Abstract
While more commonly applied in zoology, synchrotron radiation X-ray tomographic microscopy (SRXTM) is well-suited to nondestructive study of the morphology and anatomy of both fossil and modern plants. SRXTM uses hard X-rays and a monochromatic light source to provide high-resolution data with little beam-hardening, resulting in slice data with clear boundaries between materials. Anatomy is readily visualized, including various planes of section from a single specimen, as clear as in traditional histological sectioning at low magnifications. Thus, digital sectioning of rare or difficult material is possible. Differential X-ray attenuation allows visualization of different layers or chemistries to enable virtual 3-dimensional (3D) dissections of material. Virtual potential fossils can be visualized and digital tissue removal reveals cryptic underlying morphology. This is essential for fossil identification and for comparisons between assemblages where fossils are preserved by different means. SRXTM is a powerful approach for botanical studies using morphology and anatomy. The ability to gain search images in both 2D and 3D for potential fossils gives paleobotanists a tool—virtual taphonomy—to improve our understanding of plant evolution and paleobiogeography.
Keywords: anatomy, morphology, palaeobotany, synchrotron radiation X-ray tomographic microscopy
Various methods now exist for visualizing plant material in 3-dimension (3D). Confocal laser-scanning microscopy, electron tomography, and optical coherence microscopy are used for imaging thin (often sectioned) and (semi-)transparent material (1), in conjunction with modern computer programs for 3D reconstruction. Complementing this, other techniques for examining larger specimens or to avoid sectioning “difficult” material include neutron imaging, nuclear magnetic resonance (NMR) imaging, X-ray computed tomography (CT), high-resolution X-ray computed tomography (HRCT), and synchrotron radiation X-ray tomographic microscopy (SRXTM). Of these, HRCT and SRXTM are the most useful methods to visualize both internal and external morphology and anatomy in a noninvasive and nondestructive manner, and are useable for a range of specimen sizes. HRCT has been used in plant sciences to examine structure and morphology of extant flowers and fruits (1; see also the Digital Morphology digital library of the University of Texas at Austin, www.digimorph.org), density of extant woods (2, 3), spatial distribution of root systems (4–6), and light interception of the tree canopy (7, 8). In paleobotany, CT has been used in a few cases to reveal internal structures, including in Paleozoic charophytes (9), silicified Cycadeoidea stems and Araucaria mirabilis cones from the Jurassic (10), an undescribed Cretaceous gymnosperm fructification (11, 12), a Paleogene hymenophyllaceous fern rhizome (13), and Eocene myrtaceous fruits (14).
Recently, SRXTM has become increasingly used in biology, especially in paleontology (e.g., 15–20; see ref. 19 for comparative review of the various methods mentioned above and their application to paleontology); these are primarily zoological studies. SRXTM provides several advantages over CT. Modern, third-generation synchrotrons make use of hard X-rays, a monochromatic beam, and a high beam intensity to scan specimens. Thus, compared to CT, there is no beam-hardening effect that results in inaccurate portrayal of the true X-ray absorption of the specimen, potentially inaccurate measurements, and digital sections that are more difficult to segment (15). Also, SRXTM has very high resolution, up to 0.35 μm, and thus provides better resolution than HRCT. Combined with recent developments in phase contrast methods (17, 20, 21), synchrotron facilities are a powerful tool for noninvasive, volumetric investigation of a broad range of material.
There are many actual and potential uses of SRXTM for studying both fossil and extant plants. Here, we evaluate the benefits of SRXTM, and explore potential applications and the predictive value of digital preparation to (i) provide high-quality anatomical detail in multiple planes of section from a single specimen, comparable to traditional histology, but avoiding associated technical problems; and (ii) introduce a technique—“virtual taphonomy”—including production of both 2D and 3D virtual potential fossils.
Benefits of SRXTM Digital Preparation for Extant Plant Material
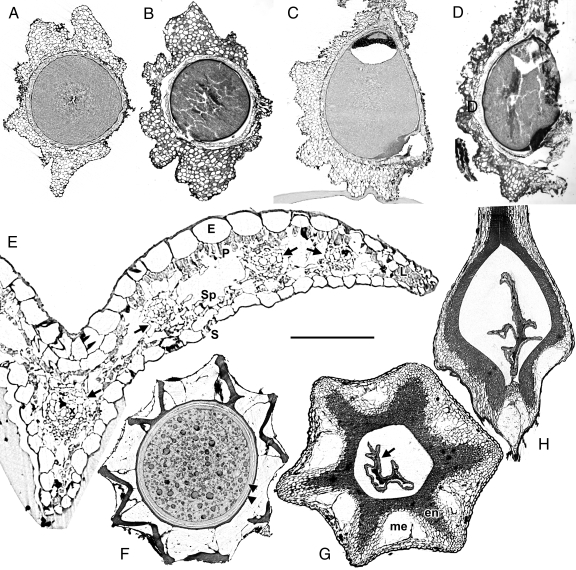
SRXTM has proven to be an excellent imaging system, not only for fossil plants [as demonstrated for charcoalified reproductive structures (17, 18, 22–24) and megaspores (25)], but also for modern plants, which we demonstrate here (Fig. 1). With SRXTM the entire sample is recorded as digital data that can be manipulated in specialized computer programs. Cellular details are reconstructed with much more resolution than has hitherto been shown by HRCT (e.g., 1; but see ref. 26 for critical-point drying/heavy metal staining technique to improve visualization). At least at lower magnifications, not only tissues but also individual cell walls are as clear as in traditional histological sections (Fig. 1 A–D), but there is no destruction of the sample and no risk of the folding, tearing, or loss of sections that can occur (Fig. 1 B and D) during the embedding, sectioning, and mounting process of traditional histology. Artifacts can also be distinguished (e.g., true vs. introduced spaces: Fig. 1 C and D). Individual xylem elements within vascular bundles are visible in digital transverse sections of leaves, even from dried (and hence relatively poorly preserved) herbarium specimens (Fig. 1E), and endosperm cells are clearly visible in fertile seeds (Fig. 1F). Digital sections can be obtained in multiple planes (Fig. 1 G and H, and Fig. 2) in contrast to the single plane per specimen in traditional histology. In some organs, there are hard layers that make them difficult to section, such as the stony endocarps in some fruits. SRXTM is a simple method that provides accurate data for both 3D reconstructions and anatomical sections of fruits otherwise difficult to section (Fig. 1 G and H, and Fig. 2), and is especially useful when dealing with dried herbarium material. Traditional histology would require differential staining (Fig. 1 B and D) to reveal differential tissue and cell wall chemistry, but with SRXTM, differences are highlighted by varying X-ray attenuation, which are shown in the reconstructed virtual sections by a visible difference in layers (e.g., Fig. 1A, C, and the distinct cuticles in F). Differential X-ray attenuation also has other applications, for example, in visualizing the difference between water and air to study how water refills xylem vessels in bamboo (27).
Fig. 1.
SRXTM results of modern plant material. (A and C) Saururus chinensis (Saururaceae), digital sections of fruit (A, transverse; C, longitudinal). (B and D) Saururus chinensis (Saururaceae), traditional paraffin-embedded sections (B, transverse; D, longitudinal) of fruits; note torn, detached, and folded parts caused by cutting. (E) Mapania monostachya (Cyperaceae), digital transverse section of leaf showing cellular detail epidermis (E), leaf margin (L) with marginal sclerenchyma, palisade mesophyll (P), stoma (S), spongy mesophyll (Sp), xylem (x), and vascular bundle sheath (arrows). (F) Cyclanthus bipartitus (Cyclanthaceae), digital transverse section of seed with different layers showing as different gray values. Thick dark lines visible in outer layer indicate wall thickenings. Two cuticular envelopes (arrowheads) are distinguishable between outer seed coat and endosperm. (G) Paramapania radians (Cyperaceae), digital transverse section of fruit, showing thin-walled outer mesocarp layer (me), thick-walled inner endocarp layer (en), and collapsed (probably sterile) seed (arrow). (H) Digital longitudinal section of same specimen as in panel G. (Scale bar: A and B, 700 μm; C and D, 465 μm; E, 125 μm; F, 410 μm; G, 255 μm; H, 675 μm.)
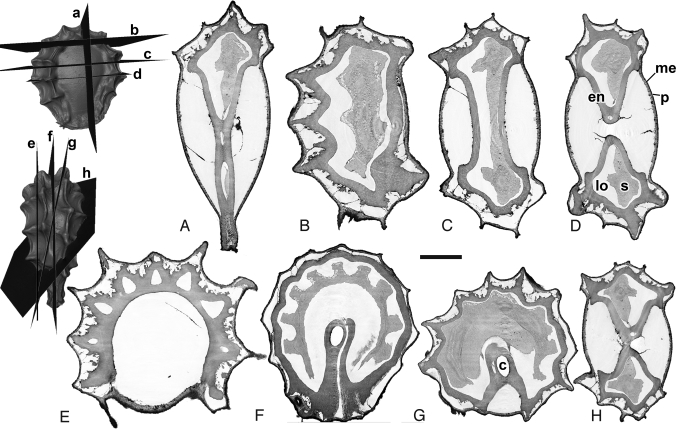
Fig. 2.
Stephania japonica (Menispermaceae), digital sections in multiple planes through a single fruit providing search images for potential fossils: Pericarp (p), mesocarp (me), endocarp (en), seed (s), locule (lo), and condyle (c). (A) Longitudinal section. (B–D) Transverse sections. (E and F) Longitudinal sections (perpendicular to that in panel A). (G and H) Oblique sections. (Scale bar: A and C–E, 1 mm; B, 0.8 mm; F–H, 1.4 mm.)
Virtual Potential Fossils from Virtual Taphonomy—A Paleobotanical Technique
The ability to digitally prepare modern fruits and seeds to remove outer layers mimicking taphonomic processes means that virtual potential fossils can be visualized, such as those that would result after physical abrasion, decomposition, or sediment/mineral infill had modified the original specimen. For example, an orange could be fossilized in a variety of ways that would make it difficult to recognize it as an orange. The entire fruit might be preserved, or only the outer peel, but with nothing inside. Alternatively, the orange segments could be held together, but lacking the outer peel. Other possible combinations include a group of segments, individual segments, part of a segment, a segment with a protruding seed, just the seed, or even a cast of the inside of the seed (28). Another example is a drupe (stone fruit), such as a plum, which could be fossilized as either the entire fruit, the endocarp (stony layer), a locule cast of the inside of the endocarp, or a seed. Virtual taphonomy enables digital removal of layers (Fig. 3B, E, I, and Fig. 4 E and F) and virtual infilling of negative space (Fig. 3 F and K), allowing paleobotanists to examine modern fruits and seeds and actually predict what potential fossils could be formed.
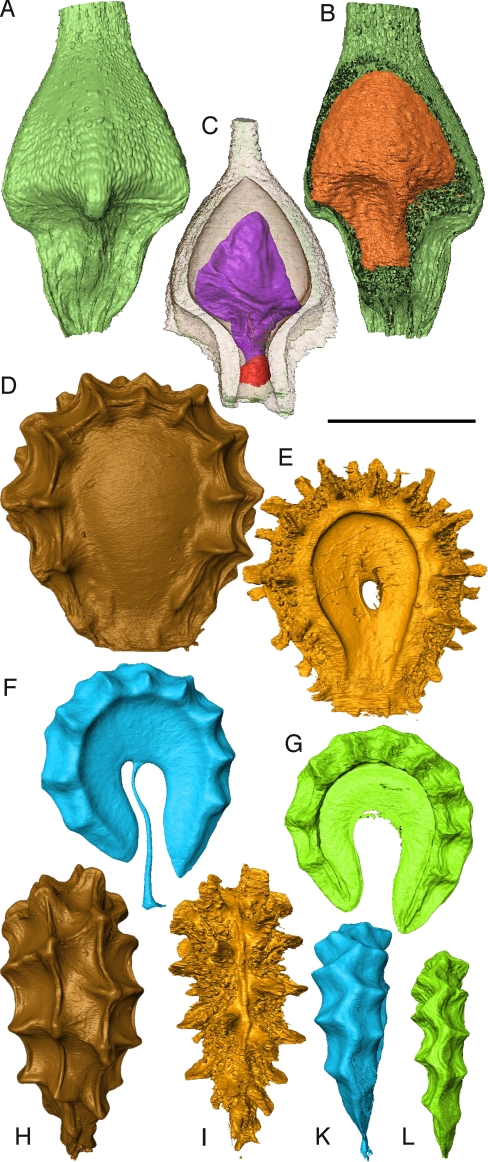
Fig. 3.
Examples of potential fossils produced by virtual taphonomy. (A–C) Paramapania radians (Cyperaceae), digital renderings of fruit. (A) Entire fruit. (B) Fruit with part of mesocarp virtually removed to show endocarp. (C) Endocarp with shrunken seed and basal plug visible. (D–L) Stephania japonica (Menispermaceae), digital renderings of fruit seen in section in Fig. 2. (D) External side view of fruit. (E) Side view of endocarp; note central hole (condyle), prominent rugose dorsal surface. (F) Virtual potential fossil of endocarp locule cast; note dorsal surface smooth except for small bumps. (G) Seed. (H) Dorsal edge of fruit showing 4 ranks of spines. (I) Dorsal edge of endocarp showing 4 ranks of spines and central small ridge. (K) Dorsal edge of locule cast showing central 2 ranks appearing united into single dorsal ridge. (L) Dorsal edge of seed with similar morphology to locule cast. (Scale bar: A–C, 815 μm; D–G, 3.1 mm; H–L, 2.9 mm.)
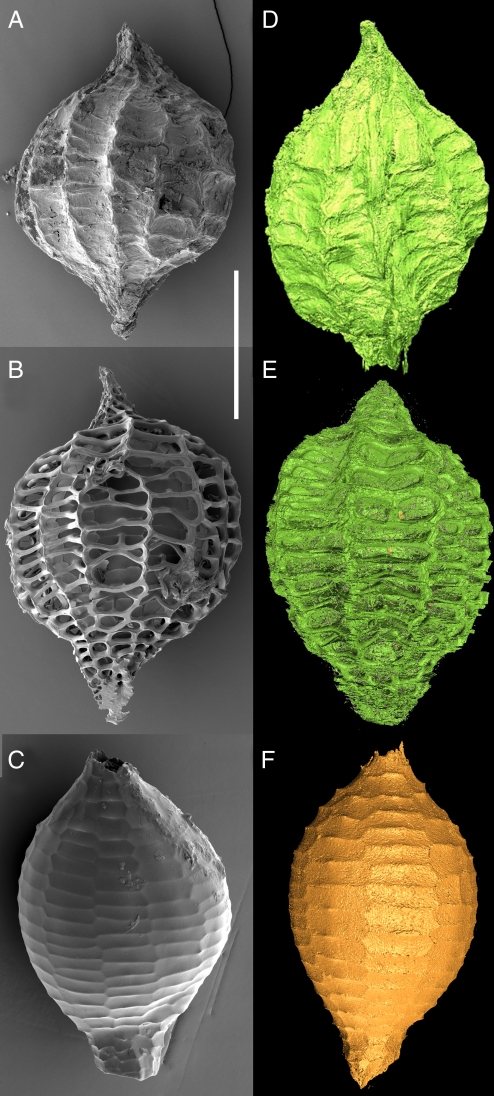
Fig. 4.
Cyclanthus bipartitus (Cyclanthaceae), taphonomy of seeds. (A–C) SEM images of seed (A) and chemically treated seeds (B and C). (D–F) Virtual reconstructions from synchrotron data. (A and D) Entire seed. (B and E) Thin-walled tissue of outer papery seed coat layer removed, with only wall thickenings remaining. (C and F) Outer seed coat entirely removed leaving cuticular layer visible. (Scale bars: A, 425 μm; B and D, 400 μm; C, 330 μm; E, 415 μm; F, 300 μm.)
Examples of Virtual Taphonomy
The high-resolution cell and tissue details in the reconstructed sections obtained by SRXTM allow easy and accurate virtual removal of layers from structures. This reveals the underlying structures that yield important systematic characters. This has been used previously for virtual dissections on an organ level; for example, the perianth was digitally removed from a Cretaceous flower of Lauraceae to reveal the androecium and gynoecium (18). Here, we demonstrate approaches including removal of tissue layers and digital formation of casts, which enable the technique of virtual taphonomy by production of virtual potential fossils. Our examples are taken from fruits and seeds. We demonstrate that removal of layers, such as fruit wall or seed coat, and virtual infills of cavities, can be used to produce virtual potential fossils that predict what fossils might look like.
Sedge Fruits.
Modern Cyperaceae (the sedge family) fruits contain both hard and soft tissues, including a thin-walled (sometimes fleshy) mesocarp, a hard endocarp, a basal “plug,” and a seed. In the paleobotanical record it is common to find only the hard, more resistant, endocarps preserved [e.g., in fruits of Caricoidea from the Eocene of England (29, 30)]. Using SRXTM, serial sections of modern fruits can readily be obtained in multiple planes (Fig. 1 G and H), rather than in a single plane as in traditional histology. Virtual taphonomy then allows us to distinguish the various layers of the fruit and “peel” them away, revealing the morphology of the endocarp alone (Fig. 3 A–C). Internally, the basal plug and dried seed are also revealed (Fig. 3C). The results can be surprising, as in Paramapania fruits where the endocarp has a more pronounced series of ridges (Fig. 3B) than would be predicted from the external appearance (Fig. 3A). Our results demonstrate that fossil Caricoidea, with a smooth endocarp, is not so closely related to Paramapania, but is more like other mapanioid sedges such as Mapania and Hypolytrum.
Menisperm Fruits.
In fruits of Menispermaceae (the moonseed family), the endocarp has intricate, complex, and highly diagnostic surface sculpturing. Modern comparative endocarp preparations are rare in some cases due to lack of fruits on herbarium sheets, presenting a serious hindrance to understanding both the systematics and the fossil history of the family. Virtual taphonomy of extant fruits (Fig. 3 D and H) reveals the endocarp (Fig. 3 E and I), allowing ready comparison with fossils that have lost fleshy fruit layers through taphonomic processes. A virtual cast of the fruit locule (Fig. 3 F and K) can be made for comparison with fossils formed by mineral infills. Seed morphology (Fig. 3 G and L) can also be visualized.
Cyclanthus Fruits and Seeds.
Exceptionally preserved fossils at the Eocene Messel World Heritage Site in Germany are readily identifiable as the unique fruiting cycles of Cyclanthus (Panama Hat plant family, Cyclanthaceae) (31). Modern Cyclanthus is distributed only from Mexico to South America, so its presence in the German Eocene suggests a much more widespread distribution in the past. Chemical treatment was used to remove the outer seed coat layers from modern Cyclanthus seeds (Fig. 4 A–C) to determine the morphologies that might result if outer layers were abraded or decomposed during fossilization, and hence facilitate recognition of dispersed fossil seeds (31). This chemical approach is unpredictable and resulted in various appearances depending on duration of treatment. Using SRXTM data and virtual taphonomy, we created a suite of comparable virtual potential fossils (Fig. 4 D–F) by sequentially removing layers of the seed. The digital images are similar to the chemical treatments but have the advantages of being precisely constrained (removal of anatomically specified known layers), repeatable (for multiple specimens and for other comparable taxa if appropriate), and nondestructive. The virtual potential fossil morphology of Fig. 4C, combined with precise knowledge of which layers were removed and evidence of distinctive layer chemistry revealed by differential X-ray attenuation (Fig. 1F), shows that only the cuticular layers of the Cyclanthus seed coat survive in the dispersed fossil seeds. This evidence explains how fossilization processes led to former misidentification of Cyclanthus seeds as fruits of Scirpus (Cyperaceae) (31). Furthermore, the search image provided by the virtual fossils will enable future researchers to identify dispersed Cyclanthus seeds elsewhere in the fossil record.
Future Potential Applications
Multiple Planes.
Digital sections in multiple planes—including oblique planes (Fig. 2)—are crucial to provide search images for paleobotanists studying slices through permineralized or petrified plant-bearing rocks. In fossils, such material is frequently sectioned randomly, because neither the presence of the organ nor its orientation are known, resulting in sections that are rarely oriented perpendicular to the central axis of the structure (e.g., fossil Juglandaceae; 32). While permineralized material offers the opportunity to understand the fossil plant at both an anatomical and morphological level, oblique sections can obscure recognition of affinities since most (modern) plant material is sectioned either transversely or longitudinally for optimal imaging. The ability to scan specimens and choose any orientation of slice therefore provides search images (Fig. 2) that can be used in the identification of permineralized plants.
Systematic Value.
The ability to virtually dissect plant organs in conjunction with visualizing internal anatomy provides an efficient means of describing and evaluating systematic characters for both extinct and extant taxa. As mentioned above, SRXTM is a nondestructive technique—an important factor when working with rare or even unique herbarium material or fossils. SRXTM allows material that is difficult to section to be examined histologically without problems such as folding or tearing of sections that can result in obscured characters. SRXTM is also a useful method to efficiently study the internal structure of multiple specimens, allowing an understanding of variation of a trait within a population or species. For example, features such as origination and distribution of resin canals and vascular tissue are important in distinguishing ovulate cones of Pinaceae (pines and relatives) (33). While many Cretaceous fossil Pinaceae cones have been described as new species within 1 of 3 extinct genera, these are often on the basis of single specimens (34). Without understanding the degree of variation (e.g., due to ecological factors) in ovulate cones of a (modern) species, we may be splitting natural, extinct species into 2 or more fossil species and hence inflating perceived biodiversity.
Comparing Fossils in Various Preservation States.
Virtual taphonomy is a technique that will facilitate comparison of differentially preserved fossils, both with each other and with modern relatives, ensuring an accurate taxonomic determination. For example, fossil fruits of Juglandaceae (the walnut family) and Annonaceae (the custard apple family) are commonly found as mineralized internal molds or casts [e.g., in the Eocene London Clay (28) and Clarno (35) floras]. These casts record the complex wrinkled topography of the internal endosperm (seed food reserves), but are very difficult to compare with purely organic compression fossils [e.g., the Eocene Messel (36) flora] or with modern seeds that retain the external seed coat or fruit wall. Digital reconstructions, like those of the Menispermaceae locules mentioned above, will allow visualization and comparison of the endosperm topography. Systematically important features of the internal organization of the fruits of Cornaceae (dogwood family) (e.g., locules, germination valves; 37) can be studied in the same way.
Permineralized fruits and seeds can be reconstructed from serial peels or thin sections in a method similar to that used for CT or SRXTM data, and then compared with mummified or compression fossils and modern material where only the external gross morphology is visible. A version of this methodology was used for flowers, fruits, seeds, and other reproductive structures from the Middle Eocene Princeton Chert of British Columbia, Canada (38, 39), and the Eocene Appian Way locality and Cretaceous Apple Bay locality of Vancouver Island, British Columbia, Canada (32, 40, 41). Preliminary results suggest that it is possible to use SRXTM on calcareous permineralizations, which would allow careful selection of appropriate specimens for peeling, and could even reduce the need for laborious serial peels, although these remain important for comparative permanent slides and study of features requiring high magnifications.
Conclusions
While SRXTM will not replace traditional histology, it does offer an improved technique for certain materials and the ability to combine 2D and 3D analyses. While others have used SRXTM for studying modern plant physiology (27, 42), here we demonstrate that SRXTM will be particularly useful for examining herbarium specimens; for examining hard material that does not infiltrate well for traditional sectioning; and for determination of systematically useful characters, especially in groups where material is rare and cannot be destroyed, but the anatomical information is needed (e.g., fruits of Menispermaceae). The great advantage of SRXTM is that it is noninvasive, nondestructive, and has relatively high resolution. Scans using SRXTM reveal cellular-level detail of modern plants that are comparable to low magnifications of traditional histological sections. Even some fine details such as stomatal guard cells, xylem elements, fruit pericarp cells, and endosperm can be digitally imaged from herbarium specimens. Chemically distinct materials are differentially visualized as a result of different X-ray attenuation, allowing recognition of cell-wall layers such as cuticle and cell inclusions such as tannins.
The digital scans can be manipulated in 3D computer programs to reveal morphology and anatomical sections in multiple planes. Digital segmentation and dissection allow various 3D internal and external morphologies to be visualized. In addition, various layers can be digitally removed, and virtual infills can be created, in effect mimicking taphonomic effects with ease, reproducibility, and without destroying specimens. Such virtual taphonomy is successful in digital visualization of endocarps, seeds, and virtual locule casts, and for comparing modern and fossil fruits and seeds, with many promising future applications. Multiple planes of section are readily obtainable, producing 2D virtual potential fossils that allow comparisons with permineralized assemblages where material is randomly oriented. Virtual taphonomy allows us to predict cryptic morphologies as would result from decomposition and abrasion, and what altered fossils of targeted groups might look like, therefore enabling us to gain better search images to identify and compare fossils. Virtual taphonomy and the production of virtual potential fossils represents a powerful tool for paleobotanical studies that depend on the recognition and accurate identification of extinct plants.
Methods
Samples were mounted onto brass stubs using polyvinyl acetate glue and imaged at the TOMCAT beamline, Swiss Light Source, Paul Scherrer Institute, Villigen, Switzerland (beamtime for research at SLS is allotted in shifts of 8 h and can be obtained by competitive application that also covers cost of beamtime). Data were acquired using the 4× or 10× objectives on an X-ray microscope, and an exposure time of 350–420 ms at 9.9 keV. A total of 1,500 projections were acquired over 180° (total scan time of ca. 10 min/sample). After data acquisition is accomplished, the rotation center is manually fine-tuned (a few seconds of time) together with the reconstruction filter. Finally, reconstruction of the whole volume can be launched on the beamline cluster (six 4-cores Linux machines) that needs less than 10 min to deliver the full, reconstructed data set. This process can be easily “scripted” so that a large number of samples can be reconstructed in a batch queue.
Reconstructed images were processed at Royal Holloway, University of London using Avizo 5.0 (Mercury Computer Systems) for Windows XP 64-bit. Digital sections are readily attainable, but 3D segmentation to obtain virtual morphology can take several days of work per specimen involving labeling of each digital section. Images were captured in Avizo and plates constructed in Adobe Photoshop CS for Mac. Digital sections were inverted and contrast adjusted; for Fig. 1 E–H and Fig. 4, the background was removed using the magic wand tool.
Acknowledgments.
We thank T. Eldridge (Royal Botanic Gardens, Kew), S. Joomun (Royal Holloway, University of London), L. McParland (Royal Holloway, University of London), and I. Steel for help in data gathering at SLS; A. Scott (Royal Holloway, University of London) for encouragement during this project; P. Donoghue and N. Gostling (Bristol University) for technical advice; W. Chaloner (Royal Holloway, University of London) and two anonymous reviewers for comments on the manuscript; and N. Sheldon (University of Michigan) for help at SLS, discussion, and comments on the manuscript. This research was supported by a Royal Society USA/Canada International Fellowship to S.Y.S., funding from the Natural Environmental Research Council Envirosynch2 to M.E.C., and funding from the Integrated Infrastructure Initiative (I3) on Synchrotrons and Free Electron Laser through Swiss Light Source to M.E.C. and S.Y.S.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.W. is a guest editor invited by the Editorial Board.
References
- 1.Stuppy WH, Maisano JA, Colbert MW, Rudall PJ, Rowe TB. Three-dimensional analysis of plant structure using high-resolution X-ray computed tomography. Trends Plant Sci. 2003;8:2–6. doi: 10.1016/s1360-1385(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 2.Lindgren LO. Medical CAT-scanning: X-ray absorption coefficients, CT numbers and their relation to wood density. Wood Sci Technol. 1991;25:341–349. [Google Scholar]
- 3.Fromm JH, et al. Xylem water content and wood density in spruce and oak trees detected by high-resolution computed tomography. Plant Physiol. 2001;127:416–425. [PMC free article] [PubMed] [Google Scholar]
- 4.Heeraman DA, Hopmans JW, Clausnitzer V. Three dimensional imaging of plant roots in situ with X-ray computed tomography. Plant Soil. 1997;189:167–179. [Google Scholar]
- 5.Pierret A, Capowiez Y, Moran CJ, Kretzschmar A. X-ray computed tomography to quantify tree rooting spatial distributions. Geoderma. 1999;90:307–326. [Google Scholar]
- 6.Gregory PJ, et al. Non-invasive imaging of roots with high resolution X-ray micro-tomography. Plant Soil. 2003;255:351–359. [Google Scholar]
- 7.Dutilleul P, Lontoc-Roy M, Prasher SO. Branching out with a CT scanner. Trends Plant Sci. 2005;10:411–412. doi: 10.1016/j.tplants.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Dutilleul P, Han L, Smith DL. Plant light interception can be explained via computed tomography scanning: Demonstration with pyramidal cedar (Thuja occidentalis, Fastigiata) Ann Bot. 2008;101:19–23. doi: 10.1093/aob/mcm273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feist M, Liu J, Tafforeau P. New insights into Paleozoic charophyte morphology and phylogeny. Amer J Bot. 2005;92:1152–1160. doi: 10.3732/ajb.92.7.1152. [DOI] [PubMed] [Google Scholar]
- 10.Pika-Biolzi M, Hochuli PA, Flisch A. Industrial X-ray computed tomography applied to paleobotanical research. Rivista italiana di paleontologia e stratigrafia. 2000;106:369–377. [Google Scholar]
- 11.Nishida H. The frontier of fossil plant studies. Gakujutu Geppou. 2001;54:1142–1144. [Google Scholar]
- 12.Nishida H. Cretaceous plants of Japan based on permineralized fossils. Kaseki. 2005;78:5–20. [Google Scholar]
- 13.Nishida H. Investigation of plant evolution using permineralized fossils. Kenbikyo. 2007;42:118–121. [Google Scholar]
- 14.Devore ML, Kenrick P, Pigg KB, Ketcham RA. Utility of high resolution X-ray computed tomography (HRXCT) for paleobotanical studies: An example using London Clay fruits and seeds. Amer J Bot. 2006;93:1848–1851. doi: 10.3732/ajb.93.12.1848. [DOI] [PubMed] [Google Scholar]
- 15.Tafforeau P, et al. Applications of X-ray synchrotron microtomography for non-destructive 3D studies of paleontological specimens. Appl Phys A. 2006;83:195–202. [Google Scholar]
- 16.Donoghue PCJ, et al. Synchrotron X-ray tomographic microscopy of fossil embryos. Nature. 2006;442:680–683. doi: 10.1038/nature04890. [DOI] [PubMed] [Google Scholar]
- 17.Friis EM, et al. Phase-contrast X-ray microtomography links Cretaceous seeds with Gnetales and Bennettitales. Nature. 2007;450:549–552. doi: 10.1038/nature06278. [DOI] [PubMed] [Google Scholar]
- 18.von Balthazar M, Pedersen KR, Crane PR, Stampanoni M, Friis EM. Potomacanthus lobatus gen. et sp nov., a new flower of probable Lauraceae from the Early Cretaceous (Early to Middle Albian) of eastern North America. Amer J Bot. 2007;94:2041–2053. doi: 10.3732/ajb.94.12.2041. [DOI] [PubMed] [Google Scholar]
- 19.Sutton MD. Tomographic techniques for the study of exceptionally preserved fossils. Proc R Soc B. 2008;275:1–7. doi: 10.1098/rspb.2008.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lak M, et al. Phase contrast X-ray synchrotron imaging: Opening access to fossil inclusions in opaque amber. Microsc Microanal. 2008;14:251–259. doi: 10.1017/S1431927608080264. [DOI] [PubMed] [Google Scholar]
- 21.Stampanoni M, et al. Trends in synchrotron-based tomographic imaging: The SLS experience. Proceedings of SPIE Developments in X-ray tomography V. ed Bonse. 2006;U 6318:6318M-1, 6318M-14. [Google Scholar]
- 22.von Balthazar M, Pedersen KR, Crane PR, Friis EM. Carpestella lacunata gen. et sp. nov., a new basal angiosperm flower from the Early Cretaceous (Early to Middle Albian) of eastern North America. Int J Plant Sci. 2008;169:890–898. [Google Scholar]
- 23.Friis EM, Pedersen KR, Crane PR. Early Cretaceous mesofossils from Portugal and eastern North American related to the Bennettitales-Erdtmannithecales-Gnetales group. Amer J Bot. 2009;96:252–283. doi: 10.3732/ajb.0800113. [DOI] [PubMed] [Google Scholar]
- 24.Scott AC, et al. Scanning electron microscopy and synchrotron radiation X-ray tomographic microscopy of 330 million year old charcoalified seed fern fertile organs. Microsc Microanal. 2009;15:166–173. doi: 10.1017/S1431927609090126. [DOI] [PubMed] [Google Scholar]
- 25.Glasspool I, et al. An ultrastructural investigation of early Middle Pennsylvanian megaspores from the Illinois Basin, USA. Rev Palaeobot Palynol. 2009;156:62–78. [Google Scholar]
- 26.Leroux O, et al. A new preparation method to study fresh plant structures with X-ray computed tomography. J Microsc. 2009;233:1–4. doi: 10.1111/j.1365-2818.2008.03088.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Kim Y. In vivo visualization of the water-filling process in xylem vessels using X-ray micro-imaging. Ann Bot. 2008;101:595–602. doi: 10.1093/aob/mcm312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collinson ME. Fossil Plants of the London Clay. Field Guide to Fossils No. 1. London: Palaeontological Association; 1983a. [Google Scholar]
- 29.Collinson ME. Palaeofloristic assemblages and palaeoecology of the Lower Oligocene Bembridge Marls, Hamstead Ledge, Isle of Wight. Bot J Linn Soc. 1983b;86:177–225. [Google Scholar]
- 30.Collinson ME. Plant macrofossils from the Bracklesham Group (Early & Middle Eocene), Bracklesham Bay, West Sussex, England: Review and significance in the context of coeval British Tertiary floras. Tert Res. 1996;16:175–202. [Google Scholar]
- 31.Smith SY, Collinson MEC, Rudall PJ. Fossil Cyclanthus (Cyclanthaceae) from the Eocene of Germany and England. Amer J Bot. 2008;95:688–699. doi: 10.3732/ajb.2007390. [DOI] [PubMed] [Google Scholar]
- 32.Elliot LL, Mindell RA, Stockey RA. Beardia vancouverensis gen. et sp. nov. (Juglandaceae): Permineralized fruits from the Eocene of British Columbia. Amer J Bot. 2006;93:557–565. doi: 10.3732/ajb.93.4.557. [DOI] [PubMed] [Google Scholar]
- 33.Miller CN., Jr Early evolution in the Pinaceae. Rev Paleobot Palynol. 1976;21:101–117. [Google Scholar]
- 34.Smith SY, Stockey RA. Permineralized pine cones from the Cretaceous of Vancouver Island, British Columbia. Int J Plant Sci. 2002;163:185–196. [Google Scholar]
- 35.Manchester SR. Fruits and seeds of the Middle Eocene Nut Beds Flora, Clarno Formation, Oregon. Palaeontographica Americana. 1994;58:1–205. [Google Scholar]
- 36.Wilde V. Aktuelle Übersicht zur Flora aus dem metteleozänen “Ölschiefer” der Grube Messel bei Darmstadt (Hessen, Deutschland) Cour Forsch-Inst Senckenberg. 2004;252:109–114. [Google Scholar]
- 37.Tiffney BH, Haggard KK. Fruits of Mastixioideae (Cornaceae) from the Paleogene of western North America. Rev Paleobot Palynol. 1996;92:29–54. [Google Scholar]
- 38.Smith SY, Stockey RA. Aroid seeds from the Middle Eocene Princeton chert (Keratosperma allenbyense, Araceae): Comparisons with extant Lasioideae. Int J Plant Sci. 2003;164:239–250. [Google Scholar]
- 39.Smith SY, Stockey RA. Establishing a fossil record for the perianthless Piperales: Saururus tuckerae sp. nov. (Saururaceae) from the Middle Eocene Princeton Chert. Amer J Bot. 2007;94:1642–1657. doi: 10.3732/ajb.94.10.1642. [DOI] [PubMed] [Google Scholar]
- 40.Rankin BD, Stockey RA, Beard G. Fruits of Icacinaceae from the Eocene Appian Way locality of Vancouver Island, British Columbia. Int J Plant Sci. 2008;169:305–314. [Google Scholar]
- 41.Stockey RA, Rothwell GW. Distinguishing angiophytes from the earliest angiosperms: A Lower Cretaceous (Valanginian-Hauterivian) fruit-like reproductive structure. Amer J Bot. 2009;96:323–335. doi: 10.3732/ajb.0800295. [DOI] [PubMed] [Google Scholar]
- 42.Verboven P, et al. Three-dimensional gas exchange pathways in pome fruit characterized by synchrotron X-ray computed tomography. Plant Physiol. 2008;147:518–527. doi: 10.1104/pp.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]