Abstract
Background and Objectives
Previous studies have concluded that transforaminal epidural steroid injections (ESIs) are more effective than interlaminar injections in the treatment of radiculopathies due to lumbar intervertebral disk herniation. There are no published studies examining the depth of epidural space using a transforaminal approach. We investigated the relationship between body mass index (BMI) and the depth of the epidural space during lumbar transforaminal ESIs.
Methods
Eighty-six consecutive patients undergoing lumbar transforaminal ESI at the L3-L4, L4-L5, and L5-S1 levels were studied. Using standard protocol, the foraminal epidural space was attained using fluoroscopic guidance. The measured distance from needle tip to skin was recorded (depth to foraminal epidural space). The differences in the needle depth and BMI were analyzed using regression analysis.
Results
Needle depth was positively associated with BMI (regression coefficient [RC], 1.13; P < 0.001). The median depths (in centimeters) to the epidural space were 6.3, 7.5, 8.4, 10.0, 10.4, and 12.2 for underweight, normal, preobese, obese I, obese II, and obese III classifications, respectively. Sex (RC, 1.3; P = 0.02) and race (RC, 0.8; P = 0.04) were also significantly associated with needle depth; however, neither factor remained significant when BMI was accounted as a covariate in the regression model. Age, intervertebral level treated, and oblique angle had no predictive value on foraminal depth (P > 0.2).
Conclusion
There is a positive association between BMI and transforaminal epidural depth, but not with age, sex, race, oblique angle, or intervertebral level.
Epidural steroid injections (ESIs) have long been used as a nonoperative treatment of low back pain and radiculopathy and are the most commonly performed procedure in pain medicine clinics in the United States.1–5 The distribution of steroid has been shown to be highly variable during interlaminar epidural injections because of a variety of factors, including needle position and history of back surgery.6 Previous studies have concluded that transforaminal ESIs are more effective than the traditional interlaminar approach in the treatment of radiculopathies due to lumbar intervertebral disk herniation.7,8 The improved efficacy is thought to be due to deposition of the steroid in the ventral epidural space near the disk herniation and concentration of the injectate in the area of pathology.4,7,9,10
There have been several studies investigating the depth from skin to the epidural space in interlaminar lumbar,11–16 thoracic,17–19 and cervical20,21 epidurals. Lumbar epidural depth studies have included obstetric13,14,16,22 and nonobstetric11,12,15 patient populations. Most studies found a correlation between body mass index (BMI) and depth of epidural space.11–14,16,22 Obesity is an increasing problem throughout the world and will certainly continue to affect clinical practice.23
Currently, there are no published studies of the depth of epidural space using a transforaminal approach. The use of an inappropriately short needle requires repeating the procedure with a longer needle and may cause increased procedural risk and patient discomfort. However, larger needles can be slightly more difficult to properly direct and are more expensive. Providing information on estimated depth based on BMI would guide pain physicians in proper needle selection and optimize patient comfort, practice efficiency, and cost-effectiveness. This is a prospective, observational study to investigate the relationship between BMI and the depth of the epidural space during lumbar transforaminal ESIs.
METHODS
Permission to conduct this study was granted by the internal review board at The Johns Hopkins Medical Institutions, Baltimore, Md. A standardized protocol was used for all patients, with recruitment and all procedures occurring between June 2007 and January 2008. Sample size estimation was performed with SAS 9.1 (SAS Institute Inc, Cary, NC) using data available in the literature. Hamza et al22 found that those with a BMI of less than 25 kg/m2 (BMI <25 group) had a mean depth of 4.3 ± 0.7 cm, whereas those with a BMI of greater than 30 kg/m2 (BMI >30 group) had a mean depth of 5.2 ± 1.1 cm. Assuming the actual difference in depth between groups is 0.9 and using a type I error rate of α = 0.05, the necessary sample size per group needed to detect an effect with 80% power is 16 patients per group in the BMI <25 and BMI >30 groups.
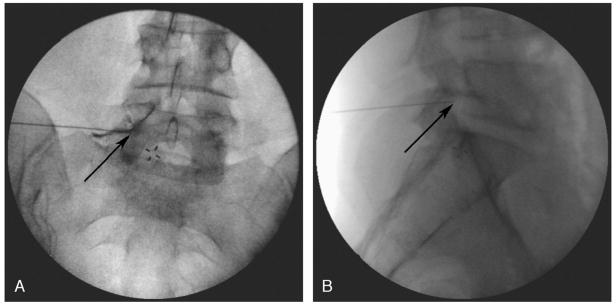
All patients 18 and older undergoing lumbar transforaminal ESI at the L3-L4, L4-L5, and L5-S1 levels at The Johns Hopkins Blaustein Pain Treatment Center were included in the study. There were no relevant exclusion criteria. As per standard protocol, patients were placed in the prone position with a pillow placed under the abdomen. The targeted intervertebral foramen was then centered under an anteroposterior (AP) view. The fluoroscope was angled until the corresponding zygapophyseal (facet) joint was positioned in the middle of the vertebral body above the targeted intervertebral foramen (oblique angle). A point was marked at the superior aspect of the intervertebral foramen, just inferior to the pedicle and inferolateral to the pars interarticularis as previously described.24 The skin overlying the site marked was anesthetized using 1% lidocaine with bicarbonate. A 5-in, 22-gauge Quincke-tip spinal needle was then advanced with intermittent fluoroscopic guidance in a coaxial direction24 toward the marked target. A 7-in needle was selected in some cases at the practitioner’s discretion based on the body habitus of the patient. Final needle position was confirmed with the needle tip within the lateral half of the pedicle on AP view and within the foramen on lateral view (Figs. 1A and B, respectively).24,25 Contrast dye was injected under live fluoroscopy in an AP view to ensure epidural spread (Fig. 1A). After final needle position and injection, the needle depth from the skin to the foramen was measured.
FIGURE 1.
Final needle position was confirmed with the needle tip within the lateral half of the pedicle on AP view and within the foramen on lateral view. Panel A shows the needle tip (arrow) below the pedicle within the intervertebral foramen. Subpedicular dye spread of contrast dye into the epidural space confirms transforaminal placement. The lateral fluoroscopic image shown in B shows the needle tip within the foramen.
The primary outcome measure was the depth of final needle placement as it related to BMI. Oblique angle from midline, age, sex, and race were analyzed as secondary outcomes.
Statistics
Statistical analyses were performed using STATA version 10.0 (StatCorp, College Station, Tex). The Shapiro-Francia W′ test for normal data was performed on outcome measures to determine data distribution. Continuous variables are reported as mean and SD, and nonparametric data are presented as median and interquartile range. Categorical data are reported as the number of subjects and percentage. Comparisons among BMI categories for nonparametric variables were performed with the Kruskal-Wallis rank test. Comparisons among BMI categories for parametric variables were performed with 2-way analysis of variance. For multiple significance testing, post hoc Bonferroni correction was used. Examination of covariates was performed with adjusted stepwise parametric linear, and nonparametric regression was performed for each outcome measure of interest.
RESULTS
Demographic and patient characteristics by BMI category as defined by the World Health Organization23 are presented in Tables 1 and 2. Age and the intervertebral level were similar between the groups. There were more women and African Americans in the obese classes II and III groups.
TABLE 1.
Demographics by BMI Category
| Underweight (BMI <18.5 kg/m2; n = 1) | Normal (BMI = 18.5–24.99 kg/m2; n = 16) | Preobese (BMI = 25–29.99 kg/m2; n = 26) | Obese Class I (BMI = 30–34.99 kg/m2; n = 22) | Obese Class II (BMI = 35–39.99 kg/m2; n = 15) | Obese Class III (BMI >40 kg/m2; n = 6) | |
|---|---|---|---|---|---|---|
| Age, mean (SD; min-max), y | 61 (—; 61–61) | 56.5 (16.4; 21–84) | 54.1 (14.3; 28–84) | 57.9 (13.2; 20–79) | 53.4 (13.0; 35–76) | 56.3 (8.3; 41–66) |
| Sex | ||||||
| Male, n (%) | 0 | 8 (50.0) | 11 (42.3) | 8 (36.4) | 1 (6.7) | 0 |
| Female, n (%) | 1 (100) | 8 (50.0) | 15 (57.7) | 14 (63.6) | 14 (93.3) | 6 (100) |
| Race | ||||||
| White, n (%) | 1 (100) | 10 (62.5) | 18 (69.2) | 11 (50) | 7 (46.7) | 1 (16.7) |
| Black, n (%) | 0 | 3 (18.8) | 7 (26.9) | 11 (50) | 8 (53.3) | 5 (83.3) |
| Asian, n (%) | 0 | 3 (18.8) | 1 (3.8) | 0 | 0 | 0 |
| BMI, mean (SD; min-max), kg/m2 | 18.3 (—; 18.3–18.3) | 22.8 (1.3; 19.7–24.3) | 27.1 (1.2; 25.1–29.6) | 31.8 (1.6; 30–34.9) | 37.5 (1.4; 35–39.7) | 50 (8.9; 40.7–66.8) |
| Level | ||||||
| L3-L4 | 0 | 0 | 1 (3.8) | 0 | 4 (26.7) | 0 |
| L4-L5 | 1 (100) | 14 (87.5) | 19 (73.1) | 17 (77.3) | 10 (66.7) | 5 (83.3) |
| L5-S1 | 0 | 2 (12.5) | 6 (23.1) | 5 (22.7) | 1 (6.7) | 1 (16.7) |
BMI categories based on classifications by the World Health Organization.23
Min indicates minimum; max, maximum; %, percentage; L, lumbar intervertebral space.
TABLE 2.
Needle Depth and Fluoroscopic Oblique Angle by Body Mass Index Category
| Underweight (n = 1) | Normal (n = 16) | Preobese (n = 26) | Obese Class I (n = 22) | Obese Class II (n = 15) | Obese Class III (n = 6) | |
|---|---|---|---|---|---|---|
| Needle depth, median (interquartile range), cm | 6.3 | 7.5 (7.0–8.5) | 8.4 (8.0–9.5) | 10 (8.9–12.5)* | 10.4 (9.3–11.8)† | 12.2 (12–12.5)‡ |
| Oblique angle, mean (SD, min-max), degrees | 15 (—; 15–15) | 35 (8.4; 20–50) | 29.23 (8.5; 12–47) | 33.8 (9.7; 15–50) | 28.72 (8.2; 15–47) | 32.0 (3.9; 27–35) |
BMI categories based on classifications by the World Health Organization.23
Obese class I patients (BMI, 30–34.99 kg/m2) required a significantly increased depth (P < 0.01) when compared with normal patients (BMI, <25 kg/m2).
Obese class II (morbid obesity) (BMI, 35–39.99 kg/m2) required a significantly increased depth (P < 0.01) when compared with normal and overweight patients.
Obese III (super-morbid obesity) (BMI, >40 kg/m2) was associated with an increased depth (P < 0.01) when compared with normal, overweight, and obese patients.
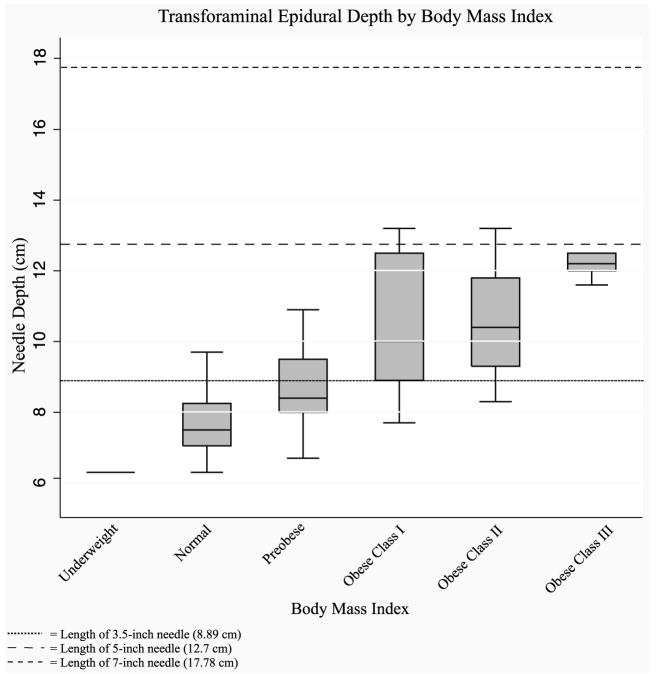
Needle depth was positively associated with BMI (regression coefficient [RC], 1.13; P < 0.001). These findings indicate the requirement of a 5-in needle in the obese classes I, II, and III (Table 2; Fig. 2). Almost half of the patients in the preobese (BMI, 25–29.99 kg/m2) group would also require a 5-in needle (11/26). Of the 43 patients in the obese classes I, II, and III groups, 5 had a transforaminal epidural depth more than the length of a 5-in needle. Sex and race were also found to be significantly associated with needle depth (sex: RC, 1.3; P = 0.02; race: RC, 0.8; P = 0.04, respectively). Neither factor remained significant when BMI was accounted as a covariate in the regression model (sex: RC, 0.3; P = 0.3; race: RC, 0.03; P = 0.9, respectively). The patient’s age, intervertebral level treated, and oblique angle had no predictive value on the foramen depth (P > 0.2). The angle of fluoroscopy necessary for the procedure was not predictable based on any factor examined.
FIGURE 2.
The depth of transforaminal epidurals is positively correlated with body mass index (BMI). A 3.5-in (8.89 cm) needle will suffice for patients with BMI of less than 25 kg/m2 (underweight and normal). A 5-in (12.7 cm) needle will be required for some patients with a BMI of 25 to 30 kg/m2 (preobese) and most patients with a BMI of greater than 30 kg/m2 (obese classes I, II, and III). Some patients with a BMI of greater than 30 kg/m2 will require a 7-in needle.
DISCUSSION
The present study supports the hypothesis that BMI correlates with the depth of transforaminal ESI. A 3.5-in (8.89 cm) needle will suffice for patients with BMI of less than 25 kg/m2. A 5-in (12.7 cm) needle will be required for some patients with a BMI of 25–30 kg/m2 (preobese) and most patients with a BMI of more than 30 kg/m2 (obese classes I, II, and III). Occasionally, a 7-in needle is necessary for patients with a BMI of greater than 30 kg/m2. The largest patient studied had a BMI of 66.8 kg/m2, and the epidural space was reached at 16.5 cm. This left an extra 1.3 cm of available needle length on a 7-in needle, indicating that the transforaminal epidural space can usually be accessed even on patients with extreme obesity. The power analysis conducted was intended to detect a difference between patients with a BMI of less than 25 kg/m2 and greater than 30 kg/m2 and calculated group sizes of at least 16 patients per group for each of these categories. There may be intergroup differences between neighboring groups (ie, obese classes I and II) that were not detected in this study.
There was no association between BMI and degree of oblique angulation, which is not surprising. The degree of angulation is based on the fluoroscopic view of the relationship between the facet joint and vertebral body. The difference in body habitus between the BMI groups would not be expected to affect the bony landmarks used for fluoroscopic positioning.
D’Alonzo et al16 found an association between ethnicity and epidural depth. Although the present study was not powered to detect a difference between different ethnic groups, there was no association between ethnicity and epidural depth when BMI was controlled. The predicted difference if BMI is equal to 30 kg/m2 found by D’Alonzo et al was the greatest when comparing African Americans and Asians and was only 0.7 cm (5.9 vs 5.2 cm, respectively). The present study did not include enough Asian patients to compare with the African American group in a subgroup analysis. Future studies with larger sample sizes may detect a difference between ethnic groups. However, given the small difference noted in the interlaminar study, the difference may not be clinically significant.
Although it is not surprising that there is a positive association between BMI and transforaminal epidural depth, the results of this study hold clinical significance. The available data allow physicians to select an appropriately sized needle and can lead to improved patient comfort and practice efficiency. Longer needles are more expensive and can be more difficult to direct. Therefore, they are not recommended in all patients. Previous interlaminar epidural depth studies do provide interesting data; however, they do not impact clinical practice in the same manner. Clinkscales et al13 showed in obstetric patients that the mean depth to the interlaminar epidural space plus 1 SD in all of the BMI groups was below the 9-cm length of a standard Tuohy needle (9 cm). In the largest group studied, the mean depth in the BMI >50 group (n = 27) was 7.5 ± 1.2 cm.13 In interventional pain medicine, however, there is a greater selection in needle length, thereby making these data important clinically.
Limitations
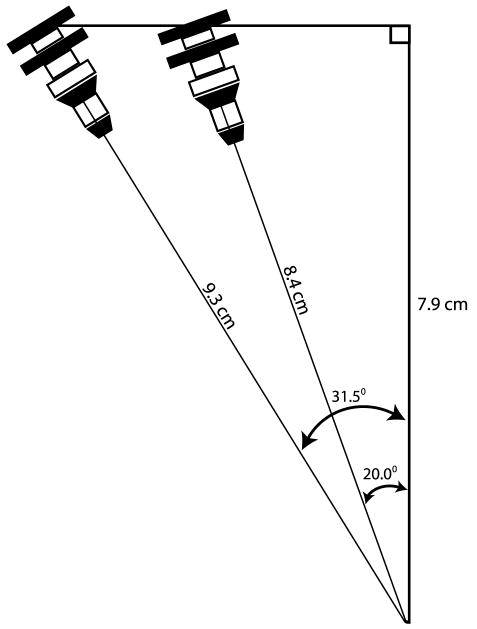
The oblique angle used in the present study is greater than that which has been described in some textbooks. In the Atlas of Image-Guided Intervention in Regional Anesthesia and Pain Medicine by Rathmell,24 the author recommends that the C-arm be “rotated obliquely 20 to 30 degrees until the facet joint and pars interarticularis are clearly visualized,” and in the corresponding figure, the zygapophyseal joint is approximately 30% of the distance from the lateral borders of the corresponding vertebral bodies.24 The International Spine Intervention Society does not specify an average degree of fluoroscopic obliquity; however, the fluoroscopic images provided do show a lesser degree of obliquity.25 In the present study, the fluoroscope was angled until the facet joint was at the midpoint of the vertebral body at the targeted level. This is the preferred technique of the authors as it allows for more medial direction of the needle and a true transforaminal injection. Lesser angles direct the needle in a more anterior direction and can lead to a selective nerve root block instead of the desired transforaminal injection. In the present study, the median needle depth was 9.3 cm, and the mean oblique angle was 31.5 ± 8.9 degrees. Assuming that the transforaminal depth represents the hypotenuse of a right triangle, the midline depth would be projected to be 7.9 cm. If the midline angle is fixed at 7.9 cm and the oblique angle is reduced to the lower 20-degree angle describe by Rathmell,24 the estimated transforaminal depth would be estimated to be 8.4 cm, representing a 9.7% decrease in estimated needle depth (Fig. 3). A less than 10% decrease in depth would be well within the interquartile range in each of the BMI groups, thereby indicating that the present data are relevant regardless of the preferred approach to the transforaminal epidural space.
FIGURE 3.
The estimated transforaminal epidural depth using a lesser oblique angle would be close to the depth found in the present study. The median depth in the present study was 9.3 cm with an oblique angle of 31.5 degrees. The midline length, assuming a right-angled triangle, is 7.9 cm (cos A = adjacent length/hypotenuse). If a lesser oblique angle of 20 degrees is used, the estimated transforaminal epidural depth is 8.4 cm (9.7% less). A 10% difference is within the interquartile ranges shown in Table 2, thereby indicating that the epidural depth data reported in the present study are relevant regardless of the approach used.
One could postulate that the transforaminal depth could be estimated using the previously established data on interlaminar lumbar epidurals, but our data show this not to be the case. Using the above trigonometric approach and interlaminar depth data published by Clinkscales et al13 (obese class I, 5.3 ± 0.9 cm), the transforaminal depth in the obese class I population would be predicted to be 6.4 cm, if assuming an oblique fluoroscopic angle of 33.8 degrees (oblique angle for obese class I; Table 2). The median transforaminal depth in this study was 10 cm, however, representing a difference of 3.6 cm. This differential can be explained by the difference in muscle and fat distribution. The paraspinous muscles and adipose tissue in the low back elevate the transforaminal entry point above that of the interlaminar insertion site. Clinkscales et al13 showed that the mean interlaminar epidural depth difference between the obese class II and normal-BMI patients was only 1.4 cm (6.2 vs 4.8 cm, respectively; 29% increased depth), whereas the difference in the median between the same groups in the present study was 2.9 cm (10.4 vs 7.5 cm, respectively; 38.6% increased depth). This again indicates that the difference in BMI has a greater impact when a transforaminal approach is selected.
There is a great deal of variability in the length and type of needles used for transforaminal ESIs. In our clinic, 3.5-, 5-, and 7-in, 22-gauge straight Quincke-tip needles are used. The data in Table 2 and Figure 2 are presented such that they can be applied to all clinical practices regardless of the needle size used.
CONCLUSION
The present study supports the hypothesis that there is a positive correlation between transforaminal epidural depth and BMI. These data can aid the pain practitioner in the selection of an appropriately sized needle, which will avoid repeat procedures and improve patient comfort. The selection of an appropriately sized needle can also save time and money. Longer needles are more expensive and can be more difficult to direct. Therefore, the use of longer needles is not recommended for all patients.
Acknowledgments
The authors thank Srinivasa Raja, MD (Department of Anesthesiology and Critical Care, The Johns Hopkins University, Baltimore, Md) for support and guidance. They also thank George C. Gettys, MD (research fellow, Department of Anesthesiology, University of Michigan, Ann Arbor, Mich) for assistance with figures.
This study was funded in part through an unrestricted educational grant from Alpharma Inc. Further support was provided by NIH grant no. MH075884 (to R.W.H) and the IASP Trainee Fellowship funded by the Scan/Design by Jens and Inger Bruun Foundation (to R.W.H.).
Footnotes
This study was performed at The Johns Hopkins Medical Institutes, Baltimore, MD.
This study was presented at the American Society of Regional Anesthesia and Pain Medicine Spring Meeting; May 2008; Cancun, Mexico.
References
- 1.Lutz GE, Vad VB, Wisneski RJ. Fluoroscopic transforaminal lumbar epidural steroids: an outcome study. Arch Phys Med Rehabil. 1998;79:1362–1366. doi: 10.1016/s0003-9993(98)90228-3. [DOI] [PubMed] [Google Scholar]
- 2.Riew KD, Yin Y, Gilula L, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am. 2000;82-A:1589–1593. doi: 10.2106/00004623-200011000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Weiner BK, Fraser RD. Foraminal injection for lateral lumbar disc herniation. J Bone Joint Surg Br. 1997;79:804–807. doi: 10.1302/0301-620x.79b5.7636. [DOI] [PubMed] [Google Scholar]
- 4.Manchikanti L. Transforaminal lumbar epidural steroid injections. Pain Physician. 2000;3:374–398. [PubMed] [Google Scholar]
- 5.Manchikanti L. The growth of interventional pain management in the new millennium: a critical analysis of utilization in the Medicare population. Pain Physician. 2004;7:465–482. [PubMed] [Google Scholar]
- 6.Whitlock EL, Bridwell KH, Gilula LA. Influence of needle tip position on injectate spread in 406 interlaminar lumbar epidural steroid injections. Radiology. 2007 doi: 10.1148/radiol.2433060983. [DOI] [PubMed] [Google Scholar]
- 7.Ackerman WE, 3rd, Ahmad M. The efficacy of lumbar epidural steroid injections in patients with lumbar disc herniations. Anesth Analg. 2007;104:1217–1222. doi: 10.1213/01.ane.0000260307.16555.7f. table of contents. [DOI] [PubMed] [Google Scholar]
- 8.Schaufele MK, Hatch L, Jones W. Interlaminar versus transforaminal epidural injections for the treatment of symptomatic lumbar intervertebral disc herniations. Pain Physician. 2006;9:361–366. [PubMed] [Google Scholar]
- 9.Abdi S, Datta S, Trescot AM, et al. Epidural steroids in the management of chronic spinal pain: a systematic review. Pain Physician. 2007;10:185–212. [PubMed] [Google Scholar]
- 10.Rosenberg SK, Grabinsky A, Kooser C, Boswell MV. Effectiveness of transforaminal epidural steroid injections in low back pain: a one year experience. Pain Physician. 2002;5:266–270. [PubMed] [Google Scholar]
- 11.Bahk JH, Kim JH, Lee JS, Lee SC. Computed tomographic study of lumbar (L3–4) epidural depth and its relationship to physical measurements in young adult men. Reg Anesth Pain Med. 1998;23:262–265. doi: 10.1016/s1098-7339(98)90052-9. [DOI] [PubMed] [Google Scholar]
- 12.Chen KP, Poon YY, Wong CH. The depth to the epidural space. Ma Zui Xue Za Zhi. 1989;27:353–356. [PubMed] [Google Scholar]
- 13.Clinkscales CP, Greenfield ML, Vanarase M, Polley LS. An observational study of the relationship between lumbar epidural space depth and body mass index in Michigan parturients. Int J Obstet Anesth. 2007;16:323–327. doi: 10.1016/j.ijoa.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 14.Segal S, Beach M, Eappen S. A multivariate model to predict the distance from the skin to the epidural space in an obstetric population. Reg Anesth. 1996;21:451–455. [PubMed] [Google Scholar]
- 15.Watts RW. The influence of obesity on the relationship between body mass index and the distance to the epidural space from the skin. Anaesth Intensive Care. 1993;21:309–310. doi: 10.1177/0310057X9302100309. [DOI] [PubMed] [Google Scholar]
- 16.D’Alonzo RC, White WD, Schultz JR, Jaklitsch PM, Habib AS. Ethnicity and the distance to the epidural space in parturients. Reg Anesth Pain Med. 2008;33:24–29. doi: 10.1016/j.rapm.2007.06.399. [DOI] [PubMed] [Google Scholar]
- 17.Carnie J, Boden J, Gao Smith F. Prediction by computerised tomography of distance from skin to epidural space during thoracic epidural insertion. Anaesthesia. 2002;57:701–704. doi: 10.1046/j.1365-2044.2002.02572_4.x. [DOI] [PubMed] [Google Scholar]
- 18.Kao MC, Tsai SK, Chang WK, et al. Prediction of the distance from skin to epidural space for low-thoracic epidural catheter insertion by computed tomography. Br J Anaesth. 2004;92:271–273. doi: 10.1093/bja/aeh053. [DOI] [PubMed] [Google Scholar]
- 19.Lai HC, Liu TJ, Peng SK, Lee KC, Luk HN, Lee SC. Depth of the thoracic epidural space in paramedian approach. J Clin Anesth. 2005;17:339–343. doi: 10.1016/j.jclinane.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Han KR, Kim C, Park SK, Kim JS. Distance to the adult cervical epidural space. Reg Anesth Pain Med. 2003;28:95–97. doi: 10.1053/rapm.2003.50025. [DOI] [PubMed] [Google Scholar]
- 21.Lin CH, Lu CH, Ning FS. Distance from the skin to the cervical epidural space. Acta Anaesthesiol Sin. 1995;33:161–164. [PubMed] [Google Scholar]
- 22.Hamza J, Smida M, Benhamou D, Cohen SE. Parturient’s posture during epidural puncture affects the distance from skin to epidural space. J Clin Anesth. 1995;7:1–4. doi: 10.1016/0952-8180(94)00018-y. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva: World Health Organization; 2000. pp. 1–254. [PubMed] [Google Scholar]
- 24.Rathmell JP. Atlas of Image-Guided Intervention in Regional Anesthesia and Pain Medicine. Philadelphia: Lippincott Williams and Wilkins; 2006. pp. 56–63. [Google Scholar]
- 25.Bogduk N. Practice Guidelines for Spinal Diagnostic and Treatment Procedures. 1. San Francisco: International Spine Intervention Society; 2004. pp. 163–187. [Google Scholar]