-hydroxysteroid dehydrogenases. J Endocrinol. 2007;195:271–279. doi: 10.1677/JOE-07-0248. [DOI] [PubMed] [Google Scholar]
- 32.Tiwary BK, Li W. Parallel evolution between aromatase and androgen receptor in the animal kingdom. Mol Biol Evol. 2009;26:123–129. doi: 10.1093/molbev/msn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osada M, Tawarayama H, Mori K. Estrogen synthesis in relation to gonadal development of Japanese scallop, Patinopecten yessoensis: gonadal profile and immunolocalization of P450 aromatase and estrogen. Comp Biochem Physiol B Biochem Mol Biol. 2004;139:123–128. doi: 10.1016/j.cbpc.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 34.Sun H, et al. Dehydrogenation of indoline by cytochrome P450 enzymes: A novel “aromatase” process. J Pharmacol Exp Ther. 2007;322:843–851. doi: 10.1124/jpet.107.121723. [DOI] [PubMed] [Google Scholar]
- 35.Oldham ML, Brash AR, Newcomer ME. The structure of coral allene oxide synthase reveals a catalase adapted for metabolism of a fatty acid hydroperoxide. Proc Natl Acad Sci USA. 2005;102:297–302. doi: 10.1073/pnas.0406352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Twan WH, Hwang JS, Chang CF. Sex steroids in scleractinian coral, Euphyllia ancora: implication in mass spawning. Biol Reprod. 2003;68:2255–2260. doi: 10.1095/biolreprod.102.012450. [DOI] [PubMed] [Google Scholar]
- 37.Kime DE, Larsen LO. Effect of gonadectomy and hypophysectomy on plasma steroid levels in male and female lampreys (Lampetra fluviatilis, L. ) Gen Comp Endocrinol. 1987;68:189–196. doi: 10.1016/0016-6480(87)90028-1. [DOI] [PubMed] [Google Scholar]
- 38.Lowartz S, et al. Blood steroid profile and in vitro steroidogenesis by ovarian follicles and testis fragments of adult sea lamprey, Petromyzon marinus. Comp Biochem Physiol A Mol Integr Physiol. 2003;134:365–376. doi: 10.1016/s1095-6433(02)00285-4. [DOI] [PubMed] [Google Scholar]
- 39.Baker ME. Xenobiotics and the evolution of multicellular animals: Emergence and diversification of ligand-activated transcription factors. Integr Comp Biol. 2005;45:172–178. doi: 10.1093/icb/45.1.172. [DOI] [PubMed] [Google Scholar]
- 40.Gilbert LI, Rybczynski R, Warren JT. Control and biochemical nature of the ecdysteroidogenic pathway. Annu Rev Entomol. 2002;47:883–916. doi: 10.1146/annurev.ento.47.091201.145302. [DOI] [PubMed] [Google Scholar]
- 41.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–1162. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 42.Moore DD, et al. International Union of Pharmacology. LXII. The NR1H and NR1I receptors: constitutive androstane receptor, pregnene X receptor, farnesoid X receptor alpha, farnesoid X receptor beta, liver X receptor alpha, liver X receptor beta, and vitamin D receptor. Pharmacol Rev. 2006;58:742–759. doi: 10.1124/pr.58.4.6. [DOI] [PubMed] [Google Scholar]
- 43.Xie W, Evans RM. Orphan nuclear receptors: The exotics of xenobiotics. J Biol Chem. 2001;276:37739–37742. doi: 10.1074/jbc.R100033200. [DOI] [PubMed] [Google Scholar]
- 44.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 45.Blumberg B, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998;12:3195–3205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mortensen AS, Arukwe A. Effects of 17alpha-ethynylestradiol on hormonal responses and xenobiotic biotransformation system of Atlantic salmon (Salmo salar) Aquat Toxicol. 2007;85:113–123. doi: 10.1016/j.aquatox.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 47.Meucci V, Arukwe A. The xenoestrogen 4-nonylphenol modulates hepatic gene expression of pregnane X receptor, aryl hydrocarbon receptor, CYP3A, and CYP1A1 in juvenile Atlantic salmon (Salmo salar) Comp Biochem Physiol C Toxicol Pharmacol. 2006;142:142–150. doi: 10.1016/j.cbpc.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Xue Y, et al. Crystal structure of the pregnane X receptor-estradiol complex provides insights into endobiotic recognition. Mol Endocrinol. 2007;21:1028–1038. doi: 10.1210/me.2006-0323. [DOI] [PubMed] [Google Scholar]
- 49.Edgar RC. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galtier N, Gouy M, Gautier C. SEAVIEW and PHYLO WIN: Two graphic tools for sequence alignment and molecular phylogeny. Comput Appl Biosci. 1996;12:543–548. doi: 10.1093/bioinformatics/12.6.543. [DOI] [PubMed] [Google Scholar]
- 51.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
- 52.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.