Abstract
A dinuclear nickel complex with methyl and thiolate ligands, Ni(dadtEt)Ni(Me)(SDmp) (2), has been synthesized as a dinuclear Nid–Nip-site model of acetyl-CoA synthase (ACS) (dadtEt is N,N′-diethyl-3,7-diazanonane-1,9-dithiolate; Dmp is 2,6-dimesitylphenyl). Complex 2 was prepared via 2 methods: (i) ligand substitution of a dinuclear Ni(II)–Ni(II) cation complex [Ni(dadtEt) Ni(tmtu)2] (OTf)2(1) with MeMgBr and KSDmp (tmtu is tetramethylthiourea), (ii) methyl transfer from methylcobaloxime Co(dmgBF2)2(Me)(Py) (5) to a Ni(II)–Ni(0) complex such as [Ni(dadtEt)Ni(cod)] (3), generated in situ from Ni(dadtEt) and Ni(cod)2, followed by addition of KSDmp (cod is 1,5-cyclooctadiene; dmgBF2 is difluoroboryl-dimethylglyoximate). Method ii models the formation of Nip–Me species proposed as a plausible intermediate in ACS catalysis. The reaction of 2 with excess CO affords the acetylthioester CH3C(O)SDmp (8) with concomitant formation of Ni(dadtEt)Ni(CO)2 (9) and Ni(CO)4 plus Ni(dadtEt). When complex 2 is treated with 1 equiv of CO in the presence of excess 1,5-cyclooctadiene, the formation of 9 and Ni(CO)4 is considerably suppressed, and instead the dinuclear Ni(II)–Ni(0) complex is generated in situ, which further affords 2 upon successive treatment with Co(dmgBF2)2(Me)(Py) (5) and KSDmp. These results suggest that (i) ACS catalysis could include the Nid(II)–Nip(0) state as the active species, (ii) The Nid(II)–Nip(0) species could first react with methylcobalamin to afford Nid(II)–Nip(II)–Me, and (iii) CO insertion into the Nip–Me bond and the successive reductive elimination of acetyl-CoA occurs immediately when CoA is coordinated to the Nip site to form the active Nid(II)–Nip(0) species.
Keywords: acetylthioester formation, dinuclear nickel-site model, N2S2 ligand, nickel acyl complex
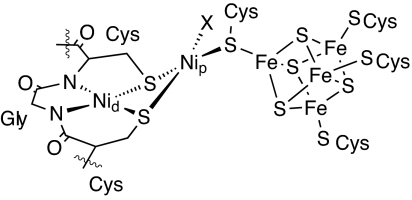
Acetyl-CoA synthase/CO dehydrogenase (ACS/CODH), a bifunctional metalloenzyme found in acetogenic, methanogenic, and sulfate-reducing bacteria, has the important function of fixing CO and CO2 in the global carbon cycle (1–3). Whereas CODH catalyzes the reversible conversion of CO2 to CO, ACS assembles acetyl-CoA from CO, CoA, and a methyl moiety that is derived from the methylcobalamin of the corrinoid iron–sulfur protein (CFeSP). Recently, the crystal structures of the ACS/CODH system from the bacteria Moorella thermoacetica and Carboxydothermus hydrogenoformans have been elucidated (4–6); the ACS active site denoted as the A-cluster contains a [Fe4S4] cubane cluster and a Nid–Nip dinuclear site as shown in Fig. 1, where the 2 nickels designated as Nid and Nip occupy distal and proximal positions, respectively, to the [Fe4S4] cluster. The geometry around Nid is square planar, composed of 2 cysteine sulfurs and 2 carboxyamide nitrogens of the tripeptide Cys-Gly-Cys from the protein backbone. Nip carries an unidentified ligand X and 3 bridging cysteine sulfurs, 2 from the aforementioned tripeptide, and 1 from the [Fe4S4] cluster.
Fig. 1.
Schematic view of the ACS active site.
Biological investigations suggest that the oxidized state of the cluster Aox should be formulated as {Nid2+–Nip2+–[Fe4S4]2+} (7–9). However, the electronic configuration of the active reduced state, probably reduced by 2 electrons from Aox, which is proposed to be {Nid2+–Nip1+–[Fe4S4]1+}, has not yet been firmly established (10–11). The order of the addition of the substrates, Me, CO, and CoA, to the active site is also controversial, and several mechanisms have been proposed on the basis of biological and computational studies (12–14). On the other hand, synthetic studies that probe the reaction mechanisms are limited in number, although various thiolato-bridged dinuclear nickel complexes have been reported as A-cluster models (15–23).
Proposed ACS mechanisms commonly involve the following steps: (i) methyl transfer from methylcobalamin to the reduced Nip(0) [or Nip(I)] site, (ii) CO insertion into the Nip–Me bond, and (iii) formation of acetyl-CoA via the reductive elimination at the Nip site (1–3, 7–14). These reactions had been studied by using mononuclear nickel complexes. CO insertion into the Ni–Me bonds of Ni(NS3R)Me [NS3R is N(CH2CH2SR)3; R is i-Pr, t-Bu], investigated by Stavropoulos et al. (24) , resulted in the formation of acetylthioesters CH3C(O)SR and Ni(0) species in high yield upon treatment with CO. Likewise, the reaction of [Ni(bpy)(SR)(Me)] with >3 equiv of CO was found to yield [Ni(bpy)(CO)2] and acetylthioesters (25). Before these reports, Kim et al. (26) had shown that the reaction of Ni(dppe)(SAr)(Me) with CO generated CH3C(O)SAr. Recently, Rampersad et al. (27) demonstrated the acetylthioester formation in the reaction of Ni–Pd dinuclear complex, Ni(bme-daco)Pd(CO)(COMe), with NaSMe. Eckert et al. (28) reported the methyl-transfer reaction from methylcobaloxime to (triphos)Ni0(PPh3) to gave [(triphos)NiMe]2+. They have also reported that the dinuclear nickel methyl complex [Ni(phma)Ni(Me)(dppe)]− reacts with CO to give [Ni(phma-CO-Me)]− and (dppe)Ni(CO)2, in which acetylation occurs at the thiolato sulfur of the phma ligand (29).
We report herein ACS model studies using a series of thiolato-bridged dinuclear nickel complexes in the Ni(II)–Ni(II) and Ni(II)–Ni(0) states, including a key Ni(II)–Ni(II) dinuclear complex carrying a methyl and a thiolate, Ni(dadtEt)Ni(Me)(SDmp) (2). Although the diaminedithiolate dadtEt ligand applied to this model study would have different electronic properties from the diamidodithiolate Cys-Gly-Cys ligand of A-cluster in ACS, the results herein would provide a clue to the reaction mechanism for the ACS catalysis.
Results and Discussion
Dinuclear Nickel Complex Having Methyl and Thiolato Ligands.
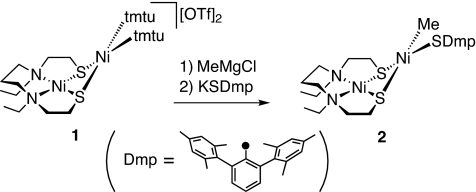
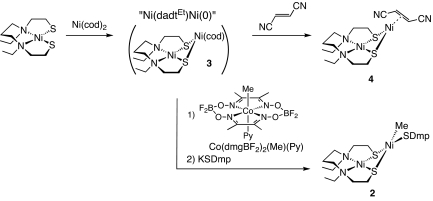
We have recently reported the synthesis of a series of thiolato-bridged dinuclear nickel complexes such as Ni(dadtEt)Ni(SAr)2 and [Ni(dadtEt)Ni(L)2]2+ (L is tmtu, tBuNC; tmtu is tetramethythiourea) (30, 31). During these studies, we found that the tmtu-substituted dinuclear complex [Ni(dadtEt)Ni(tmtu)2](OTf)2 (1) reacted with MeMgCl and KSDmp stepwise in THF at −60 °C to afford the dinuclear nickel complex [Ni(dadtEt)Ni(Me)(SDmp)] (2) carrying a methyl and a thiolate in 76% yield as a reddish-purple powder (Scheme 1). Complex 2 is an intriguing entity modeling the dinuclear nickel site of the A-cluster. The Me and SDmp moieties closely resemble the substrates of the ACS mediating reaction, a methyl group and CoA, respectively. The 1H NMR spectrum of 2 shows a singlet signal at −0.66 ppm that is attributable to the Ni–Me group (25, 26, 29).
Scheme 1.
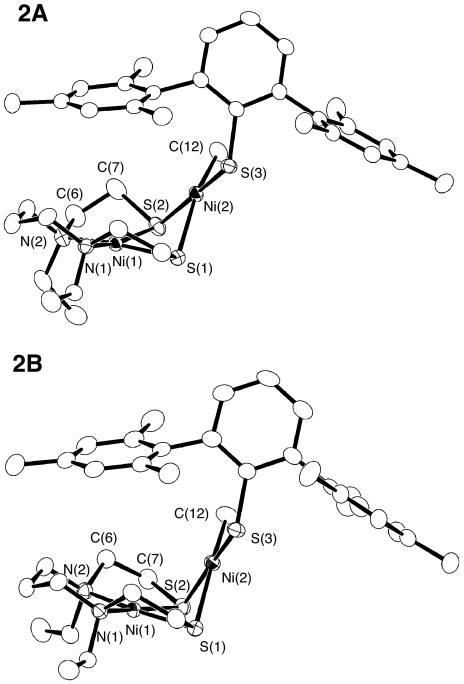
The molecular structure of 2 was confirmed by X-ray crystallography. Complex 2 crystallized with 2 crystallographically independent molecules, 2A and 2B, and Fig. 2 shows their structures. The metric parameters of 2A and 2B are mostly similar. One of the structural differences between 2A and 2B derives from the geometry of dadtEt ligand; the conformation of the Ni(1)-N(2)-C(6)-C(7)-S(2) ring and the orientation of 1 ethyl group differ between 2A and 2B. The other intriguing structural disparity is the folding of the 2 Ni-coordination planes along the μS–μS vector, where the dihedral angle defined by the 2 Ni(μ-S)2 planes is 119.4° for 2A and 103.5° for 2B. As a result, the Ni–Ni distance of 2A [2.9029(3) Å] is markedly longer than that of 2B [2.6935(3) Å]. This observation points to a soft nature of the potential energy curve for the folding motion. The Ni–Me bond lengths of 2A and 2B are comparable to that of [Ni(phma)Ni(Me)(dppe)]− [1.966(2) Å] (28). Because of the strong trans influence of the methyl group, the Ni(2)–S(1) distances are elongated by 0.10 and 0.12 Å compared with the Ni(2)–S(2) distances.
Fig. 2.
ORTEP drawing of Ni(dadtEt)Ni(Me)(SDmp) (2A, 2B). Thermal ellipsoids are shown at 50% probability.
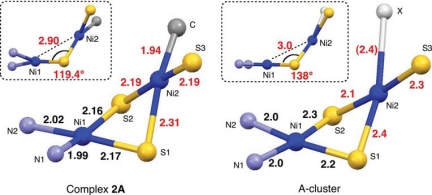
The bond lengths and angles of 2A and 2B also compared well with the relevant structural parameters reported for the A-clusters of ACSMt (from Moorella thermoacetica) and ACSCh (from Carboxydothermus hydrogenformans) (5, 6), as shown schematically in Fig. 3. Although the Cys-Gly-Cys tripeptide of the A-cluster must have different geometrical/electronic properties from the dadtEt model ligand, the core structures of 2A (and 2B) and the A-cluster are very much alike. Like 2A, B, the Ni(2)-μS bonds are longer than the Ni(1)–μS bonds, except for Ni(2)–S(2) of ACSMt,, which is trans to the Ni(2)–S(3) bond. The dihedral angle between the Ni(μ-S)2 planes is somewhat larger for the A-clusters. Of note are the elongated Ni(2)–S(1) distances observed for both 2A, B, and the A-clusters, compared with the Ni(2)–S(2) lengths. This elongation indicates that the unidentified ligand X of ACS has a strong trans influence, which may be as strong as a Me group.
Fig. 3.
Core structures of Ni(dadtEt)Ni(Me)(SDmp) (2A) and A-cluster in ACSMt. In square, a view of structure from the S1–S2 vector.
Synthesis of 2 via a Nid(II)–Nip(0) Complex.
A plausible mechanism of ACS catalysis includes the Nid(II)–Nip(0) state of the A-cluster as an active, reduced form (1, 3, 9). The formation of “[Ni(S2N2′)Ni(cod)]2−” in situ from [Ni(S2N2′)]2− and Ni(cod)2 [S2N2′ is N,N′-ethylenebis(3-mercaptopropionamide)] has been proposed by Rauchfuss and coworkers (16), and here we report the synthesis of a similar Nid(II)-Nip(0) model by the reaction of Ni(dadtEt) and Ni(cod)2. When an acetonitrile solution of Ni(dadtEt) was added to a toluene solution of Ni(cod)2 at −20 °C, the solution immediately turned dark purple. Although we have not yet isolated this species because of its thermal instability, the formation of the Ni(II)–Ni(0) complex such as Ni(dadtEt)Ni(cod) (3) was strongly indicated by a trapping reaction with fumaronitrile, affording Ni(dadtEt)Ni(η2-(E)-NCCH=CHCN) (4) in 77% yield (Scheme 2). The molecular structure of 4 was determined by X-ray crystallography [see supporting information (SI) Text]. To get further insights into the Ni(II)–Ni(0) complex formed in situ, we examined the reaction at low temperature and monitored by 1H-NMR. Because the dissociation of 1,5-cyclooctadiene and the consumption of Ni(dadtEt) and Ni(cod)2 were confirmed, the formation of the Ni(II)–Ni(0) complex is indicated. However, the signals except for those of the dissociated 1,5-cyclooctadiene are considerably broad, and therefore the Ni(II)–Ni(0) complex has not yet completely characterized. The broadness of the signals may suggest the fractional cod coordination to the Ni(0) site.
Scheme 2.
Modeling the methyl-transfer step from methylcobalamin to the Nip site, proposed for the ACS catalytic mechanism, the reaction of the Ni(II)–Ni(0) complex with methylcobaloxime Co(dmgBF2)2(Me)(Py) (5) was examined (28). Because of the thermal instability, the reaction of the Ni(II)–Ni(0) complex and 5 was conducted at −30 °C, although the reaction was rather sluggish at this temperature. After stirring the solution containing the Ni(II)–Ni(0) complex and 5 for 37 h, addition of KSDmp and workup gave 2 in 60% yield (Scheme 2). This result indicates that ACS catalysis could include the Nid(II)–Nip(0) state as an active state, reacting with methylcobalamin to afford the Nid(II)–Nip(II)–Me species.
Methylation of Ni(0) can also be achieved by oxidative addition of MeI to the Ni(II)–Ni(0) complex, affording complex 2 upon successive treatment with KSDmp (Scheme 3). However, this route produces an inferior yield of <20%, probably because the MeI addition to the Ni(0) site competes with electrophilic addition of MeI to the μ-thiolates of the dadtEt ligand as observed for analogous reactions of Ni(N2S2) complexes (32). The selective methylation of the Nip site in ACS could be due to the steric bulk of the methylcobalamin, which may hinder the nucleophilic methylation of the cystein sulfurs bridging the 2 nickel centers. Although the putative MeI adduct of the Ni(II)–Ni(0) complex, [Ni(dadtEt)Ni(Me)I], was not isolated, a similar reaction of the Ni(II)–Ni(0) complex with iodopentafluorobenzene confirms the oxidative addition process, giving the analogous adduct Ni(dadtEt)Ni(C6F5)I (6) in 24% yield. Complex 6 was further converted to Ni(dadtEt)Ni(C6F5)(SDmp) (7) via subsequent addition of KSDmp (Scheme 3). The molecular structures of 6 and 7 were analyzed by X-ray crystallography (see SI Text).
Scheme 3.
Acetylthioester Formation.
To model the formation of acetyl-CoA at the ACS active site, we examined the reaction of 2 with CO. Treatment of 2 with an atmospheric pressure of CO in THF at room temperature resulted in an immediate color change from reddish purple to reddish brown. After removal of volatiles, the materials soluble to ether were subjected to silica gel column chromatography to afford the acetylthioester CH3C(O)SDmp (8) in 89% yield. This result proves that the dinuclear nickel complex 2 can carry out a reaction similar to that of ACS. The residual reddish brown solid, insoluble to ether, was identified as Ni(dadtEt), which was recovered in quantitative yield.
When CO gas was charged to a THF solution of 2 at −60 °C, it was noticed that the color first changed from reddish purple to a dark green before becoming reddish brown. After stirring the solution for 3 min at −60 °C, a dark-green solid was precipitated from the reaction mixture upon addition of hexane. The IR spectrum of the nearly colorless THF/hexane solution showed νCO bands at 2,044 and 1,710 cm−1, which are attributable to Ni(CO)4 and 8, respectively. The dark-green precipitate was crystallized from acetonitrile/ether, and the resulting dark-green needles were subjected to X-ray structural analysis. The crystals were found to contain the dinuclear nickel dicarbonyl complex Ni(dadtEt)Ni(CO)2 (9) and Ni(dadtEt) in a 1:1 ratio (Scheme 4).
Scheme 4.
The formation of the acetylthioester, CH3C(O)SDmp (8), probably proceeds via insertion of CO into the Ni–Me bond of 2 with successive reductive elimination. The latter step, where Ni(2) in Fig. 2 is reduced from NiII to Ni0, would be facilitated by coordination of CO at Ni(2), to generate 9 and Ni(CO)4. In an attempt to detect the possible nickel(II) acetyl intermediate, we examined the reaction of 2 with 1 equiv of CO at −60 °C. However, the detectable products were the same as those obtained from the reaction of 2 with atmospheric CO, i.e., CH3C(O)SDmp (8), 9, and Ni(dadtEt), except for the presence of unreacted starting complex 2. The result indicates that the reductive elimination of the acetylthioester facilitated by CO coordination is faster than the preceding CO insertion into the Ni–Me bond.
An ORTEP drawing of 9 is given in Fig. 4. The coordination geometries of Ni(1) and Ni(2) are square planar and tetrahedral, typical for Ni(II) and Ni(0) oxidation states, respectively. The long Ni(2)–μS bonds of 2.3847(8)–2.3577(8) Å corroborate the Ni(0) state for Ni(2), and the Ni(1)–μS bond lengths of 2.1866(7)–2.1956(6) Å are similar to those of the Ni(II)–Ni(II) dinuclear nickel models (15, 21, 30, 31). Interestingly, the dihedral angle of 97.6° between the 2 Ni(μ-S)2 planes is notably smaller than those of Ni(II)–Ni(II) dinuclear nickel models (10, 18–23). It is also smaller than that (109.7°) of the closely related complex, [Et4N]2[{Ni(S2N2′)}{Ni(CO)2}], reported by Linck et al. (16). The IR spectrum of the dark-green needles shows 2 νCO bands at 1,971 and 1,890 cm−1, which are reasonable for a Ni(0) dicarbonyl such as 9; the νCO bands of [Et4N]2[{Ni(S2N2′)}{Ni(CO)2}] were reported to appear at 1,948 and 1,866 cm−1. This shift of νCO to lower wave numbers indicates greater back donation to CO for the negatively charged dinuclear complex.
Fig. 4.
ORTEP drawing of Ni(dadtEt)Ni(CO)2 (9). Thermal ellipsoids are shown at 50% probability.
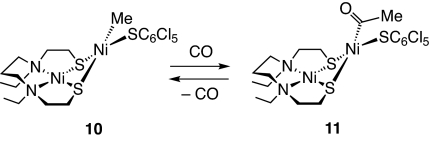
To obtain further insight into the CO insertion into the Ni–Me bond, the dinuclear complex carrying an electron-withdrawing benzenethiolate, Ni(dadtEt)Ni(Me)(SC6Cl5) (10), was prepared in a similar fashion from 1, MeMgCl, and KSC6Cl5 (25). When a dichloromethane solution of 10 was treated with atmospheric CO at room temperature, MeC(O)SC6Cl5 was produced in 36% yield. However, when the same reaction was performed at −40 °C, the IR spectrum exhibits an intense absorption at 1,629 cm−1 attributable to an acyl CO stretch. X-ray structural analysis of brown crystals obtained at −40 °C under a CO atmosphere confirms the formation of the acyl complex Ni(dadtEt)Ni(COMe)(SC6Cl5) (11) (see SI Text). Notably CO insertion is reversible in this particular case. The ν(CO) band in the IR spectra weakened and then disappeared upon evacuation of the solution at −40 °C, from which complex 10 was recovered quantitatively (Scheme 5). In ACS catalysis, CO insertion into the Nip–Me species would be reversible, and the reductive elimination of acetyl-CoA would immediately occur when the CoA is coordinated to Nip site.
Scheme 5.
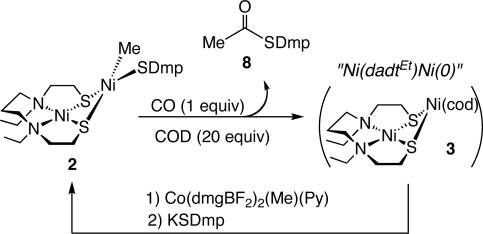
Because complex 9 obtained after treatment of 2 with excess CO does not react with methyl cobaloxime, the acetylthioester formation reported here is stoichiometric. However, the active Ni(II)–Ni(0) complex such as 3 can be regenerated when complex 2 is treated with 1 equiv of CO in the presence of 20 equiv of 1,5-cyclooctadiene. This significantly suppresses the formation of 9 and Ni(CO)4, and complex 2 and CH3C(O)SDmp (8) were obtained in 46% and 93% yields, respectively, upon treatment with methylcobalamin 5 and KSDmp (Scheme 6). According to these results, CO addition to the Nip site in ACS catalysis must be strictly regulated to prevent the formation of inactive Ni(0)-carbonyl species, such as 9, and therefore CO transfer may perhaps be controlled by opening and closing the tunnel connecting CODH and ACS (5, 6). The cystein thiolate of the [4Fe-4S] cluster located at the Nip site may be important for the regulation of CO coordination and promotion of reductive elimination of acetyl-CoA, a role played by 1,5-cyclooctadiene in our model reaction.
Scheme 6.
Concluding Remarks.
We have synthesized the dinuclear nickel complex Ni(dadtEt)Ni(Me)(SDmp) (2), having a methyl group and a thiolate, which is a good mimic of the structure of the dinuclear nickel site of the A-cluster, although the diamidodithiolate Cys-Gly-Cys ligand of the A-cluster is different from the diaminedithiolate dadtEt ligand. Complex 2 has been synthesized from the Ni(II)–Ni(0) species such as [Ni(dadtEt)Ni(cod)] (3) via methyl transfer from Co(dmgBF2)2(Me)Py (5), which models the reaction of the active state of the A-cluster with methylcobalamin. Notably an ACS model reaction was found to take place on the Nip site of 2, featuring CO insertion into the Ni–Me bond, successive reductive elimination of the acetylthioester, CH3C(O)SDmp (8), and generation of such Nid(II)–Nip(0) species. These results are consistent with an ACS mechanism, in which the Nip site in the A-cluster first takes up a Me moiety and CO insertion follows (12). However, the alternative mechanism, which involves initial CO coordination at Nip(I) and subsequent reaction with Me, remains to be examined (13). In either case, Nid is not the catalytic site but plays a structural role to hold the 2 bridging cysteinyl sulfurs of the Cys-Gly-Cys backbone in a favorable and perhaps flexible position for coordination to Nip (33–35).
Materials and Methods
All reactions and the manipulations were performed under nitrogen or argon atmospheres by using standard Schlenk techniques. Solvents were dried, degassed, and distilled from CaH2 (CH2Cl2), or from Mg turnings (methanol) under nitrogen. Hexane, ether, toluene, THF, and CH3CN were purified by columns of activated alumina and a supported copper catalyst supplied by Hansen.
All experimental procedures and details of characterization of complexes are given in SI Text. Molecular structures of 4, 6, 7, 10, and 11 are given in Figs. S1–S5, and selected bond distances and angles for 2, 4, 6-7, 9, 10-11 are listed in Tables S1–S5, respectively. Crystal data are summarized in Table S6.
Supplementary Material
Acknowledgments.
We are grateful to Prof. Roger E. Cramer for discussions and careful reading of the manuscript. This work was supported by Grants-in-Aid for Scientific Research 18GS0207 and 18065013 from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited with the Cambridge Crystallographic Data Centre, Cambridge CB2 1EK, U.K. (CSD accession nos. 716127–716134).
This article contains supporting information online at www.pnas.org/cgi/content/full/0900433106/DCSupplemental.
References
- 1.Lindahl PA, Graham DE. In: Nickel and Its Surprising Impact in Nature: Metal Ions in Life Science. Sigel A, Sigel H, Sigel RKO, editors. Vol 2. Chichester, UK: Wiley; 2007. pp. 357–411. [Google Scholar]
- 2.Ragsdale SW. Life with carbon monoxide. Crit Rev Biochem Mol Biol. 2004;39:165–195. doi: 10.1080/10409230490496577. [DOI] [PubMed] [Google Scholar]
- 3.Riordan CG. Acetyl coenzyme A synthase: New insights into one of nature's bioorganometallic catalysts. J Biol Inorg Chem. 2004;9:509–510. [Google Scholar]
- 4.Doukov T, Iverson TM, Seravalli J, Ragsdale SW, Drennan CL. A Ni-Fe-Cu center in a bifunctional carbon monoxide dehydrogenase/acetyl-CoA synthase. Science. 2002;298:567–572. doi: 10.1126/science.1075843. [DOI] [PubMed] [Google Scholar]
- 5.Darnault C, et al. Ni-Zn-[Fe4-S4] and Ni-Ni-[Fe4-S4] clusters in closed and open subunits of acetyl-CoA synthase/carbon monoxide dehydrogenase. Nat Struct Biol. 2003;10:271–279. doi: 10.1038/nsb912. [DOI] [PubMed] [Google Scholar]
- 6.Svetlitchnyi V, et al. A functional Ni-Ni-[4Fe-4S] cluster in the monomeric acetyl-CoA synthase from Carboxydothermus hydrogenoformans. Proc Natl Acad Sci USA. 2004;101:446–451. doi: 10.1073/pnas.0304262101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia J, Hu Z, Popescu CV, Lindahl PA, Münck E. Mössbauer and EPR study of the Ni-activated α-subunit of carbon monoxide dehydrogenase from Clostridium themoaceticum. J Am Chem Soc. 1997;119:8301–8312. [Google Scholar]
- 8.Bramlett MR, et al. Mössbauer and EPR study of recombinant acetyl-CoA synthase from Moorella thermoacetica. Biochemistry. 2006;45:8674–8685. doi: 10.1021/bi060003+. [DOI] [PubMed] [Google Scholar]
- 9.Lindahl PA. Acetyl-coenzyme A synthase: The case for a Nip0-based mechanism of catalysis. J Biol Inorg Chem. 2004;9:516–524. doi: 10.1007/s00775-004-0564-x. [DOI] [PubMed] [Google Scholar]
- 10.Tan X, Martinho M, Stubna A, Lindahl PA, Münck E. Mössbauer evidence for an exchange-coupled {[Fe4S4]1+ Nip1+} A-cluster in isolated α subunits of acetyl-coenzyme A synthase/carbon monoxide dehydrogenase. J Am Chem Soc. 2008;130:6712–6713. doi: 10.1021/ja801981h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gencic S, Grahame DA. Two separate one-electron steps in the reductive activation of the A cluster in subunit β of the ACDS complex in Methanosarcina thermophila. Biochemistry. 2008;47:5544–5555. doi: 10.1021/bi7024035. [DOI] [PubMed] [Google Scholar]
- 12.Tan X, Surovtsev IV, Lindahl PA. Kinetics of CO insertion and acetyl group transfer steps, and a model of the acetyl-CoA synthase catalytic mechanism. J Am Chem Soc. 2006;128:12331–12338. doi: 10.1021/ja0627702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George SJ, Seravalli J, Ragsdale SW. EPR and infrared spectroscopic evidence that a kinetically competent paramagnetic intermediate is formed when acetyl-coenzyme A synthase reacts with CO. J Am Chem Soc. 2005;127:13500–13501. doi: 10.1021/ja0528329. [DOI] [PubMed] [Google Scholar]
- 14.Hegg EL. Unraveling the structure and mechanism of acetyl-coenzyme A synthase. Acc Chem Res. 2004;37:775–783. doi: 10.1021/ar040002e. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, et al. Structure and electronic properties of an asymmetric thiolate-bridged binuclear complex: A model for the active site of acetyl CoA synthase. Chem Commun. 2003:3012–3013. doi: 10.1039/b310183e. [DOI] [PubMed] [Google Scholar]
- 16.Linck RC, Spahn CW, Rauchfuss TB, Wilson SR. Structural analogues of the bimetallic reaction center in acetyl CoA synthase: A Ni-Ni model with bound CO. J Am Chem Soc. 2003;125:8700–8701. doi: 10.1021/ja035606c. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan R, Riordan C. Cys-Gly-Cys tripeptide complexes of nickel: Binuclear analogues for the catalytic site in acetyl coenzyme A synthase. J Am Chem Soc. 2004;126:4484–4485. doi: 10.1021/ja038086u. [DOI] [PubMed] [Google Scholar]
- 18.Rao PV, Bhaduri S, Jiang J, Holm RH. Sulfur bridging interactions of cis-planar NiII-S2N2 coordination units with nickel(II), copper(I,II), zinc(II), and mercury(II): A library of bridging modes, including NiII(μ2-SR)2MI,II rhombs. Inorg Chem. 2004;43:5833–5849. doi: 10.1021/ic040055s. [DOI] [PubMed] [Google Scholar]
- 19.Harrop TC, Olmstead MM, Mascharak PK. Structural models of the bimetallic subunit at the A-cluster of acetyl coenzyme A synthase/CO dehydrogenase: Binuclear sulfur-bridged Ni-Cu and Ni-Ni complexes and their reactions with CO. J Am Chem Soc. 2004;126:14714–14715. doi: 10.1021/ja045284s. [DOI] [PubMed] [Google Scholar]
- 20.Duff SE, Barclay JE, Davies SC, Evans DJ. Homonuclear dinickel complexes: Structural mimics for the dinickel subsite of the A-cluster of acetyl-CoA synthase. Inorg Chem Commun. 2005;8:170–173. [Google Scholar]
- 21.Rao PV, Bhaduri S, Jiang J, Hong D, Holm RH. On [Fe4S4]2+-(μ2-SR)-MII bridge formation in the synthesis of an A-cluster analogue of carbon monoxide dehydrogenase/acetylcoenzyme A synthase. J Am Chem Soc. 2005;127:1933–1945. doi: 10.1021/ja040222n. [DOI] [PubMed] [Google Scholar]
- 22.Duff SE, Barclay JE, Davies SC, Hitchcock PB, Evans DJ. Synthesis and structural characterisation of group 10 homo- and hetero-dimetallic sulfur-bridged complexes of the [Ni(ema)(μ-S,S)M(diphos)](M = Ni, Pd, Pt) type. Eur J Inorg Chem. 2005:4527–4532. [Google Scholar]
- 23.Harrop TC, Olmstead MM, Mascharak PK. Synthetic analogues of the active site of the A-cluster of acetyl coenzyme A synthase/CO dehydrogenase: Syntheses, structures, and reactions with CO. Inorg Chem. 2006;45:3424–3436. doi: 10.1021/ic0520465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stavropoulos P, Carrie M, Muetterties MC, Holm RH. Structural and reaction chemistry of nickel complexes in relation to carbon monoxide dehydrogenase: A reaction system simulating acetyl-coenzyme A synthase activity. J Am Chem Soc. 1991;113:8485–8492. [Google Scholar]
- 25.Tucci GC, Holm RH. Nickel-mediated formation of thio esters from bound methyl, thiols, and carbon monoxide: A possible reaction pathway of acetyl-coenzyme A synthase activity in nickel-containing carbon monoxide dehydrogenases. J Am Chem Soc. 1995;117:6489–6496. [Google Scholar]
- 26.Kim Y-J, Osakada K, Sugita K, Yamamoto T, Yamamoto A. Preparation and properties of new methyl-(alkoxo)- and methyl-(thiolato)nickel and methyl(alkoxo)- and methyl(thiolato)-palladium complexes. Carbon monoxide and carbon disulfide insertion into the alkoxo-palladium bond. Organometallics. 1988;7:2182–2188. [Google Scholar]
- 27.Rampersad MV, et al. N2S2Ni metallothiolates as a class of ligands that support organometallic and bioorganometallic reactivity. Angew Chem Int Ed. 2005;44:1217–1220. doi: 10.1002/anie.200461747. [DOI] [PubMed] [Google Scholar]
- 28.Eckert NA, Doughety WG, Yap GPA, Riordan CG. Methyl transfer from methylcobaloxime to (triphos)Ni(PPh3): Relevance to the mechanism of acetyl coenzyme A synthase. J Am Chem Soc. 2007;129:9286–9287. doi: 10.1021/ja072063o. [DOI] [PubMed] [Google Scholar]
- 29.Dougherty WG, Rangan K, O'Hagan MJ, Yap GPA, Riordan CG. Binuclear complexes containing a methylnickel moiety: Relevance to organonickel intermediates in acetyl coenzyme A synthase catalysis. J Am Chem Soc. 2008;130:13510–13511. doi: 10.1021/ja803795k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito M, Kotera M, Song Y, Matsumoto T, Tatsumi K. Structural models for the active site of acetyl-CoA synthase: Synthesis of dinuclear nickel complexes having thiolate, isocyanide, and thiourea on the Nip site. Inorg Chem. 2009;48:1250–1256. doi: 10.1021/ic801967e. [DOI] [PubMed] [Google Scholar]
- 31.Song Y, Ito M, Kotera M, Matsumoto T, Tatsumi K. Cationic and anionic dinuclear nickel complexes [Ni(N2S2)Ni(dtc)]n (n = −1, +1) modeling the active site of acetyl-CoA synthase. Chem Lett. 2009;38:184–185. [Google Scholar]
- 32.Farmer PJ, Reibenspies JH, Lindahl PA, Darensbourg MY. Effects of sulfur site modification on the redox potentials of derivatives of [N,N′-bis(2-mercaptoethyl)-1,5-diazacyclooctanato]nickel(II) J Am Chem Soc. 1993;115:4665–4674. [Google Scholar]
- 33.Rampersad MV, et al. Characterization of steric and electronic properties of NiN2S2 complexes as S-donor metallodithiolate ligands. J Am Chem Soc. 2005;127:17323–17334. doi: 10.1021/ja055051g. [DOI] [PubMed] [Google Scholar]
- 34.Webster CE, Darensbourg MY, Lindahl PA, Hall MB. Structures and energetics of models for the active site of acetyl-coenzyme A synthase: Role of distal and proximal metals in catalysis. J Am Chem Soc. 2004;126:3410–3411. doi: 10.1021/ja038083h. [DOI] [PubMed] [Google Scholar]
- 35.Amara P, Volbeda A, Fontecilla-Camps JC, Field MJ. A quantum chemical study of the reaction mechanism of acetyl-coenzyme A synthase. J Am Chem Soc. 2005;127:2776–2784. doi: 10.1021/ja0439221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.