Abstract
Using the Unc93b1 3d mutation that selectively abolishes nucleic acid-binding Toll-like receptor (TLR) (TLR3, -7, -9) signaling, we show these endosomal TLRs are required for optimal production of IgG autoAbs, IgM rheumatoid factor, and other clinical parameters of disease in 2 lupus strains, B6-Faslpr and BXSB. Strikingly, treatment with lipid A, an autoAb-inducing TLR4 agonist, could not overcome this requirement. The 3d mutation slightly reduced complete Freund's adjuvant (CFA)-mediated antigen presentation, but did not affect T-independent type 1 or alum-mediated T-dependent humoral responses or TLR-independent IFN production induced by cytoplasmic nucleic acids. These findings suggest that nucleic acid-sensing TLRs might act as an Achilles' heel in susceptible individuals by providing a critical pathway by which relative tolerance for nucleic acid-containing antigens is breached and systemic autoimmunity ensues. Importantly, this helps provide an explanation for the high frequency of anti-nucleic acid Abs in lupus-like systemic autoimmunity.
Keywords: autoimmunity, SLE, Unc93b1, innate immunity
Systemic lupus erythematosus (SLE) is characterized by autoAbs to nuclear and cytoplasmic material that contain RNA, DNA, or both. AutoAbs typically arise before overt manifestations of disease, and high titers of anti-dsDNA are associated with greater severity (1, 2). Similar findings are observed in lupus-prone mice and, importantly, passively administered anti-DNA mAbs can produce lupus-like immune complex kidney deposits (3). Thus, evidence points to a direct role of anti-nuclear Abs in SLE.
Recent studies show that production of anti-nuclear Abs depends, to varying degrees, on endosomal Toll-like receptors (TLRs) that bind dsDNA (TLR9) or ssRNA (TLR7) (4, 5). Indeed, in vitro experiments have consistently found that either TLR can enhance activation of B cells and dendritic cells (DC) following antigen receptor (B cell receptor, BCR)- or FcγRIIa (FcγRIII in mice)-mediated endocytosis of nucleic acid-containing material or immune complexes (4). Remarkably, chromatin-containing immune complexes, presumably because of combined engagement of BCR and TLR, can stimulate B cells 100-fold more effectively than complexes without nucleic acids (6). On the basis of these findings, Leadbetter et al. (4, 7) proposed the novel hypothesis that these processes might explain the induction and prevalence of anti-nuclear Abs in lupus. Interestingly, these TLRs are not only B cell activators, but also potent inducers in DCs and plasmacytoid (p)DCs of the SLE-promoting type I interferons (IFNs) (8, 9).
When lupus-prone mice were examined, however, TLR9 deficiency had mixed effects on anti-DNA or anti-chromatin and, to a lesser extent, end-organ damage. In MRL-Faslpr mice, lack of TLR9 had different effects on anti-nuclear Ab specificity in different studies, yet overall disease was inexplicably exacerbated (10–12). TLR9-deficiency also enhanced disease in MRL-Faswt (11), B6-Faslpr (13), and mutant Plcg2+/Ali5 mice (14). In striking contrast, lupus-prone FcγRIIB−/− mice expressing a high-affinity anti-DNA transgene had reduced disease (15), although this may be related to dependence on a single transgenic anti-DNA specificity. Taken together, despite uncertainty about the role of TLR9 in the production of anti-DNA-related Abs, the majority of studies indicate that TLR9 has an overall lupus-suppressing function.
In contrast, studies have consistently documented a lupus-promoting function for TLR7, most clearly shown by the discovery that an extra copy of TLR7 on the Y chromosome of BXSB mice explained the lupus-enhancing Yaa mutation (16–19). Moreover, TLR7-deficient MRL-Faslpr mice had reduced anti-RNP, less lymphoproliferation, and a slightly lower composite renal disease score, but similar levels of anti-nucleosome and anti-dsDNA (12). Thus, in MRL-Faslpr mice, TLR7 appears to play a major role in the induction of RNA-related autoAbs, but only a modest role in overall disease severity. In the tetramethylpentadecane-induced model of lupus, TLR7 is also required for the production of both RNP autoAbs and disease-promoting type I IFNs (20). In contrast, TLR3 deficiency did not significantly affect autoAbs, lymphoproliferation, or glomerulonephritis (GN) in MRL-Faslpr mice (12).
A possible limitation of studying single TLR deletions, however, is that immune complexes of nucleic acid-containing material, such as apoptotic debris, are likely to contain both RNA and DNA. Therefore, Abs to either DNA or RNA could potentially form complexes that activate both TLR7 and TLR9, and deleting any one of these TLRs would provide only partial, if any, inhibition. Thus, assessing the impact of completely blocking all nucleic acid-sensing TLRs on autoAb production and lupus pathogenesis is important. To this end, we studied the induction and progression of lupus-like disease in mice in which endosomal TLR (eTLR) signaling was abolished by the 3d mutation in Unc93b1 (21). Unc93b1 encodes an endoplasmic reticulum (ER)-resident protein that physically associates with TLR3, -7, and -9 and is required for the trafficking of these TLRs from the ER to the endolysosomes where encounter with their cognate ligands occurs (22). We found that the 3d mutation virtually abolished IgG anti-nuclear Abs and markedly reduced disease in 2 different lupus strains, providing direct evidence that signaling by self-nucleic acid-recognizing TLR is central to the production of autoAbs to nucleic acid-containing material and disease pathogenesis.
Results
The 3d Mutation Reduces IgG AutoAbs and Lymphoproliferation in B6-Faslpr Mice.
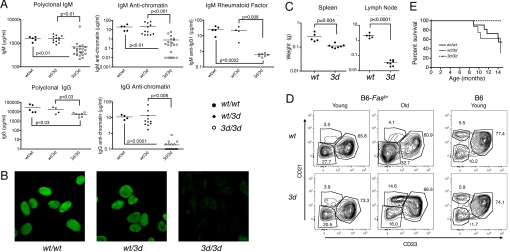
To study the effects of blocking TLR3, -7, and -9 in lupus, we backcrossed the 3d mutation onto B6-Faslpr mice, which develop significant lymphoproliferation and autoAbs, but have limited susceptibility to end-organ injury. Compared with wild-type (wt) or heterozygous (wt/3d) animals, 3d B6-Faslpr mice had modest suppression of polyclonal IgM and IgG levels, but much greater suppression of IgM and IgG anti-chromatin [Fig. 1A, supporting information (SI) Fig. S1]. Strikingly, while all wt B6-Faslpr mice were strongly positive for anti-nuclear Abs (ANA), 6- to 8-month-old 3d mice were all negative, and only 2 of 7 of the 14- to 15-month-old 3d mice had weak ANA staining (Fig. 1B, Table S1). Moreover, these weaker ANAs exhibited atypical patterns: one with essentially metaphase chromosome staining and the other similar to human anti-proliferating cell nuclear antigen (anti-PCNA). Also, another 14- to 15-month-old B6-Faslpr 3d mouse was ANA negative, but had Golgi apparatus staining (Table S1). IgM rheumatoid factor (RF), another autoAb found in high titers in this strain, was also markedly lower in 3d mice (Fig. 1A, Fig. S1). Thus, eTLRs appear to play a critical role in the production of classical anti-nuclear and RF Abs.
Fig. 1.
Immunopathology of B6-Faslpr 3d mice. (A) IgM and IgG polyclonal and autoAbs from 6- to 8-month-old B6-Faslpr wild-type (wt/wt), heterozygous (wt/3d), and mutant (3d/3d) mice determined by ELISA. IgM RF was anti-IgG1, 4–22 mice/group. (B) Representative ANA results from 6- to 7-month-old mice (1/100 dilution), 4–7/group. (C) Lymphoid organ weights: spleen and LN (cervical, axillary, inguinal, and mesenteric) weights from 10-month-old mice, 5–7/group. (D) Representative flow cytometry analyses of splenic B cell subsets in young (1 month) or old (15 month) mice with the Faslpr and 3d mutations. Follicular (CD21lo CD23hi), marginal zone (CD21hi CD23lo), and CD21lo CD23lo populations are gated (see Table S3). (E) Cumulative survival, 8–11/group. P < 0.04 for 3d/3d versus wt/wt or wt/3d.
The lack of eTLRs in B6-Faslpr 3d mice also significantly suppressed lymphoproliferation with substantial reductions in splenomegaly and especially in the characteristic aggressive lymphadenopathy (Fig. 1C). B6-Faslpr mice do not develop significant GN, but succumb to complications secondary to massive lymphoproliferation. Highlighting the significant enhancement of long-term survival imparted by the effect of 3d mutation on lymphoid hypertrophy, mortality at 14–15 months of age was 45% in wt/wt, 50% in wt/3d, and 0% in 3d/3d B6-Faslpr (P < 0.04 for 3d/3d compared with either wt/wt or wt/3d, Fig. 1E). Substantial lymphadenopathy, however, was readily detectable in 14- to 15-month-old 3d mice, indicating that the reduction in lymphoproliferation was not from correction of the defective Fas-mediated apoptosis per se, but from the lack of self and foreign nucleic acid sensing by eTLRs (Table S2).
When T cell subsets in the spleen and lymph node (LN) of young mice (1 month) were characterized, the only major difference was an ≈30% reduction in the CD44hi subset of CD8 T cells (Table S2). In contrast, old 3d mice (≈15 months) had a lower percentage of T cells in the spleen and, in the LNs, reduced percentages of CD4 and CD8 T cells associated with an increased percentage of double negative (DN, CD4−CD8−) T cells. In both spleen and LNs, reductions in the naive CD62L+ subset of CD8+ T cells were also observed. Analysis of splenic B cells in young mice showed a significant 27% decrease in the CD21lo CD23lo population, which is expanded in Faslpr mice (Table S3, Fig. 1D). In old 3d mice, this population was reduced even more (77% decrease) and the percentage of CD138+ plasma cells was lower (65% decrease). These findings are consistent with suppression of autoimmunity in older 3d B6-Faslpr mice.
Reduced Lupus Pathology in 3d Male BXSB Background Mice.
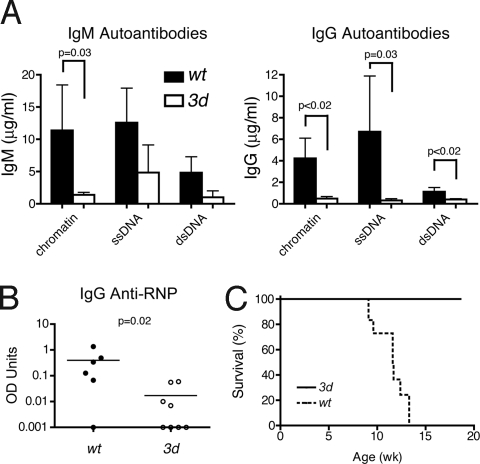
The 3d mutation was next backcrossed to the lupus-prone BXSB background to further examine the effects of blocking nucleic acid-sensing TLRs on systemic autoimmunity. Similar to the B6-Faslpr 3d mice, 3d-deficient male BXSB mice had marked reductions in autoAbs, including IgM and IgG anti-chromatin, -ssDNA, and -dsDNA and IgG anti-ribonuclear protein (RNP) (Fig. 2 A and B). The reductions were greatest for IgG autoAbs, and ANAs were undetectable (Table S4). GN in 3- to 4-month-old mice was also suppressed (kidney disease score: wt, 2.4 ± 0.7; 3d, 0.4 ± 0.4; P = 0.04, n = 4–5/group) as reflected by a 100% survival in the BXSB 3d group up to 18.6 weeks compared to a median survival of 11.6 weeks in wt mice (Fig. 2C). Thus, eTLR signaling was critical for both anti-nuclear production and disease-associated pathology and mortality.
Fig. 2.
AutoAbs and survival of 3d BXSB background mice. (A) Serum IgM and IgG anti-nuclear Abs from 3- to 4-month-old mice (mean ± SE, 4–5/group). (B) Serum IgG anti-RNP from 3- to 4-month-old mice, 7–8 mice/group. (C) Cumulative survival, 10–12 mice/group, P < 0.0001.
Effect of TLR4 Stimulation of B6-lpr 3d Mice on AutoAb Production.
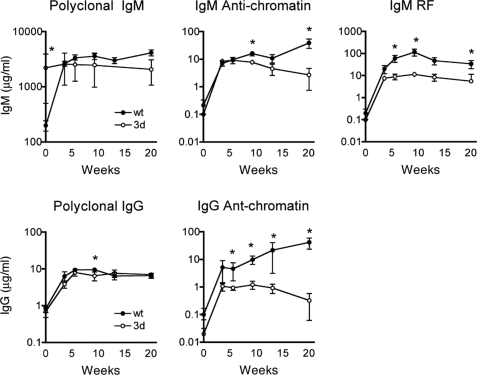
To further study the role of eTLRs in the generation of anti-nuclear Abs, we treated 3d mutant B6-Faslpr mice with the TLR4 ligand, lipid A, a nonimmunogenic form of LPS. TLR4 signaling is not affected by the 3d mutation (21) and its engagement, like that of eTLRs, significantly enhances disease in lupus-prone strains, including B6-Faslpr (9, 23). B6-Faslpr 3d and wt mice were given 50 μg lipid A i.p. 2 times per week for 20 weeks during which time serum immunoglobulins and autoAbs were measured serially (Fig. 3). The polyclonal IgM and IgG Ab responses to lipid A treatment in wt and 3d mice were very similar and consistent with the activation of a large number of B cells. In contrast, although lipid A initially (4 weeks after injection) induced increases in IgM and IgG and anti-chromatin autoAbs in 3d mice that were similar to wt B6-Faslpr mice, thereafter autoAb levels remained constant or reduced in 3d mice compared with significantly increasing concentrations of autoAbs in wt mice, which was much more pronounced in the IgG isotype (Fig. 3). ANA analysis also showed no detectable amounts of IgG autoAbs in the lipid A-treated 3d group (Table S5). The IgM RF response to TLR4 engagement was similarly suppressed in 3d mice (Fig. 3). Thus, activation of the innate immune system by TLR4 stimulation failed to overcome the suppression of autoAbs by 3d.
Fig. 3.
IgM and IgG polyclonal and autoAbs from lipid A-treated B6-Faslpr wt and 3d mice. Six-week-old mice were given 50 μg lipid A i.p. 2 times per week for 20 weeks. Ig amounts are by ELISA (mean ± SE for 3–6/group at each time point). *, P < 0.05.
T-Independent Type 1 (TI-1) and T-Dependent (TD) Responses to Trinitrophenol (TNP) in B6-Faslpr 3d Mice.
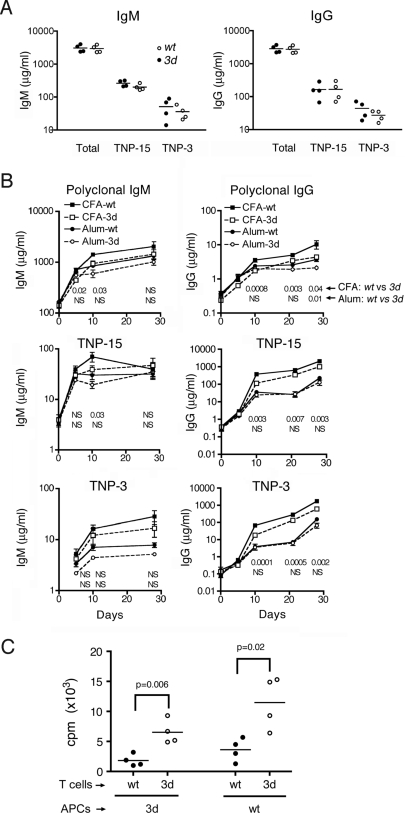
We next investigated whether Ab responses to foreign antigens are also affected by the 3d mutation by measuring TI-1 (TNP-LPS) and TD (TNP keyhole limpet hemocyanin (KLH)) humoral responses in B6-Faslpr mice. For the former, similar levels of polyclonal and anti-TNP IgM or IgG were induced in both wt and 3d mice, consistent with the normal TLR4 signaling in 3d mice (Fig. 4A). The TD response was assessed in mice immunized on days 0 and 21 with TNP-KLH plus either of 2 types of adjuvants, alum or sequential complete Freund's adjuvant (CFA) and incomplete Freund's adjuvant (IFA) (CFA/IFA) (Fig. 4B). Both alum and CFA/IFA induced IgM and IgG responses after the initial and recall immunizations in both wt and 3d mice, although Ab concentrations were modestly higher with CFA/IFA, particularly for the IgG isotype. With alum, the overall polyclonal and TNP-specific IgM and IgG levels in wt and 3d mice were very similar after both the first and the second immunizations, indicating no impairment of T cell helper activity. With CFA/IFA, however, levels of Abs in 3d mice, particularly those of the IgG isotype, were often slightly, but nevertheless significantly (P < 0.05), lower than corresponding concentrations in wt mice. Thus, a slight reduction in TD humoral response in 3d mice was detected with CFA, an adjuvant that contains ligands for the nucleic acid-sensing TLRs, but not with alum, which lacks nucleic acids.
Fig. 4.
B and T cell responses in B6-Faslpr 3d mice. (A) TI-1 anti-TNP response. Sera were from 7 days after 50 μg TNP-LPS i.p. Total Ig and Abs to high-density (TNP-15) or low-density (TNP-3) TNP conjugates were measured by ELISA. P > 0.05 for wt vs. 3d in all groups. (B) TD anti-TNP response was measured serially in mice immunized with TNP-KLH on days 0 and 21 either in CFA for the first dose and IFA for the second (CFA/IFA) or in alum for both doses (mean ± SE from 3–11/group). P-values comparing wt and 3d groups are shown below their respective time points: Upper line for CFA/IFA and Lower line for alum-injected mice. (C) Recall of T cell proliferation of wt and 3d T cells to OVA with either wt or 3d APCs. Splenic T cells and APCs were isolated 10 days after immunization with OVA in CFA and proliferation was assessed by thymidine uptake after 4 days. One of 2 independent experiments is shown.
To examine the relative roles of 3d antigen-presenting cells (APCs) and T cells in recall T cell activation, we used antigen (ovalbumin, OVA) plus APCs from either wt or 3d B6-Faslpr mice to stimulate wt or 3d T cells (Fig. 4C). As previously reported (21), 3d APCs were less effective than wt APCs in stimulating wt T cells and 3d T cells, although interestingly, 3d T cells had slightly greater thymidine incorporation than wt T cells regardless of whether the APCs were wt or 3d. Thus, 3d T cells are fully capable of responding to Ags despite reduced APC function.
3d Mutation Does Not Affect TLR-Independent Nucleic Acid-Induced Cell Activation.
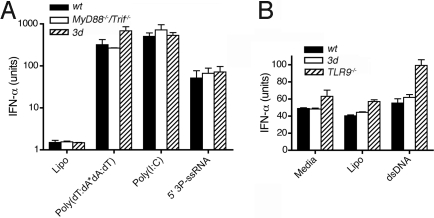
Recently described cytoplasmic nucleic acid receptor or signaling molecules, such as retinoic acid-inducible gene I (RIG-I, sensing 5′ triphosphate RNA), melanoma differentiation-associated gene 5 (Mda5, sensing dsRNA), absent in melanoma 2 (AIM2, sensing DNA), and stimulator of IFN genes (STING, sensing B-DNA), can also in some cases activate cells to produce proinflammatory responses, such as IFN-α/β, and to upregulate costimulatory molecules (24–32). Because the role of these cytoplasmic sensors in lupus is not known, we sought to determine whether the 3d mutation affected cytoplasmic nucleic acid recognition. When wt, 3d, MyD88−/− Trif−/− double knockout, or Tlr9−/− DCs were transfected with 5′ triphosphate RNA, dsRNA, or mammalian dsDNA, no suppression of TLR-independent cytokine production by the 3d mutation was detected (Fig. 5 A and B). Thus, the effects of the 3d mutation on autoAb production and other lupus manifestations cannot be attributed to defective TLR-independent nucleic acid sensing.
Fig. 5.
TLR-independent activation of DCs. (A) DC activation with cytoplasmic dsRNA and 5′ (3P)-RNA. Bone marrow (BM)-derived wt, 3d, and MyD88−/−/Trif−/− double-knockout DCs were transfected with 250 μg/mL poly(I:C) alone or by Lipofectamine with 10 μg/mL poly(dT:dA*dA:dT) or 200 ng 5′ (3P)-dsRNA. Poly(I:C) in high concentrations directly enters cells. P < 0.009 for all wt, 3d, and MyD88−/−/Trif−/− double-knockout DCs transfected with RNA versus Lipofectamine-treated (Lipo) DCs. (B) DC activation with cytoplasmic double-stranded mammalian DNA. DCs from wt, 3d, and TLR9−/− mice were transfected with 10 μg/mL calf thymus DNA. P < 0.05 for dsDNA-transfected wt, 3d, and TLR9−/− DCs versus Lipo alone DCs. A and B are mean ± SEM of triplicates.
Discussion
Herein we show that the Unc93b13d mutation, which abolishes nucleic acid-sensing TLR signaling, markedly suppressed spontaneous anti-nuclear, anti-RNP, and RF Ab production, GN, and mortality in lupus-prone strains. Moreover, this suppression was not overcome by treating mice with lipid A, a TLR4 agonist that promotes autoAbs and lupus. We further show that 3d had no effect on TI-1 humoral responses or TD Ab responses with alum as the adjuvant, but slight, although significant, reducing effects in CFA/IFA-mediated TD responses. TLR-independent nucleic acid sensing was also unaffected by the 3d mutation. Thus, eTLRs are largely dispensable for humoral responses to foreign protein antigens (Ags), but are, for all practical purposes, necessary for the generation of autoAbs to nucleic acid and nucleic acid-containing material and IgM RF in systemic autoimmunity. These results definitively demonstrate that the eTLRs play a key and essential role in the pathogenesis of lupus in 2 susceptible strains and, in conjunction with previous studies (6, 7, 12, 18, 20, 33), provide an explanation for the frequent and dominant presence of ANAs in SLE.
Previous studies, showing reduced APC function in 3d mice, suggested that Unc93b1 might play a direct role in Ag presentation to both CD8 and to a lesser extent CD4 T cells in addition to trafficking eTLRs (21, 22). Our finding, however, that the 3d mutation did not impair TD Ab responses when nucleic acid-free alum was the adjuvant indicates that T helper function in these mutant mice is not significantly altered. Thus, reduced 3d APC activity for 3d T cells cannot account for the marked suppression of autoAbs in the lupus-prone mice. The finding that eTLR deficiency did not affect TD humoral responses is consistent with a recent study showing that absent TLR signaling in Myd88/Trif double-deficient mice did not significantly reduce adjuvant-enhanced Ab responses (34). Nonetheless, we found that the 3d mutation slightly reduced the humoral response in 3d mice when CFA was the adjuvant. This suggests that nucleic acids in CFA contribute to the overall adjuvant effect of CFA in wt mice, whereas the adjuvanticity of the alum-based mixture is not affected by eTLR deficiency because the response is mediated primarily through the Nalp3 inflammasome (35).
Despite the normal TD humoral response, our findings confirmed reduced activity of APC from 3d mice for stimulating wt T cells (21, 22). However, we found that the activation of OVA-primed 3d T cells by 3d APC was not impaired, and 3d T cells exhibited greater stimulation than wt T cells when activated by wt APC. These findings, combined with normal TD humoral responses in 3d mice (this study) and the lack of T cell population changes in nonautoimmune 3d mice (21), suggest that 3d T cells compensate for the slightly reduced function of 3d APCs. Possible explanations for this are dynamic tuning of T cells for which a large number of different molecular mechanisms have been identified, including cell signaling feedback, level of CD5 expression, sialylation, and miRNA expression (36–40), or modification of the T cell receptor repertoire. We are currently generating T cell receptor transgenic 3d mice to address this issue. Overall, our findings indicate that eTLRs play a limited, but significant role in determining the overall steady state of APC activity, possibly because of constant exposure of APC to subactivating amounts of nucleic acid-containing material from endogenous sources such as apoptotic cells or from exogenous commensal organisms and dietary substances. This possibility is supported by the observation that the copy number of TLR7 can alter response to self and foreign Ags (18).
Previous studies in lupus-prone mice showed that lack of TLR7 specifically inhibited RNP Ab production, whereas TLR9 deficiency had varying effects on DNA-related Abs (4, 9, 33). Our finding that complete elimination of eTLR signaling inhibits the specific production of IgG anti-nucleic acid-associated Abs in lupus-prone mice indicates that these autoAb specificities are strongly dependent on TLR engagement. Therefore, the combined data suggest that anti-RNP B cells require TLR7 ligands for activation, whereas anti-DNA B cells can be activated through either TLR7 or TLR9. Thus, the major Ags for anti-RNP B cells most likely contain primarily RNA and little DNA, whereas the major antigenic targets for anti-DNA B cells must contain both DNA and RNA, with only the DNA-related Ags accessible to BCRs (otherwise anti-RNP B cells could take up these targets and be activated by TLR9 in TLR7-deficient mice). Apoptosis-derived blebs and particles, considered to be the major source of self Ag in SLE, contain varying amounts of nucleosomes, cytoplasmic RNA, and RNPs (41, 42). Among these, large apoptotic blebs, known to contain both nucleosomes and RNA, would appear to be a major self Ag for DNA and nucleosome-specific B cells, whereas the major self Ag for RNP-specific B cells may be smaller RNP particles (42).
The critical importance of eTLRs in the production of anti-nucleic acid Abs was strongly supported by 2 key findings. First, atypical ANA staining of 2 of 7 old B6-Faslpr 3d mice was detected only at an age where there were no significant differences in lymphoid hypertrophy and hyperIgG compared to wt mice. Second, chronic TLR4 stimulation of 3d mice could not overcome the requirement for eTLRs in autoAb production. TLR4 is the only nonnucleic acid-sensing TLR known to induce type I IFNs and to enhance lupus-like autoimmunity (23). Moreover, TLR4 is similar to the eTLRs in its signaling through both MyD88 (TLR7 and -9) and TRIF (TLR3) and in activating B cells and other APCs (9). Thus, the inability of autoimmune-prone 3d mice to sustain high levels of anti-nuclear Abs and RF in old lupus-prone mice or after TLR4 stimulation must be related to the specific recognition of nucleic acids by eTLRs or less likely by another unique property of these TLRs.
The lipid A treatment initially (day 3) increased concentrations of anti-nuclear Abs and RF in 3d mice commensurate to wt mice and consistent with polyclonal B cell activation, but no further increases in autoAbs occurred. Thus, it can be deduced that eTLRs were not required for activation of anti-nucleic acid recognizing B cells and the initial production of autoAbs, but were required for the subsequent amplification of this response. This is consistent with our previously hypothesized 2-phase paradigm of SLE (9), in which the first TLR-independent phase, triggered by activation of pDCs and DCs by apoptotic cell debris and associated nucleic acids, leads to the elaboration of activating cytokines and low levels of autoAb production. For sustained autoAb production and disease, however, a second TLR-dependent amplification phase is required, mediated by the engagement of eTLRs by nucleic acid-containing material taken up either directly via Ag receptors in B cells or as autoAb complexes in pDCs and DCs. This second phase is likely to involve a positive feedback loop that results in substantial magnification of the initial response.
Previous in vitro studies showed that activation of anti-IgG2a RF-expressing AM14 B cells by IgG2a anti-DNA/DNA complexes required TLR9, while activation by IgG2a anti-RNP/RNA complexes required TLR7, consistent with endocytosis of the nucleic acid complexes via the surface-expressed RF and subsequent engagement of eTLRs by their cognate ligand (7, 43). More recent in vivo studies in AM14 IgH-chain transgenic MRL-Faslpr lupus mice showed that direct activation of low-affinity RF B cells was not dependent on T cell help, but required the presence of TLR7 and TLR9 (33). Here, we extend this finding to show that dependence on nucleic acid-sensing TLRs also applies to spontaneous production of RF and, by inference, for RF regardless of affinity. Moreover, we show that, similar to the production of anti-nucleic acid Abs, TLR4 stimulation by lipid A administration in B6-Faslpr 3d mice cannot bypass the dependence of sustained RF production on nucleic acid-recognizing TLRs.
The 3d mutation reduced, but did not ameliorate the lymphoid hypertrophy associated with defective Faslpr, although the degree of suppression was probably underestimated because of deaths in the more severely affected wt group before the final analysis. Examination of the cellular composition of the spleen and lymph nodes revealed some differences suggesting reduced activation of 3d T cells, including less splenic T cells, less CD4 T cells in lymph nodes, and more naive (CD62L+) and fewer activated (CD44hi) CD8 T cells in both spleen and lymph node. Another finding was a reduction in the CD21loCD23lo B cell population in 3d mice, which is abnormally increased in B6-Faslpr mice with age. This, along with the finding of a reduction in the CD138+ B220−, possibly more mature, plasmablasts/plasma cell population, is consistent with a general reduction in overall B cell activation and autoimmunity. Thus, although it is not known to what extent endogenous self-nucleic acid Ags and foreign nucleic acid material might be responsible, we clearly document a major, but not essential, role for eTLRs in the lymphoid hypertrophy that develops in Faslpr mice.
Finally, our findings strongly support the therapeutic targeting of nucleic acid-sensing TLRs in SLE (44) and demonstrate the dramatic benefit of simultaneously inhibiting all such TLR family members. These findings also raise the possibility that eTLR signaling may play a critical role in other autoimmune diseases that have anti-nucleic acid Abs.
Materials and Methods
Mice.
C57BL/6 (B6)-Unc93b13d (3d), B6- Faslpr, BXSB, B6-MyD88−/−, B6-TriffLps2/Lps2, and B6-Tlr9CpG1,CpG1 mice were bred and maintained at The Scripps Research Institute. Experiments followed approved Institutional Animal Care and Use Committee protocols. Faslpr and 3d were identified by PCR (45) or sequencing. 3d BXSB mice were N2 or greater, Yaa+, and fixed for BXSB on chromosome 1 between 19.8 and 174.9 Mb (D1Mit3, D1Mit21, D1Mit387, and D1Mit206). Lupus was assessed as described (46). Fifty micrograms of lipid A (Calbiochem) in PBS were given 2 times per week i.p. for the indicated durations.
Immunopathology and Serology.
Zinc formalin-fixed and PAS/hematoxylin-stained tissue sections were scored blindly for GN on a 0–4 scale (47). Ab concentrations were measured by ELISA (48). RNP plates were from Inova Diagnostics. ANAs were detected on HEp-2 slides (Bion Enterprises) using 1/100 serum and 1/200 Alexa Fluor 488-goat anti-mouse IgG dilutions (Invitrogen).
Flow Cytometry.
Isolated splenic and LN cells, blocked with anti-CD16/CD32, were stained with combinations of dye-conjugated Abs to B220, CD4, CD5, CD8, CD19, CD21, CD23, CD44, CD62L, CD86, CD90.2, CD138, F4/80, IgM, and I-A/I-E (BD Biosciences or Biolegend). Data were acquired on a LSRII (BD Biosciences) and analyzed by Flowjo (Tree Star).
Humoral Responses.
For the TI-1 response, mice were immunized once with 50 μg TNP-LPS (Biosearch Technologies) in PBS i.p. and for the TD response twice with 50 μg TNP-KLH on days 0 and 21 either in alum or in CFA on day 0 and in IFA on day 21 (CFA/IFA). Total, anti-TNP3, and anti-TNP15 IgM and IgG were measured by ELISA (49).
T Cell Proliferation.
A total of 105 T cells and 6 × 105 APCs (non-T cells), isolated from spleens 10 days after immunization with 100 μg OVA in CFA, were incubated for 4 days with or without 20 μg OVA and harvested 18 h after addition of tritiated thymidine. Data are sample cpm minus media-alone cpm.
TLR-Independent Pathway Induction.
BM-derived DCs, generated by culturing BM cells with 10 ng/mL GM-CSF for 7 days and then by CD11c microbead (MACSMiltenyi Biotech) isolation, were transfected with poly(I:C) or poly(dA:dT*dT:dA) (Sigma), 5′ (3P)-transcribed RNA (pGEM express positive control, Riboprobe system T7, Promega), or calf-thymus DNA (Sigma) by Lipofectamine (Invitrogen). After 24-h cultures, IFN type I in supernatants was determined (50).
Statistical Analysis.
Group comparisons used unpaired 2-tailed t tests. Survival was analyzed by Kaplan-Meier plots with a log-rank test.
Supplementary Material
Acknowledgments.
This is publication no. 20017-IMM from the Department of Immunology & Microbial Science, The Scripps Research Institute. We thank M. K. Occhipinti for editing and C. Thompson for technical assistance. Work was supported by National Institutes of Health grants AR42242, AR31203, AR053228, AI059777, AR053731, AR39555, GM67759, and ES07511.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0905441106/DCSupplemental.
References
- 1.Arbuckle MR, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 2.Reveille JD. Predictive value of autoantibodies for activity of systemic lupus erythematosus. Lupus. 2004;13:290–297. doi: 10.1191/0961203303lu1015oa. [DOI] [PubMed] [Google Scholar]
- 3.Vlahakos DV, et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 1992;41:1690–1700. doi: 10.1038/ki.1992.242. [DOI] [PubMed] [Google Scholar]
- 4.Marshak-Rothstein A, Rifkin IR. Immunologically active autoantigens: The role of toll-like receptors in the development of chronic inflammatory disease. Annu Rev Immunol. 2007;25:419–441. doi: 10.1146/annurev.immunol.22.012703.104514. [DOI] [PubMed] [Google Scholar]
- 5.Martin DA, Elkon KB. Autoantibodies make a U-turn: The toll hypothesis for autoantibody specificity. J Exp Med. 2005;202:1465–1469. doi: 10.1084/jem.20052228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viglianti GA, et al. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 7.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 8.Lovgren T, Eloranta ML, Bave U, Alm GV, Ronnblom L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004;50:1861–1872. doi: 10.1002/art.20254. [DOI] [PubMed] [Google Scholar]
- 9.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 10.Christensen SR, et al. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–342. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- 12.Christensen SR, et al. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Lartigue A, et al. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–1354. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- 14.Yu P, et al. Toll-like receptor 9-independent aggravation of glomerulonephritis in a novel model of SLE. Int Immunol. 2006;18:1211–1219. doi: 10.1093/intimm/dxl067. [DOI] [PubMed] [Google Scholar]
- 15.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian S, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deane JA, et al. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santiago-Raber ML, et al. Evidence for genes in addition to Tlr7 in the Yaa translocation linked with acceleration of systemic lupus erythematosus. J Immunol. 2008;181:1556–1562. doi: 10.4049/jimmunol.181.2.1556. [DOI] [PubMed] [Google Scholar]
- 20.Lee PY, et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J Exp Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabeta K, et al. The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol. 2006;7:156–164. doi: 10.1038/ni1297. [DOI] [PubMed] [Google Scholar]
- 22.Kim YM, Brinkmann MM, Paquet ME, Ploegh HL. UNC93B1 delivers nucleotide-sensing toll-like receptors to endolysosomes. Nature. 2008;452:234–238. doi: 10.1038/nature06726. [DOI] [PubMed] [Google Scholar]
- 23.Hang LM, Aguado MT, Dixon FJ, Theofilopoulos AN. Induction of severe autoimmune disease in normal mice by simultaneous action of multiple immunostimulators. J Exp Med. 1985;161:423–428. doi: 10.1084/jem.161.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita T. Virology. Sensing viral RNA amid your own. Science. 2006;314:935–936. doi: 10.1126/science.1135756. [DOI] [PubMed] [Google Scholar]
- 25.Pichlmair A, et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science. 2006;314:997–1001. doi: 10.1126/science.1132998. [DOI] [PubMed] [Google Scholar]
- 26.Hornung V, et al. 5′-Triphosphate RNA is the ligand for RIG-I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- 27.Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 28.Ishii KJ, et al. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol. 2006;7:40–48. doi: 10.1038/ni1282. [DOI] [PubMed] [Google Scholar]
- 29.Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008;455:674–678. doi: 10.1038/nature07317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornung V, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burckstummer T, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- 33.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and Toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grossman Z, Paul WE. Autoreactivity, dynamic tuning and selectivity. Curr Opin Immunol. 2001;13:687–698. doi: 10.1016/s0952-7915(01)00280-1. [DOI] [PubMed] [Google Scholar]
- 37.Acuto O, Bartolo VD, Michel F. Tailoring T-cell receptor signals by proximal negative feedback mechanisms. Nat Rev Immunol. 2008;8:699–712. doi: 10.1038/nri2397. [DOI] [PubMed] [Google Scholar]
- 38.Azzam HS, et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starr TK, Daniels MA, Lucido MM, Jameson SC, Hogquist KA. Thymocyte sensitivity and supramolecular activation cluster formation are developmentally regulated: a partial role for sialylation. J Immunol. 2003;171:4512–4520. doi: 10.4049/jimmunol.171.9.4512. [DOI] [PubMed] [Google Scholar]
- 40.Laufer TM. T-cell sensitivity: A microRNA regulates the sensitivity of the T-cell receptor. Immunol Cell Biol. 2007;85:346–347. doi: 10.1038/sj.icb.7100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Radic M, Marion T, Monestier M. Nucleosomes are exposed at the cell surface in apoptosis. J Immunol. 2004;172:6692–6700. doi: 10.4049/jimmunol.172.11.6692. [DOI] [PubMed] [Google Scholar]
- 42.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lau CM, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrat FJ, Coffman RL. Development of TLR inhibitors for the treatment of autoimmune diseases. Immunol Rev. 2008;223:271–283. doi: 10.1111/j.1600-065X.2008.00630.x. [DOI] [PubMed] [Google Scholar]
- 45.Feeney AJ, Lawson BR, Kono DH, Theofilopoulos AN. Terminal deoxynucleotidyl transferase deficiency decreases autoimmune disease in MRL-Faslpr mice. J Immunol. 2001;167:3486–3493. doi: 10.4049/jimmunol.167.6.3486. [DOI] [PubMed] [Google Scholar]
- 46.Vidal S, Kono DH, Theofilopoulos AN. Loci predisposing to autoimmunity in MRL-Faslpr and C57BL/6-Faslpr mice. J Clin Invest. 1998;101:696–702. doi: 10.1172/JCI1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kono DH, et al. Lupus susceptibility loci in New Zealand mice. Proc Natl Acad Sci USA. 1994;91:10168–10172. doi: 10.1073/pnas.91.21.10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haraldsson MK, et al. Autoimmune alterations induced by the New Zealand Black Lbw2 locus in BWF1 mice. J Immunol. 2005;174:5065–5073. doi: 10.4049/jimmunol.174.8.5065. [DOI] [PubMed] [Google Scholar]
- 49.Haraldsson MK, et al. The lupus-related Lmb3 locus contains a disease-suppressing Coronin-1A gene mutation. Immunity. 2008;28:40–51. doi: 10.1016/j.immuni.2007.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Z, et al. CD14 is required for MyD88-independent LPS signaling. Nat Immunol. 2005;6:565–570. doi: 10.1038/ni1207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.