Abstract
Background
Crystalloid fluid resuscitation after hemorrhagic shock (HS) that restores/maintains central hemodynamics often culminates in multi-system organ failure and death due to persistent/progressive splanchnic hypoperfusion and end-organ damage. Adjunctive direct peritoneal resuscitation (DPR) using peritoneal dialysis solution reverses HS-induced splanchnic hypoperfusion and improves survival. We examined HS-mediated hepatic perfusion (galactose clearance), tissue injury (histopathology), and dysfunction (liver enzymes).
Methods
Anesthetized rats were randomly assigned (n=8/group): (1) sham (no HS); (2) HS (40% mean arterial pressure for 60 min) plus conventional i.v. fluid resuscitation (CR; shed blood + 2 volumes saline); (3) HS + CR + 30 mL intraperitoneal (IP) DPR; or (4) HS + CR + 30 mL IP saline. Hemodynamics and hepatic blood flow were measured for 2 h after CR completion. In duplicate animals, liver and splanchnic tissues were harvested for histopathology (blinded, graded), hepatocellular function (liver enzymes), and tissue edema (wet–dry ratio).
Results
Group 2 decreased liver blood flow, caused liver injuries (focal to submassive necrosis, zones 2 and 3) and tissue edema, and elevated liver enzymes (alanine aminotransferase (ALT), 149±28 μg/mL and aspartate aminotransferase (AST), 234±24 μg/mL; p<0.05) compared to group 1 (73±9 and 119±10 μg/mL, respectively). Minimal/no injuries were observed in group 3; enzymes were normalized (ALT 89±9 μg/mL and AST 150±17 μg/mL), and tissue edema was similar to sham.
Conclusions
CR from HS restored and maintained central hemodynamics but did not restore or maintain liver perfusion and was associated with significant hepatocellular injury and dysfunction. DPR added to conventional resuscitation (blood and crystalloid) restored and maintained liver perfusion, prevented hepatocellular injury and edema, and preserved liver function.
Keywords: Hemorrhagic shock, Direct peritoneal resuscitation, Liver blood flow, Liver injury
Introduction
Despite advances in treatment and therapies, hemorrhagic shock remains a major cause of morbidity and mortality following trauma.1 Management of hemorrhagic shock comprises bleeding control and correction of the vascular volume deficit with intravenous fluid replacement. The resuscitation process is clinically assessed by the restoration and maintenance of central hemodynamics. Recent evidence suggests that despite return of central hemodynamics by aggressive fluid resuscitation, the gut and liver experience a progressive vasoconstriction and hypoperfusion.2–4 This end-organ tissue hypoperfusion is linked to several mechanistic factors including endothelial cell dysfunction, compounding tissue hypoxia, compromised capillary filling and fluid exchange, deranged electrolyte handling, and the release of mediators that produce an exaggerated systemic inflammatory response.2,5–9 These factors result in cellular and end-organ tissue injury that translate clinically into organ dysfunction and, potentially, organ failure. The complexity of the pathogenesis of shock-induced end-organ tissue damage and multi-system organ failure necessitates a continued search for treatment modalities to prevent the course of hemorrhagic shock pathophysiology, alleviate end-organ damage, and improve survival in trauma patients.
Recent studies have shown that topical exposure on the small intestine with commercially available peritoneal dialysis solution during resuscitation from hemorrhagic shock, termed direct peritoneal resuscitation, can prevent or reverse the vasoconstriction and hypoperfusion commonly associated with conventional resuscitation.10 In those experimental studies, direct peritoneal resuscitation produced a rapid and sustained vasodilation and hyperperfusion of the gut regardless of the timing of direct peritoneal resuscitation (i.e., at the time of or delayed for 4 h after conventional volume replacement).10,11 Other studies have examined the effects of adjunctive direct peritoneal resuscitation by analyzing whole-organ blood flow distribution (colorimetric microsphere technique); the exaggerated systemic inflammatory cytokine response often associated with resuscitated hemorrhagic shock (cytokine enzyme-linked immunosorbent assays) and survival.12,13 Adjunctive peritoneal resuscitation caused splanchnic hyperperfusion that was associated with increased lung blood flow (>100% increase). These changes in organ blood flow distribution were associated with down-regulation of the systemic inflammatory response and increased survival compared to conventional resuscitation therapies.12,13 Hepatic artery blood flow was constant in those studies, in large part due to the dual liver blood supply which does not allow for the measurement of total liver blood flow. Microspheres are cleared from the arterial circulation via the gastrointestinal microvasculature, and thus the contribution of portal vein blood flow from the gastrointestinal circulation cannot be determined by the microsphere technique. The purpose of the current study was to focus on the effects of adjunctive direct peritoneal resuscitation from hemorrhagic shock on total effective liver blood flow as measured by galactose clearance, hepatocellular function, histopathology, and organ edema formation. We hypothesized that direct peritoneal resuscitation exposure in the peritoneal cavity would stabilize liver blood flow and prevent liver injury, in much the same manner that had been previously seen with direct peritoneal resuscitation-mediated protection of gut perfusion during resuscitated hemorrhagic shock.
Materials and Methods
Animals were maintained in a facility approved by the American Association for the Accreditation of Laboratory Animal Care. The research protocol was approved by the Institutional Animal Care and Use Committee and Biohazard Safety Committee at the Louisville Veterans Administration Medical Center. Male Sprague–Dawley rats (200–220 g) were acclimated for 1–2 weeks prior to experimental use during which time the animals received standard rat chow (20 g/day) and water ad libitum. Animal weights were recorded daily to ensure positive weight gain.
Surgery and Animal Preparation
All animal and experimental interventions were performed under aseptic conditions. Anesthesia was induced with intraperitoneal pentobarbital (50 mg/kg), and supplemental subcutaneous injections (25% of the original dose) were given as needed to maintain a surgical plane of anesthesia throughout the experimental protocol. After induction of anesthesia, 2 mL of normal saline was injected subcutaneously to maintain body fluid homeostasis during surgery and equilibration. Body temperature was maintained at 37±1°C with a rectal probe and a servo-controlled heating pad. Surgery was carried out after the loss of blink and withdrawal reflexes. A tracheotomy was performed (Intramedic PE-240 polyethylene tubing, Clay Adams Division of Becton Dickinson & Company, Parsipanny, NJ, USA), and animals were allowed to spontaneously breathe room air. The right femoral artery and vein and the left femoral artery were cannulated with PE-50 catheters for blood pressure measurement, blood withdrawal, and resuscitation. Animals were allowed to equilibrate for 45–60 min following the completion of surgery. Mean arterial pressure (MAP) and heart rate (HR) were continuously monitored and recorded every 15 min throughout the experimental protocol (Digi-Med Signal Analyzers, Louisville, KY, USA). All animals had repeated baseline MAP and HR values within 10% prior to initiation of shock protocol.
After equilibration, animals were randomly assigned to undergo hemorrhage and resuscitation or sham protocol as outlined in the experimental groups. The end point for all groups was 2 h after completion of resuscitation. Two sets of animals for each experimental group were completed, one set (n=8/group) for liver blood flow determination by galactose clearance and a second set (n=6/group) for the measurement of liver enzymes, histopathology, and tissue edema. At the 2-h post-resuscitation (RES) time point in the second set of animals, serum was obtained from arterial blood samples for liver function tests (i.e., aspartate aminotransferase (AST) and alanine aminotransferase, (ALT)) using commercially available kits (Sigma Chemical Company, St. Louis, MO, USA). Tissue samples of liver, lung, and abdominal muscle were harvested, immediately weighed (wet weight), and then dried in an oven at 50°C until constant weight was obtained (dry weight). Tissue wet weight to dry weight ratio was calculated to serve as an index of total tissue water. Separate liver specimens were collected for histopathological staining (hematoxylin and eosin (H&E)). Each tissue sample was processed in triplicate for later blinded analysis.
Hemorrhagic Shock Model
The standard model of resuscitated hemorrhagic shock we utilized has been previously described.2 Briefly, hemorrhagic shock was achieved with blood withdrawal (1 mL/min) from the femoral artery into a syringe pre-rinsed with heparin until 40% of baseline MAP was attained. Hypovolemia was maintained for 60 min with blood withdrawal or return to maintain the 40% MAP. On average, the hemorrhage volume required to maintain the target MAP was 6.11 mL. Conventional resuscitation was initiated with the return of the shed blood via the femoral vein over 5 min, followed by normal saline infusion of two times the volume of shed blood over the next 25 min. Adjunctive direct peritoneal resuscitation was initiated at the start of intravenous fluid resuscitation with intraperitoneal injection of 30 mL of 2.5% glucose-based clinical peritoneal dialysis solution (Delflex®, Fresenius USA, Inc. Ogden, UT, USA) that contained 5.67 g/L sodium chloride, 3.92 g/L sodium lactate, 0.257 g/L calcium chloride, 0.152 g/L magnesium chloride at a pH of 5.5, and osmolality of 398 mOsm/L. As a volume control for adjunctive direct peritoneal resuscitation, 30 mL of normal saline was injected intraperitoneally immediately following the initiation of intravenous fluid resuscitation in a separate group of animals. All peritoneal resuscitation solutions were pre-warmed to 37°C prior to injection.
Animal Groups
As already mentioned, two sets of experiments were performed in each of the following groups: group 1, sham animals which underwent surgical cannulations but no hemorrhagic shock or resuscitation; group 2, hemorrhagic shock plus conventional i.v. fluid resuscitation; group 3, hemorrhagic shock plus conventional i.v. fluid resuscitation and 30 mL of peritoneal dialysis solution injected intraperitoneally; and group 4, hemorrhagic shock plus conventional i.v. fluid resuscitation and 30 mL of normal saline injected intraperitoneally. All animals in groups 3 and 4 were checked at the end of the experimental protocol for peritoneal bleeding, which was an exclusion criterion for the study.
Liver Blood Flow Determination
The galactose clearance method has been used to assess effective hepatic blood flow in both experimental animals and humans.23 The assumptions inherent in this technique are that systemic galactose is solely metabolized and thus cleared from the plasma by the liver, and therefore, the steady-state galactose clearance accurately measures liver blood flow. To measure effective hepatic blood flow (EHBF), a steady-state galactose concentration was obtained by the bolus infusion of galactose (2.6 mg/1 mL/5 min) via the femoral vein catheter followed by a constant infusion of galactose (13 mg/mL/h). Steady-state systemic galactose concentration was achieved in 30–40 min after the initial bolus, verified with two successive blood samples (0.2 mL each) collected at 40 and 55 min after bolus. When the variation between samples was less than 10%, the experimental protocol was initiated. No rats were excluded due to unstable steady-state galactose concentrations. EHBF was determined every 30 min throughout the protocol by blood galactose determination in triplicate. EHBF was calculated by measuring low steady-state galactose concentration (GCss) at a known infusion rate (I) as described by the equation EHBF=I/GCss.
Histology Scoring
H&E-stained liver specimens were evaluated for signs of liver injury according to a predetermined tissue injury score based on previously published injury score criteria in blinded fashion.14,15 Specimens with no or minimal injury were scored 0, focal necrosis scored 1, centrilobular necrosis (zone 3) scored 2, submassive necrosis (zone 2 and 3) scored 3, and massive necrosis scored 4. Each sample was identified only by number such that the pathologists were blinded to the experimental protocol and animal groups. Two pathologists (M.E.C. and J.R.P) evaluated each tissue specimen independently and scored the tissue using the scoring system.
Statistical Analysis
Data are expressed as mean±standard error of the mean, and differences between groups were determined by two-way analysis of variance (ANOVA). The null hypothesis was rejected a priori at P<0.05. When differences were found using ANOVA, one of the following post hoc tests was performed (as indicated in the figure legends): Tukey–Kramer honestly significant different test, Bonferroni's test, or repeated measures ANOVA and Dunnett's test.
Results
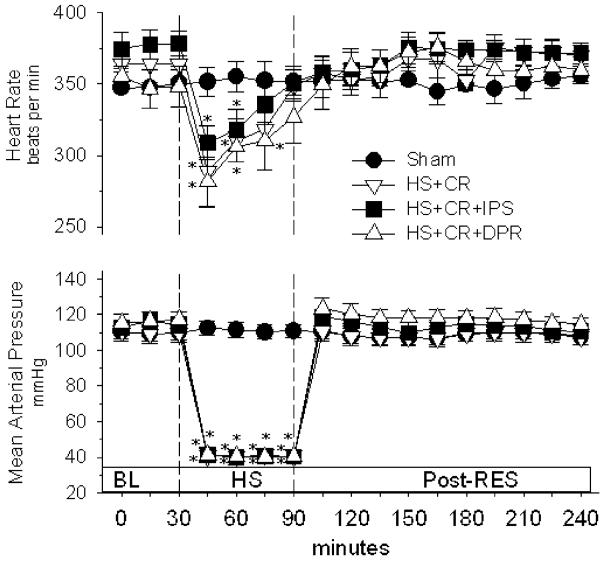
Figure 1 shows that there were no differences between groups in mean arterial blood pressure or pulse rate during the post-resuscitation period. In the hemorrhagic shock groups, mean arterial pressure was held at 40% of baseline during the 60-min hypovolemic period per the experimental protocol. Conventional resuscitation restored and maintained mean arterial pressure at baseline levels for all hemorrhagic shock groups without additional fluid infusion. Heart rate was decreased during in all hemorrhage groups during hypovolemia, which was completely reversed by resuscitation in groups 2–4.
Figure 1.
Heart rate and mean arterial pressure. BL baseline; groups: Sham surgical cannulations but no hemorrhage shock or resuscitation; HS + CR hemorrhagic shock plus conventional intravenous fluid resuscitation; HS + CR + DPR hemorrhagic shock and resuscitation plus 30 mL of clinical peritoneal dialysis solution (Delflex™) intraperitoneally at the time of resuscitation; HS + CR + IPS hemorrhagic shock and resuscitation plus 30 mL of normal saline intraperitoneally at the time of resuscitation. *p<0.01 versus corresponding baseline by repeated measures ANOVA and Dunnett's post-test.
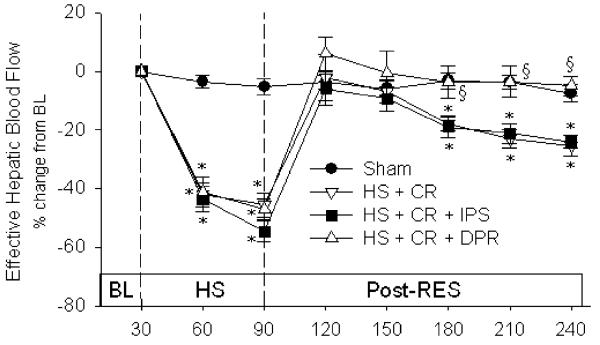
There were no differences between groups in baseline liver blood flow (Fig. 2). During the period of hypovolemia, effective hepatic blood flow was reduced approximately 40–45% compared to group baseline values in the three hemorrhage groups: conventional resuscitation (3.39±0.29 mL/min/100 g; −43%), saline (3.29±0.17; −44%), and direct peritoneal resuscitation (3.54±0.30, −41%), and these levels were also significantly lower than sham animals (5.70±0.29 mL/min/100 g). All resuscitation protocols restored effective hepatic blood flow to baseline levels at the completion of resuscitation. However, the conventional resuscitation (group 2) and IP saline (group 4) rats displayed a slow, progressive decline in effective hepatic blood flow that was not observed in the direct peritoneal resuscitation group (group 3). Direct peritoneal resuscitation rats had normalized effective hepatic blood flow throughout the 2-h post-resuscitation period. At the 30-min end point of resuscitation, effective hepatic blood flow (mL/min/100 g) was 4.35±0.21 in conventional resuscitation (p<0.05 versus direct peritoneal resuscitation), 4.52±0.30 in IP saline (p<0.05 versus direct peritoneal resuscitation), 5.74±0.26 in direct peritoneal resuscitation, and 5.20±0.27 in sham animals.
Figure 2.
Effective hepatic blood flow. Groups: Sham surgical cannulations but no hemorrhage shock or resuscitation; HS + CR hemorrhagic shock plus conventional intravenous fluid resuscitation; HS + CR + DPR hemorrhagic shock and resuscitation plus 30 mL of clinical peritoneal dialysis solution (Delflex™) intraperitoneally at the time of resuscitation; HS + CR +IPS hemorrhagic shock and resuscitation plus 30 mL of normal saline intraperitoneally at the time of resuscitation. Baseline values in mL/min/100 g body weight were: Sham, 5.6±0.3; HS + CR, 6.3±0.5; HS + CR + IPS, 5.9±0.4; and HS + CR + DPR, 6.1±0.4. *p<0.01 versus corresponding baseline by repeated-measures ANOVA and Dunnett's post-test. Section sign p<0.01 versus the HS + CR group by two-way ANOVA and Bonferroni post-test.
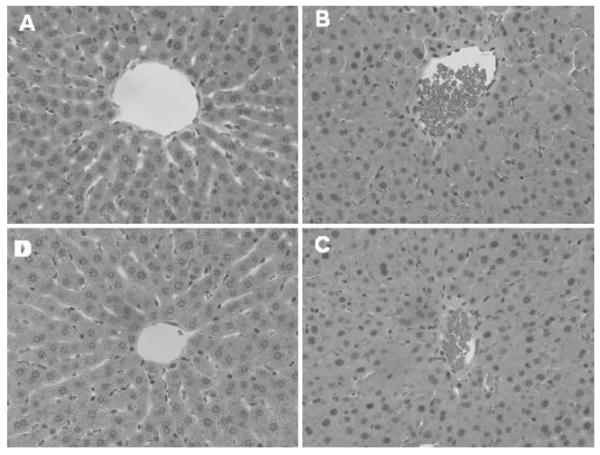
Histopathologic scoring of the sham (group 1) liver specimens revealed no/minimal injury. Fifty percent (three of six) of IP saline (group 4) liver specimens were scored as having focal or centrilobular necrosis. Similarly, the conventional resuscitation rats (group 2) had 50% (3/6) of livers scored as having focal, centrilobular, or submassive necrosis. The direct peritoneal resuscitation group (group 3) had a decreased incidence of microscopic liver injury compared to the other hemorrhagic shock groups. In the direct peritoneal resuscitation liver specimens, only one in six was scored as having focal necrosis, the remaining specimens (5/6) were scored as no/minimal injury. Representative liver micrographs are depicted in Fig. 3. As seen in the micrographs, liver architectural pattern was lost in the conventional resuscitation and IP saline groups due to near obliteration of the sinusoids, presumably due to edema in endothelial cells and hepatocytes. Multiple areas of focal necrosis (zones 2 and 3) along with stasis and red blood cell aggregation in central veins were seen in the conventional resuscitation (group 2) and IP saline (group 4) specimens.
Figure 3.
Liver histopathology (blinded scoring). a Sham; b HS + CR; c HS + CR + IPS; d HS + CR + DPR. HS hemorrhagic shock, CR conventional intravascular fluid resuscitation, IPS intraperitoneal saline (30 mL) at time of the fluid resuscitation, DPR adjunct direct peritoneal resuscitation (30 mL Delflex™ with 2.5% glucose) at the time of the fluid resuscitation.
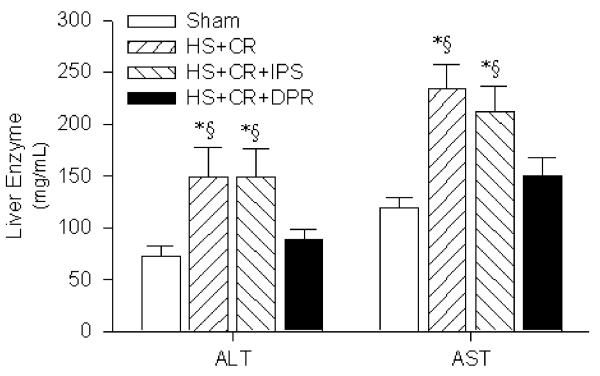
Direct peritoneal resuscitation-mediated histopathological changes in conventional resuscitation and IP saline groups correlated with impaired hepatocellular function as assessed by liver enzyme assays at 120 min post-RES (Fig. 4). Conventional resuscitation liver enzyme levels (μg/mL) were significantly elevated (ALT 149±28 and AST 234±24) compared to sham rats (73±9 and 119±10, respectively). Adjunct peritoneal resuscitation restored liver enzyme levels to near sham control levels (ALT 89±9 and AST 150±17). However, peritoneal saline installation in the IP saline rats did not improve liver enzymes (ALT 149± 27 and AST 212±25).
Figure 4.
Liver function test. Groups: Sham surgical cannulations but no hemorrhage shock or resuscitation; HS + CR hemorrhagic shock plus conventional intravenous fluid resuscitation; HS + CR + DPR hemorrhagic shock and resuscitation plus 30 mL of clinical peritoneal dialysis solution (Delflex™) intraperitoneally at the time of resuscitation; HS + CR + IPS hemorrhagic shock and resuscitation plus 30 mL of normal saline intraperitoneally at the time of resuscitation. *p<0.01 versus Sham control by one-way ANOVA and Bonferroni post-test. Section sign p<0.01 versus the HS + CR group by two-way ANOVA and Bonferroni post-test.
Finally, tissue edema (total tissue water) was assessed by wet–dry ratios (Fig. 5) at the 120-min resuscitation end point. Total tissue water of all organs investigated was significantly greater in the conventional fluid resuscitation (group 2) and IP saline (group 4) rats compared to the peritoneal resuscitation (group 3) or sham control (group 1), suggesting significant compartmental fluid shifts and total water sequestration in the conventional resuscitation and IP saline groups compared to shams, which was prevented in the direct peritoneal resuscitation rats.
Figure 5.
Total tissue water. AAM anterior abdominal muscle; Groups: Sham surgical cannulations but no hemorrhage shock or resuscitation; HS + CR hemorrhagic shock plus conventional intravenous fluid resuscitation; HS + CR + DPR hemorrhagic shock and resuscitation plus 30 mL of clinical peritoneal dialysis solution (Delflex™) intraperitoneally at the time of resuscitation; HS + CR + IPS hemorrhagic shock and resuscitation plus 30 mL of normal saline intraperitoneally at the time of resuscitation. *p<0.01 versus Sham control by one-way ANOVA and Bonferroni post-test. Section sign p<0.01 versus the HS + CR group by two-way ANOVA and Bonferroni post-test.
Discussion
Liver dysfunction and failure associated with resuscitated hemorrhagic shock is a significant clinical problem that is driven by ischemic stress. This ischemic stress is thought to be mediated by multiple factors including pro-inflammatory cytokines and chemokines, reactive oxygen species, and eicosanoids, which in combination cause hepatic microcirculatory dysfunction, leukocyte infiltration, damage to cell membranes, development of fibrosis, and stasis of biliary flow. It is proposed that three major pathophysiological events occur during intravenous volume resuscitated hemorrhagic shock: (1) persistent liver and gut vasoconstriction and hypoperfusion despite restoration of central hemodynamic variables; (2) a liver- and gut-derived inflammatory response; and (3) tissue fluid sequestration and failure of early fluid mobilization within the intestinal fluid compartment. The addition of adjunctive direct peritoneal resuscitation with glucose-based peritoneal dialysis solutions to a volume replacement resuscitation strategy has been shown to improve or prevent these changes in the gut8–10,17,18 and perhaps the liver.
Liver injury and dysfunction constitute a driving component of multi-system organ failure following resuscitation from hemorrhagic shock. Hepatocellular and sinusoidal endothelial cell dysfunction occurs early during hemorrhage and persists despite adequate fluid resuscitation.16–21 The pathogenesis of end-organ damage appears to be initiated and maintained by a cascade of events involving multiple mediators and pathways (i.e., pro-inflammatory cytokines, reactive oxygen radicals, lipooxygenase derivatives, intracellular Ca2+ signaling, and hypoxia). A central event that could initiate such interactions might be compromised hepatic nutritive blood flow during hemorrhage and resuscitation. The current study supports this idea because restoration of hepatic blood flow by adjunctive direct peritoneal resuscitation was associated with improved hepatocellular function, tissue edema, and histopathological tissue injury score. Post-resuscitation splanchnic hypoperfusion occurs following correction of the intravascular volume deficit and accounts for the portal component of the deficit in nutrient hepatic flow.22–26 In addition, endothelial cell dysfunction appears to play a role in gut hypoperfusion since protection of endothelial cell function through pharmacologic means either before or during resuscitation was associated with normal end-organ blood flow.22,27–31 Thus, direct peritoneal resuscitation appears to maintain hepatic nutrient blood flow through mechanisms that preserve gut perfusion and subsequent portal flow.
Direct peritoneal resuscitation is a nonpharmacologic adjunctive resuscitation strategy to reverse splanchnic end-organ hypoperfusion by endothelium-dependent mechanisms. In the intestinal microvasculature, these mechanisms are thought to include activation of glibenclamide-sensitive K+ channels (KATP), adenosine A1 receptor activation, and nitric oxide release.32 Additional therapeutic benefits of adjunctive peritoneal resuscitation that would preserve hepatic perfusion and function include early fluid mobilization of gut tissue edema, prevention of endothelial cell swelling, and the down-regulation of the gut-associated systemic inflammatory response.12,33,34 Of particular significance to end-organ tissue perfusion and function is hemorrhage-induced endothelial cell swelling and interstitial edema.33 Endothelial cell swelling appears to occur early during hemorrhagic shock by activation of amiloride-sensitive (Na+/H+ channels) and the rapid exchange of intracellular H+ for extracellular Na+ and water.33,35 The resultant narrowing of the end-organ tissue capillaries in the gut and presumably in the sinusoids could physically impede filling and compromise liver nutritive perfusion.
The mechanism of hepatic injury after hemorrhage and resuscitation is not fully understood but has been extrapolated from ischemia-reperfusion models.36 It has been proposed that, during ischemia-reperfusion injury, neutrophil hepatotoxicity causes liver injury and dysfunction.37 Neutrophil-mediated injury involves chemokine-mediated neutrophil extravasation and subsequent interstitial degranulation. Our studies have demonstrated that liver myeloperoxidase levels, an index of neutrophil sequestration, remained low during the first 24 h following resuscitation from hemorrhagic shock.34 These studies did not address mechanisms of neutrophil-mediated hepatocellular injury but suggest that neutrophil hepatotoxicity is not the predominant mechanism of liver injury in this model of hemorrhage resuscitation.
The gut and liver have been implicated as the primary source of cytokines following hemorrhagic shock.38–41 Pro-inflammatory cytokines (TNF-α, IL-1, IL-6, IFN-γ) are released following trauma and shock and activate the cellular immune response, while anti-inflammatory cytokines (IL-4, IL-10) appear to modulate this pro-inflammatory response. The degree of activation of the systemic inflammatory response syndrome response and subsequent immunocompetence depends on the balance of the two processes and the pattern of cytokine expression. Direct peritoneal resuscitation has been shown to down-regulate the pro-inflammatory response noted in the liver and gut with volume resuscitation alone by decreasing IL-6 and TNF-α 24 h after hemorrhagic shock.12 At the same time, IL-10 levels were significantly increased with direct peritoneal resuscitation. In this study, mortality correlated with the cytokine patterns ranging from 10% to 40% in the resuscitated groups versus 100% survival in the direct peritoneal resuscitation animals. The mechanisms behind the cytokine pattern presumably relate to persistent ischemic changes in the gut which lead to loss of mucosal barrier integrity and the release of pro-inflammatory cytokines. direct peritoneal resuscitation down-regulates the pro-inflammatory response as a result of maintaining perfusion of the liver and gut during resuscitation.
In conclusion, the present data demonstrate that conventional volume resuscitation from hemorrhagic shock that restores and maintains central hemodynamics does not restore or maintain effective hepatic blood flow and is associated with significant hepatocellular injury and dysfunction. Adjunctive direct peritoneal resuscitation initiated during resuscitation maintains effective hepatic blood flow, prevents hepatocellular injury, and improves liver function.
Acknowledgments
Grant support: This project was supported by a VA Merit Review grant and by NIH research Grant # 5R01 HL076160-03, funded by the National Heart, Lung, and Blood Institute and the United States Army Medical Resources and Material Command.
Footnotes
Presented at the Digestive Disease Week, American Association for the Study of Liver Diseases, Los Angeles, CA, USA, May 2006.
No conflicts of interest exist.
References
- 1.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417–422. doi: 10.1097/00024382-199812000-00007. doi:10.1097/00024382-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Wang P, Hauptman JG, Chaudry IH. Hemorrhage produces depression in microvascular blood flow which persists despite fluid resuscitation. Circ Shock. 1990;32:307–318. [PubMed] [Google Scholar]
- 4.Zakaria ER, Spain DA, Harris PD, Garrison RN. Resuscitation regimens for hemorrhagic shock must contain blood. Shock. 2002;18:567–573. doi: 10.1097/00024382-200212000-00014. doi:10.1097/00024382-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Deitch EA, Xu D, Franko L, Ayala A, Chaudry IH. Evidence favoring the role of the gut as a cytokine-generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–145. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Wang P, Hauptman JG, Chaudry IH. Hepatocellular dysfunction occurs early after hemorrhage and persists despite fluid resuscitation. J Surg Res. 1990;48:464–470. doi: 10.1016/0022-4804(90)90014-s. [DOI] [PubMed] [Google Scholar]
- 7.Wang P, Ba ZF, Chaudry IH. Endothelial cell dysfunction occurs very early following trauma-hemorrhage and persists despite fluid resuscitation. Am J Physiol. 1993;265:H973–H979. doi: 10.1152/ajpheart.1993.265.3.H973. [DOI] [PubMed] [Google Scholar]
- 8.Zakaria ER, Garrison RN, Spain DA, Harris PD. Impairment of endothelium-dependent dilation response after resuscitation from hemorrhagic shock involved postreceptor mechanisms. Shock. 2004;21:175–181. doi: 10.1097/00024382-200402000-00014. doi:10.1097/00024382-200402000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Zweifach BW. Mechanisms of blood flow and fluid exchange in microvessels: hemorrhagic hypotension model. Anesthesiology. 1974;41:157–168. doi: 10.1097/00000542-197408000-00006. doi:10.1097/00000542-197408000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Zakaria ER, Garrison RN, Spain DA, Matheson PJ, Harris PD, Richardson JD. Intraperitoneal resuscitation improves intestinal blood flow following hemorrhagic shock. Ann Surg. 2003;237:704–711. doi: 10.1097/01.SLA.0000064660.10461.9D. doi:10.1097/00000658-200305000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zakaria ER, Garrison RN, Kawabe T, Harris PD. Direct peritoneal resuscitation from hemorrhagic shock: effect of time delay in therapy initiation. J Trauma. 2005;58:499–506. doi: 10.1097/01.TA.0000152892.24841.54. doi:10.1097/01. TA.0000152892.24841.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrison RN, Conn AA, Harris PD, Zakaria ER. Direct peritoneal resuscitation as adjunct to conventional resuscitation from hemorrhagic shock: a better outcome. Surgery. 2004;136:900–908. doi: 10.1016/j.surg.2004.06.027. doi:10.1016/j.surg.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Zakaria ER, Hurt RT, Matheson PJ, Garrison RN. A novel method of peritoneal resuscitation improves organ perfusion after hemorrhagic shock. Am J Surg. 2003;186:443–448. doi: 10.1016/j.amjsurg.2003.07.006. doi:10.1016/j.amjsurg.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Chojkier M, Fierer J. D-Galactosamine hepatotoxicity is associated with endotoxin sensitivity and mediated by lymphoreticular cells in mice. Gastroenterology. 1985;88:115–121. doi: 10.1016/s0016-5085(85)80142-6. [DOI] [PubMed] [Google Scholar]
- 15.Shiratori Y, Kawase T, Shiina S, Okano K, Sugimoto T, Teraoka H, Matano S, Matsumoto K, Kamii K. Modulation of hepatotoxicity by macrophages in the liver. Hepatology. 1988;8:815–821. doi: 10.1002/hep.1840080420. doi:10.1002/hep.1840080420. [DOI] [PubMed] [Google Scholar]
- 16.Wang P, Ba ZF, Biondo A, Chaudry I. Liver endothelial cell dysfunction occurs early following hemorrhagic shock and persists despite crystalloid resuscitation. J Surg Res. 1996;63:241–247. doi: 10.1006/jsre.1996.0255. doi:10.1006/jsre.1996.0255. [DOI] [PubMed] [Google Scholar]
- 17.Bauer M, Marzi I, Ziegenfuss T, Seeck G, Buhren V, Larsen R. Comparative effects of crystalloid and small volume hypertonic hyperoncotic fluid resuscitation on hepatic microcirculation after hemorrhagic shock. Circ Shock. 1993;40:187–193. [PubMed] [Google Scholar]
- 18.Corso CO, Okamoto S, Leiderer R, Messmer K. Resuscitation with hypertonic saline dextran reduces endothelial cell swelling and improves hepatic microvascular perfusion and function after hemorrhagic shock. J Surg Res. 1998;80:210–220. doi: 10.1006/jsre.1998.5426. doi:10.1006/jsre.1998.5426. [DOI] [PubMed] [Google Scholar]
- 19.Dart RC, Liebler DC, Sipes IG. Hepatic injury and lipid peroxidation during hemorrhagic shock and resuscitation. Life Sci. 1993;53:1685–1690. doi: 10.1016/0024-3205(93)90205-h. doi:10.1016/0024-3205(93)90205-H. [DOI] [PubMed] [Google Scholar]
- 20.Holliday RL, Illner HP, Shires GT. Liver cell membrane alterations during hemorrhagic shock in the rat. J Surg Res. 1981;31:506–515. doi: 10.1016/0022-4804(81)90189-x. doi:10.1016/0022-4804(81)90189-X. [DOI] [PubMed] [Google Scholar]
- 21.Jarrar D, Wang P, Cioffi WG, Bland KI, Chaudry IH. Critical role of oxygen radicals in the initiation of hepatic depression after trauma hemorrhage. J Trauma. 2000;49:879–885. doi: 10.1097/00005373-200011000-00015. doi:10.1097/00005373-200011000-00015. [DOI] [PubMed] [Google Scholar]
- 22.Flynn WJ, Cryer HG, Garrison RN. Pentoxifylline restores intestinal microvascular blood flow during resuscitated hemorrhagic shock. Surgery. 1991;110:350–356. [PubMed] [Google Scholar]
- 23.Flynn WJ, Cryer HG, Garrison RN. Pentoxifylline but not saralasin restores hepatic blood flow after resuscitation from hemorrhagic shock. J Surg Res. 1991;50:616–621. doi: 10.1016/0022-4804(91)90051-m. doi:10.1016/0022-4804(91)90051-M. [DOI] [PubMed] [Google Scholar]
- 24.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Selective microvascular endothelial cell dysfunction in the small intestine following resuscitated hemorrhagic shock. Shock. 1998;10:417–422. doi: 10.1097/00024382-199812000-00007. doi:10.1097/00024382-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Wang P, Hauptman JG, Chaudry IH. Hemorrhage produces depression in microvascular blood flow which persists despite fluid resuscitation. Circ Shock. 1990;32:307–318. [PubMed] [Google Scholar]
- 26.Zakaria ER, Spain DA, Harris PD, Garrison RN. Resuscitation regimens for hemorrhagic shock must contain blood. Shock. 2002;18:567–573. doi: 10.1097/00024382-200212000-00014. doi:10.1097/00024382-200212000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Flynn WJ, Jr, Gosche JR, Garrison RN. Intestinal blood flow is restored with glutamine or glucose suffusion after hemorrhage. J Surg Res. 1992;52:499–504. doi: 10.1016/0022-4804(92)90318-t. doi:10.1016/0022-4804(92)90318-T. [DOI] [PubMed] [Google Scholar]
- 28.Flynn WJ, Jr, Pilati D, Hoover EL. Effect of allopurinol on venous endothelial dysfunction after resuscitated hemorrhagic shock. Int J Surg Investig. 1999;1:11–18. [PubMed] [Google Scholar]
- 29.Fruchterman TM, Spain DA, Wilson MA, Harris PD, Garrison RN. Complement inhibition prevents gut ischemia and endothelial cell dysfunction after hemorrhage/resuscitation. Surgery. 1998;124:782–791. doi: 10.1067/msy.1998.91489. doi:10.1067/msy.1998.91489. [DOI] [PubMed] [Google Scholar]
- 30.Wang P, Ba ZF, Chaudry IH. Endothelial cell dysfunction occurs after hemorrhage in nonheparinized but not in preheparinized models. J Surg Res. 1993;54:499–506. doi: 10.1006/jsre.1993.1077. doi:10.1006/jsre.1993.1077. [DOI] [PubMed] [Google Scholar]
- 31.Watkins JM, Spain DA, Krysztopik RJ, Downard PJ, Wilson MA, Garrison RN. Heparan preserves intestinal perfusion after hemorrhage and resuscitation. J Surg Res. 1996;66:154–158. doi: 10.1006/jsre.1996.0388. doi:10.1006/jsre.1996.0388. [DOI] [PubMed] [Google Scholar]
- 32.Zakaria ER, Li N, Garrison RN. Mechanisms of direct peritoneal resuscitation-mediated splanchnic hyperperfusion following hemorrhagic shock. Shock. 2007;27:436–442. doi: 10.1097/01.shk.0000245017.86117.4e. doi:10.1097/01.shk.0000245017.86117.4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zakaria ER, Li N, Matheson PJ, Garrison RN. Cellular edema regulates tissue capillary perfusion after hemorrhage resuscitation. Surgery. 2007;142:487–496. doi: 10.1016/j.surg.2007.08.007. doi:10.1016/j.surg.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zakaria ER, Campbell JE, Peyton JC, Garrison RN. Postresuscitation tissue neutrophil infiltration is time-dependent and organ-specific. J Surg Res. 2007;143:119–125. doi: 10.1016/j.jss.2007.04.008. doi:10.1016/j.jss.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzoni MC, Intaglietta M, Cragoe EJ, Jr, Arfors KE. Amiloride-sensitive Na+ pathways in capillary endothelial cell swelling during hemorrhagic shock. J Appl Physiol. 1992;73:1467–1473. doi: 10.1152/jappl.1992.73.4.1467. [DOI] [PubMed] [Google Scholar]
- 36.Chang JX, Chen S, Ma LP, Jiang LY, Chen JW, Chang RM, Wen LQ, Wu W, Jiang ZP, Huang ZT. Functional and morphological changes of the gut barrier during the restitution process after hemorrhagic shock. World J Gastroenterol. 2005;11:5485–5491. doi: 10.3748/wjg.v11.i35.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jaeschke H. Mechanisms of Liver Injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemiareperfusion and other acute inflammatory conditions. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1083–G1088. doi: 10.1152/ajpgi.00568.2005. doi:10.1152/ajpgi.00568.2005. [DOI] [PubMed] [Google Scholar]
- 38.Chang TW. Improvement of survival from hemorrhagic shock by enterectomy in rats: finding to implicate the role of the gut for irreversibility of hemorrhagic shock. J Trauma. 1997;42:223–230. doi: 10.1097/00005373-199702000-00007. doi:10.1097/00005373-199702000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. doi:10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Matsutani T, Kang SC, Miyashita M, Sasajima K, Choudhry MA, Bland KI, Chaudry IH. Liver cytokine production and ICAM-1 expression following bone fracture, tissue trauma, and hemorrhage in middle-aged mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G268–G274. doi: 10.1152/ajpgi.00313.2006. doi:10.1152/ajpgi.00313.2006. [DOI] [PubMed] [Google Scholar]
- 41.Roumen RM, Hendriks T, Van der Ven-Jongekrijg J, Nieuwenhuijzen GA, Sauerwein RW, van der Meer JW, Goris RJ. Cytokine patterns in patients after major vascular surgery, hemorrhagic shock, and severe blunt trauma. Relation with subsequent adult respiratory distress syndrome and multiple organ failure. Ann Surg. 1993;218:769–776. doi: 10.1097/00000658-199312000-00011. doi:10.1097/00000658-199312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]