Abstract
Zebrafish (zebra danio) are becoming increasingly popular in behavioral neuroscience and behavior genetics. This small vertebrate may be utilized in modeling human brain disorders. One of the major neuropsychiatric conditions still not well understood is abnormally increased fear and anxiety. Zebrafish may be an appropriate organism with which these human diseases can be modeled and their biological mechanisms investigated. Predator induced anxiety paradigms have been suggested as useful methods in translational research. Shoaling fish, such as zebrafish, are known to respond to alarm substances with antipredatory or alarm reactions. However, these responses are not well characterized in zebrafish. In the current paper, we investigate the behavioral responses of zebrafish elicited by its alarm substance. Using observation-based as well as video-tracking aided behavior quantification methods we demonstrate significant alarm substance-induced behavioral changes that are independent of the presence of a predatory fish stimulus. The results suggest that, once refined, the use of alarm substance with zebrafish will allow the development of high throughput behavioral paradigms for drug and mutation screening aimed at the analysis of the biological mechanisms of fear in vertebrates.
Keywords: Alarm substance, Antipredatory behavior, Anxiety, Danio rerio, Fear, Zebrafish, Shoaling
1. Introduction
Zebrafish, or zebra danio (Danio rerio), has been a popular subject of developmental biology for the past three decades. A considerable amount of genetic information has been accumulated for this species [22]. As a result, this small vertebrate has become amenable to large-scale mutagenesis screening and to many other genetic applications that may be utilized by disciplines other than developmental biology. One of these disciplines is neurobehavioral genetics [16]. The utility of zebrafish in forward genetic applications is clear when one considers the characteristics of this species: a large number of offspring may be generated (a single female zebrafish can spawn 200 eggs every other day), and the small zebrafish (maximum length is 4 cm) can be kept with ease efficiently and in large numbers [37]. In addition to the generally accepted view on the utility of zebrafish in mutagenesis screening, examples already suggest that this species may also be useful in pharmacological screens [2]. Although the number of studies using zebrafish has been exponentially increasing for the past few years, this species is still understudied and underutilized compared to other model organisms [33]. Perhaps, the most problematic area of research is the understanding of the behavior and brain function of zebrafish. This is particularly noteworthy because the foundation of genetic and pharmacological screens is phenotypical characterization, and behavioral analysis is perhaps the best window to the brain allowing the quantification of changes in its function [17,15,4].
A prevalent human psychiatric disease is the anxiety cluster including numerous forms and levels of severity of anxiety and pervasive phobias [35] and [9]. Arguably, these psychiatric conditions are the result of abnormally functioning neurobiological processes (pathways, circuits, connections, and/or molecular mechanisms) that originally evolved to support adaptive fear responses [5] including predator avoidance [3]. The use of “predation models” in anxiety research is gaining acceptance because this naturalistic approach is not only practical but also better allows the analysis of the biological and genetic aspects of the abnormalities (see e.g. [24]; also see [17] for general discussion of this question). In this context “fear” is defined in an operational way and means a collection of behavioral responses that are elicited by negative stimuli associated with imminent danger such as the presence of a predator.
Fear reactions, including antipredatory behavior, have been rarely studied in zebrafish. Nevertheless, pioneering work with this species [36] and [23] implies that it has a potential for being a good model organism for the analysis of the biological mechanisms and genetics of vertebrate fear in general and human anxiety in particular. Furthermore, recently zebrafish has been shown to respond to sympatric and allopatric predators differentially without any prior exposure to these predatory species [1], a finding that demonstrates genetic predisposition in antipredatory behavior in zebrafish. Innate fear responses are particularly interesting as they can be found in several vertebrate species [25,8,28,32,3] and may also underlie natural tendencies that manifest in an exaggerated or abnormal manner in anxiety related pathological cases in the human clinic [5,3].
Systematic analysis of the characteristics of the sympatric predator, the Indian leaf fish (Nandus nandus), to which zebrafish has been found particularly sensitive [1] is being performed in our laboratory with the hope to create a computer generated visual stimulus set that elicits fear in a consistent and controlled manner, a prerequisite for future forward genetic or drug screening paradigms. However, fear may be induced in zebrafish in a reliable manner using a different, and simpler, methodology. Alarm substance, originally described in the min-now (Phoxinus phoxinus) [14], is known to induce fear responses in a range of fish species [29]. Alarm substance is made in specialized epidermal club cells and is released when the skin of the prey fish is damaged. The release of the alarm substance causes an alarm reaction in neighboring fish which, using their sensitive chemoreceptors [36], detect (smell) the substance. The reaction the substance elicits is often described as “random darting about” which is followed by “tightening of the fish shoal” as well as swimming away from the predator [36]. Although the alarm substance is initially detected by olfaction, the darting motion of fish that come into contact with the pheromone can be perceived visually or by the lateral line of other fish in the shoal and thus may serve as an effective and fast signaling mechanism [14]. This darting movement has also been described in the literature as “erratic movement” or “zig-zagging” and may occur in a species-specific manner on the bottom of the tank or near the surface [29]. Erratic movement has been described in two South Asian fish species, the paradise fish (Macropodus opercularis) [18] and the zebrafish [19,20] under fear inducing conditions. Fish may exhibit additional responses to fear inducing stimuli, for example, freezing or crowding [29,19,18,26]. Importantly, the species-specific alarm reactions are often found identical in appearance in the aquarium and in the field [29] and thus may serve as a biologically meaningful method for quantifying the level of fear induced in the laboratory.
In the current paper, using the alarm substance, we will attempt to induce the above explained behaviorally well defined fear reactions in zebrafish. We will consider and discuss the issues associated with different behavior quantification methods and test procedures including the use of predators and the application of observation-based and computer aided tracking techniques. Our goal is to develop experimental methods that will be applicable to forward genetic and drug screening.
2. Methods
2.1. Animals and housing
Five hundred wild type zebrafish (50:50% males and females) of heterogeneous genetic background were obtained from a local pet store (Big Al’s Aquarium Warehouse Outlets Inc., Mississauga). The rationale for the choice of this outbred population is explained in detail elsewhere [1]. Briefly, this population is expected to approximate the genetic heterogeneity found in wild zebrafish populations and is not expected to be altered by inbreeding-induced random genetic drift. Thus, this population may reflect the species-typical characteristics of zebrafish better than those maintained by closed breeding systems. Three hundred zebrafish were used for the first experiment, and 200 were used for the second (for sample sizes representing the number of five member zebrafish shoals, see figure legends). All zebrafish were sexually mature, fully developed, 4 cm long (S.D. = ±0.2 cm) adults between 6 and 12 months of age at the time of the experiments. The fish were housed in groups of 10 in 3-L acrylic tanks that were part of a high-density rack system specifically designed for zebrafish by Aquaneering (San Diego, USA). The water of these tanks was filtered using mechanical (sponge), chemical (activated carbon), and biological (fluidized glass bed) filtration units. The system water used in the rack was generated via reverse osmosis, and the purified water was reconstituted with the addition of a salt mixture (Instant Ocean) to achieve 700–900 ppm salt concentration. The zebrafish were fed live nauplii of brine shrimp (O.S.I., Utah) and TetraMin flakes (Melle, Germany) mixed with ground freeze dried krill (Aquatic Ecosystems, Inc. Florida) three times a day. The water temperature was maintained at 26 ± 1 °C and the light cycle was controlled with lights on at 7:00 h and off at 19:00 h. In addition to zebrafish, live Cichlids measuring twice the body length of zebrafish (8 cm long Astronotus ocellatus and Tilapia buttikoferi) were also used. These fish served as the predatory stimulus. They were obtained from local pet stores (Big Al’s Aquarium Warehouse Outlet, Mississauga and Dragon aquarium, Mississauga) and were housed in 100 L tanks. The effect of the employed different predatory species on the behavior of zebrafish was statistically indistinguishable and thus their data were pooled for the analyses presented in this paper.
2.2. General experimental procedure
The general experimental set up and procedure was similar to that described in detail elsewhere [1]. Briefly, the observation tank (50.8 cm × 25.5 cm × 30.5 cm, length × width × height) was placed in a compartment that provided visual isolation from all directions except the front. The tank was illuminated from above by a fluorescent light tube (Eclipse 13 W) placed directly above the tank. The water temperature in the observation tank was held at 26 °C. The observation tank was filled with fresh system water identical to what was used for the maintenance of zebrafish (reverse osmosis purified salt reconstituted oxygenated water). A video-camera (Sony DCR-HC20, Sony Corporation, Japan) was positioned in front of the tank and all experiments were recorded onto Sony MiniDV tapes and later replayed for analysis. The experimenter was not visible to the fish during the recording. Five experimental zebrafish (a small shoal) were placed in the observation tank as described before [1]. The rationale for this was that zebrafish is a shoaling species and the presence of shoal-mates accelerated habituation to the test tank. After each recording session, the experimental zebrafish were returned to the high-density racks and maintained there for future experiments and breeding. All experiments were conducted with experimentally naïve, previously untested, fish and all fish were tested only once, a between subject design. In all experiments, four observation tanks were used, two in parallel, i.e. at the same time. In the first set of experiments, four alarm substance dose groups were tested. Each observation tank was assigned to a particular dose group. The physical location of the tanks was randomized. Similarly to the first set of experiments, in the second set of experiments, four tanks were employed, two of them were used for high and two for zero alarm substance dose and particular tanks were assigned to these dose groups but again the physical location of the tanks was randomized.
2.3. Alarm substance extraction and administration
The chemical makeup of the alarm substance has not been identified in zebrafish and it is not known whether zebrafish could respond to alarm substances of other species. Nevertheless, it is know that in several fish species this substance is released from specialized epidermal cells once the cells are damaged [29]. For this reason, fresh alarm substance was directly extracted from zebrafish every morning of testing. The procedure for extracting the pheromone and creating dilutions followed the recommendations of Waldman [36] and was as follows: excess water was removed from the skin of donor zebrafish with a paper towel. The fish were quickly sacrificed by decapitation using surgical scissors (approved by the University Animal care Committee following the guidelines of the Canadian Council of Animal Care). Fifteen shallow cuts were made on each side of the trunk of 10 donor fish and the cuts were washed with distilled water. Alarm substance is known to be released upon skin damage and superficial cuts were found sufficient [29]. Blood was made certain not to contaminate the solution. At the end of the process a total of 100 mL alarm substance solution was collected in a 500 mL beaker. During the collection process and until further use the solution was kept on ice.
In the first set of experiments, four alarm substance doses were used: high, medium, low dose, and a freshwater control. Although the exact concentration of the substance could not be determined, the relative concentration differences were maintained throughout the experiments as follows: the high concentration was the same concentration as the solution obtained at the time of extraction. The medium concentration was obtained by a 50% dilution of the high concentration and the low concentration was obtained by a 10% dilution of the high concentration. The control solution was composed of distilled water. All alarm substance solutions were kept on ice throughout testing to minimize denaturation [36]. In the second set of experiments, only two doses, high concentration and freshwater control, were used.
To avoid disturbing the fish the alarm substance was delivered via a 5.0 mm diameter aquarium tubing (Pennplex) attached to the observation tank with one end of the tube 1.5 mm above the water line and the other hidden from the fish behind the tank. A 3.0 mL syringe filled with 2.5 mL of the appropriate alarm substance solution was connected to the plastic tubing and the content of the syringe was slowly injected into each observation tank (30 L water volume). The order in which the alarm substance was administered to the observation tanks was randomized. For each concentration the solution was administered with a different syringe.
2.4. Procedure for Experiment 1
In this experiment, we compared the effects of different doses of the alarm substance on zebrafish. In order to present a naturalistic set up and to elicit a potentially maximal alarm substance effects, we allowed the experimental zebrafish to view a live predatory fish [1]. The predator’s presence in the current experiment was expected to potentiate the effect of the alarm substance and increase the sensitivity of the dose response analysis.
The effects of three doses of the alarm substance and a control (water) treatment were compared. To quantify the reactions of zebrafish we measured the motor and posture patterns erratic movement and freezing, known to be emitted in a number of fish species in response to predators or fear inducing stimuli including the alarm substance [29,18,19]. In addition, we also quantified crowding, i.e. shoal cohesion, which is expected to increase in response to fear inducing stimuli [29,19,26,27].
The predatory (stimulus) fish was placed in a tank (30.5 cm × 20.3 cm × 15.2 cm) adjacent to the zebrafish observation tank. On the other side of the observation tank, an empty water filled stimulus tank was placed. The position of the predator and the empty stimulus tank was varied randomly across sessions. Five experimental zebrafish, a small shoal, were placed in the observation tank at a time, and approximately fifteen shoals were tested per concentration. Zebrafish form small shoals in nature [11] and five-member shoals have been employed before [1,19] making the current methods comparable to other studies. The experimental test fish and stimulus fish were allowed to habituate to their tank for 20 min, a length of time set based on prior studies [19,27]. Sub-sequently, recording started, and within 30 s the alarm substance solution was administered and the recording concluded 7 min later. The order in which the different alarm substance concentrations were administered was randomized.
2.5. Procedure for Experiment 2
Given the multimodal and multi-cue integration demonstrated in predator-prey interaction in fishes [34], one may assume the alarm substance to be effective only in combination with other cues. For example, mild pain inducing electric shocks elicited an avoidance reaction in paradise fish only in the presence of eye spots or live stimulus fish [10,21]. Alternatively, the alarm substance may be an unconditioned key stimulus that is capable of eliciting a full-fledged fear reaction.
To distinguish these two possibilities, in the second experiment we examined whether the presence of a predatory fish is required for the alarm substance effects to manifest. To maximize the potential difference between the presence and the absence of the predator, now the experimental zebrafish had full access to all stimuli from the predator, i.e. could detect its presence using not only vision but also olfaction as well as the lateral line and hearing. The procedural details of this experiment were identical to those of the first except that the predator was placed inside of the experimental tank of the zebrafish. To avoid physical injury to the experimental zebrafish the subjects were separated from the predator by a transparent perforated acrylic sheet. Briefly, the following treatment groups were tested: high alarm substance concentration group with no predator, high alarm substance concentration group with predator, control (zero concentration) group with no predator, control (zero concentration) group with predator.
2.6. Quantification of behavioral responses
We digitally transferred the recorded videos to the computer using Windows Movie Maker and stored the files on DVD disks for later replay and analysis. We quantified behavior using the Observer ColorPro event recorder (Noldus, Wageningen, The Netherlands) and a custom tracking software application developed in our laboratory [27]. Two motor patterns known to change in response to alarm substance treatment or to other fear inducing stimuli, erratic movement and freezing [18,19,29], were quantified and analyzed. In addition, we also quantified the amount of time the fish spent in the lower quadrant of the observation tank because zebrafish have been found to respond to fear inducing stimuli with moving away from the surface of water [19,29]. Last, we measured changes in shoal cohesion as the distance between members of fish shoals has been found to decrease in response to fear inducing stimuli [36].
Erratic movement was defined as swimming associated with fast directional changes, or “zig-zagging”. Although the speed of swimming and the frequency of direction changes were not precisely quantifiable and thus the decision whether the observed behavior was indeed erratic movement or not rested upon the human observer, such observation-based techniques have been found highly reliable and replicable (inter-and intra-observer reliability) and have been employed in a range of species including zebrafish and rodents (see e.g. [4,13,19]). Freezing was defined as complete immobility whereby only the gills and occasionally the eyes move. The duration of time the fish spent performing the above behaviors was measured and values are expressed as percent of the total session length. A group of five experimental zebrafish was tested in each observation session. In most instances the behavior of the shoal members was highly synchronized. Thus, we decided to follow the recording method employed in another study [1] and quantified what the majority of fish did: the onset of erratic movement and freezing was recorded when at least three of the five experimental fish were exhibiting the behavior, and recording of the behavior ended when at least three fish stopped exhibiting the behavior. In addition, we also quantified the location of the fish. The lower quadrant was defined as the area of the tank that was within 7.6 cm from the bottom of the tank. To analyze the duration of time the zebrafish spent in the lower quadrant of the observation tank, the amount of time each fish spent there relative to the total session length was calculated and the relative durations of the five fish of the shoal were averaged (giving values that could range between 0 and 100%).
A custom software application developed in our laboratory [27] was used to quantify shoal cohesion, i.e. to measure the distance of zebrafish from each other. Digital images were taken at every 1 s for the first experiment and every 5 s for the second experiment for the entire duration of the 7-min observation session using Sony DCR HC20 camcorders. The center of the tank and the distance to the edge of the tank from the center was identified for calibration purposes. The position of each individual zebrafish was identified on the image as described before [27]. The distances between pairs of fish of all combination, i.e. a total of 10 distances in the five-fish shoal, were calculated by the software and averaged for each image [27]. The results were averaged for 1-min intervals of the recording session and were statistically analyzed.
2.7. Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL). The effect of alarm substance concentration and other factors was analyzed using univariate variance Analysis (ANOVA), and the potential changes of behavior across the 7 min of the recording session was analyzed using repeated measures ANOVA. Significant ANOVA terms were further investigated using post hoc Tukey HSD multiple comparison test as appropriate.
3. Results
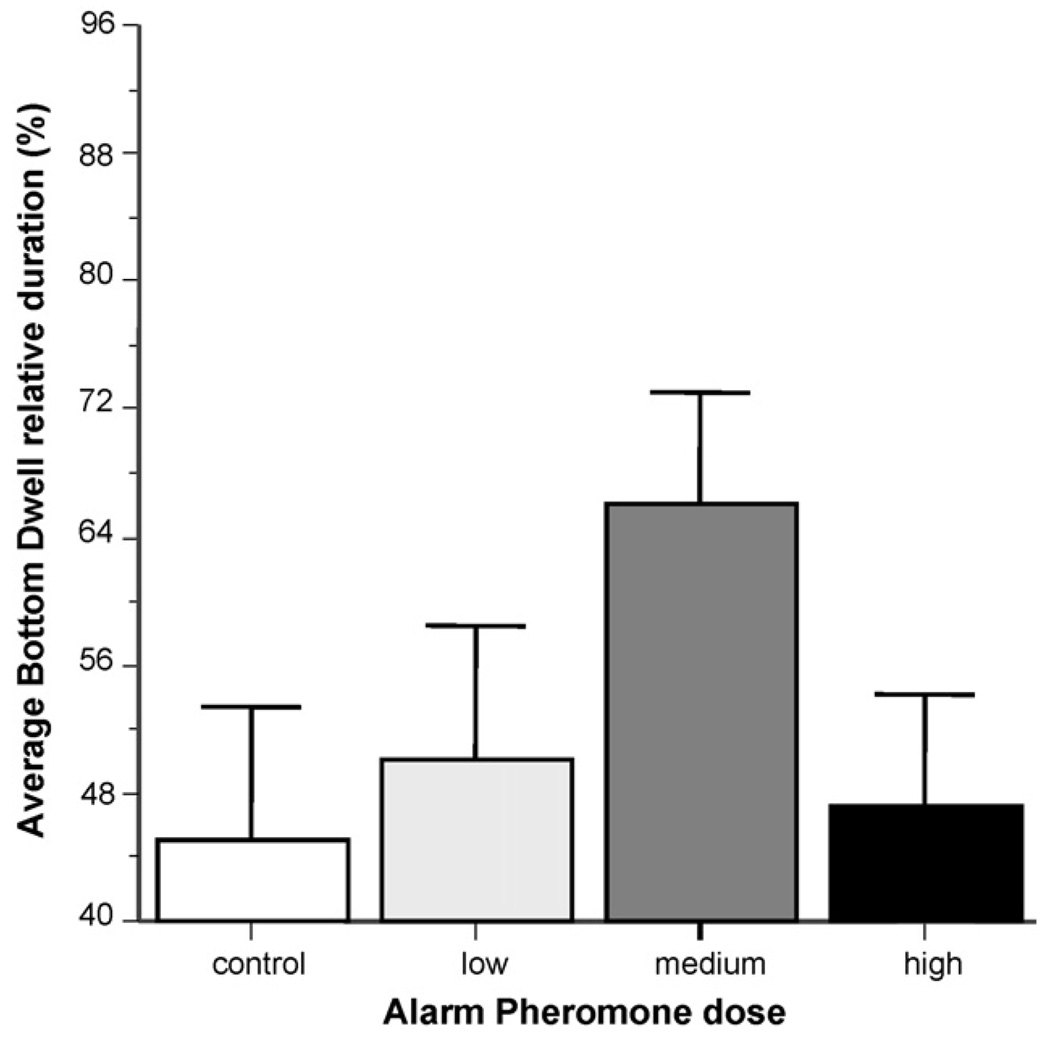
Fig. 1 shows the percent of time zebrafish spent in the bottom quadrant of the tank. The data represent the average of the responses of the five members of each experimental shoal. Although it appears the medium dose of alarm substance increased the bottom dwell time, ANOVA found no significant alarm substance effects (F(3, 47) = 1.55, p > 0.05). The distance between members of the shoal, however, did change significantly (Fig. 2) in response to alarm substance treatment. ANOVA revealed a significant alarm substance dose effect (F(3, 50) = 14.470, p < 0.001). The distance between members of the shoal also significantly changed across the seven 1-min intervals of the recording session (ANOVA Time F(6, 300) = 2.233, p < 0.05). Importantly, the interaction between alarm substance dose and time was also significant (ANOVA F(18, 300) = 2.580, p < 0.001). Post hoc Tukey HSD tests confirmed these findings and showed that the highest dose group significantly (p < 0.05) decreased their shoaling distance with time whereas the control group increased it. It also showed that by the end of the session all groups differed from each other (p < 0.05) in their shoaling distance with the highest dose group having the smallest, the control the largest, and the two intermediate dose groups medium distance values.
Fig. 1.
Alarm substance treatment did not significantly affect the length of time experimental zebrafish spent near the bottom of their observation tank. Mean + S.E.M. is shown. Sample sizes (n) represent the number of five-fish shoals tested and are as follows: control (zero alarm substance) = 13; low concentration = 15; medium concentration = 13; high concentration 15. Note that the figure shows the average of the percent of time each zebrafish of the five-fish shoal spent in the lowest quarter of the tank during the 7 min observation session. Also note that throughout the session zebrafish could view a fish predator swimming in a stimulus tank adjacent to the observation tank. For procedural details see Section 2 and for details of the results of the statistical analysis see Section 3.
Fig. 2.
Alarm substance treatment significantly reduced the distance among zebrafish. Mean + S.E.M. is shown. Sample sizes (n) represent the number of five-fish shoals tested and are as follows: control (zero alarm substance) = 13; low concentration = 15; medium concentration = 13; high concentration = 15. The distance among shoal mates is calculated as the average of distances, expressed in cm, between all possible pairs of zebrafish of the shoal. Note that while zebrafish in the highest concentration group reduced the distance (increased their shoal cohesion) with time, the control fish (zero alarm substance) did not. Also note that throughout the session zebrafish could view a fish predator swimming in a stimulus tank adjacent to the observation tank. For procedural details, see Section 2. For details of the results of the statistical analysis, see Section 3.
The alarm substance also affected the amount of erratic movement performed by zebrafish in a dose dependent manner (Fig. 3A). In order to homogenize variances, the data were logarithm transformed. Variance analysis demonstrated that the alarm substance effect was highly significant (ANOVA F(3, 47) = 5.28, p < 0.01). Tukey HSD post hoc multiple comparison test confirmed this finding and showed that the control and low concentration groups were significantly (p < 0.05) different from the high concentration group and that the control group was also significantly (p < 0.05) different from the medium concentration group. Analysis of the frequency of erratic movement (Fig. 3B), i.e. the number of times it was exhibited, also showed significant alarm substance induced changes (ANOVA (F(3, 47) = 2.95, p < 0.05). Tukey HSD showed that the control and the high dose groups differed from each other (p < 0.05).
Fig. 3.
Alarm substance treatment increased erratic movement in zebrafish in a dose dependent manner. (Panel A) The percent of time zebrafish performed Erratic movement during the observation session is shown. (Panel B) The number of times (frequency) Erratic movement was performed during the observation session is shown. Mean + S.E.M. is shown. Sample sizes (n) represent the number of five-fish shoals tested and are as follows: control (zero alarm substance) = 13; low concentration = 15; medium concentration = 13; high concentration = 15. Note the quasi-linear dose dependent increase of the behavioral measure on both panels. Also note that throughout the session zebrafish could view a fish predator swimming in a stimulus tank adjacent to the observation tank. For procedural details, see Section 2. For details of the results of the statistical analysis, see Section 3.
Contrary to our expectations, the amount of freezing did not change in response to the alarm substance treatment (Fig. 4A). ANOVA revealed no significant effect of the alarm substance concentration (F(3, 47) = 0.51, p > 0.05). The percent of time the zebrafish spent freezing was very low in all groups. Analysis of the frequency of freezing (number of times it occurred) confirmed this (Fig. 4B). Although it appears that the medium alarm substance concentration group showed freezing somewhat more frequently, the number of times this behavior occurred was very low in all groups and ANOVA found no significant effect of the alarm substance treatment (F(3, 47) = 1.05, p > 0.05).
Fig. 4.
Alarm substance treatment did not significantly change freezing (immobility) in zebrafish. (Panel A) The percent of time zebrafish performed freezing during the observation session is shown. (Panel B) The number of times (frequency) freezing was performed during the observation session is shown. Mean + S.E.M. is shown. Sample sizes (n) represent the number of five-fish shoals tested and are as follows: control (zero alarm substance) = 13; low concentration = 15; medium concentration = 13; high concentration = 15. Note that freezing appeared only for very short duration of time and very infrequently (see scale of the Y axis on Panels A and B, respectively). Also note that throughout the session zebrafish could view a fish predator swimming in a stimulus tank adjacent to the observation tank. For procedural details, see Section 2. For details of the results of the statistical analysis, see Section 3.
In the second set of experiments we asked the question whether the presence or absence of a live predator influenced the fear inducing effects of the alarm substance treatment. Variance analysis revealed that the alarm dose (high vs. zero) had a close to significant effect (F(3, 34) = 2.10, p = 0.056) on the amount of time the members of the five-zebrafish shoal spent in the bottom quarter of the observation tank (Fig. 5), but the predator treatment had no effect (F(1, 34) = 1.33, p > 0.05), and there was no interaction between alarm dose and predator treatment (F(1, 34) = 1.05, p > 0.05).
Fig. 5.
Alarm substance treatment had a marginally significant effect on the duration of time zebrafish spent near the bottom of their observation tank but the presence or absence of the predator had no significant effect. Mean + S.E.M. is shown. Sample sizes (n) represent the number of five-fish shoals tested and are as follows: control (zero alarm substance) with predator present = 10; control (zero alarm substance) with predator absent = 10; high concentration alarm substance with predator present = 10; high concentration alarm substance with predator absent = 10. Note that the figure shows the average of the percent of time each zebrafish of the five-fish shoal spent in the lowest quarter of the tank during the 7 min observation session. Also note that zebrafish in the ‘predator present’ groups could not only view a fish predator but could also use their olfaction hearing and lateral line to detect its presence. For procedural details see Section 2 and for details of the results of the statistical analysis see Section 3.
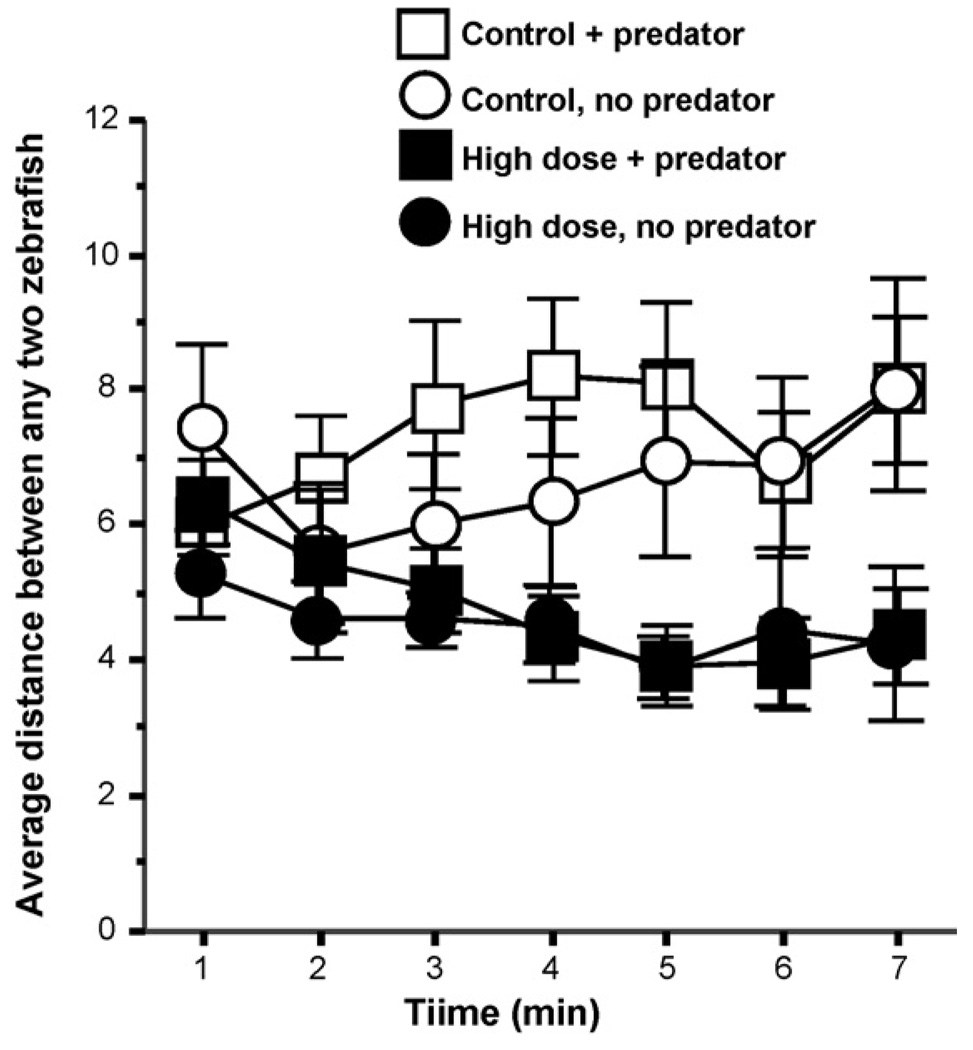
Shoal cohesion (Fig. 6) was affected by the alarm substance treatment. Variance analysis found a significant alarm substance dose effect (F(1, 34) = 5.13, p < 0.05). But again, the effect of predator was non-significant (F(1, 34) = 0.33, p > 0.05). The effect of interval was also non-significant (F(6, 204) = 1.11, p > 0.05) but the alarm substance dose × interval interaction was significant (F(6, 204) = 4.35, p < 0.001). The other interaction terms including the predator × interval and the alarm substance dose × predator × interval triple interaction was non-significant. Tukey HSD post hoc comparisons as well as Fig. 6 demonstrate that the distance of fish within the shoal treated with the high concentration of alarm substance was significantly (p < 0.05) smaller by the fifth minute of the recording session as compared to the control (0 alarm substance treatment) groups irrespective of whether the predator was present or absent in the observation tank of the zebrafish.
Fig. 6.
Alarm substance treatment significantly reduced the distance among zebrafish irrespective of the presence or absence of the predator. Mean + S.E.M. is shown. Sample sizes (n) represent the number of five-fish shoals tested and are as follows: control (zero alarm substance) with predator present = 10; control (zero alarm substance) with predator absent = 10; high concentration alarm substance with predator present = 10; high concentration alarm substance with predator absent = 10. The distance among shoal mates is calculated as the average of distances, expressed in cm, between all possible pairs of zebrafish of the shoal. Note that with time, the difference between the alarm substance treated and the control groups increased: the former showed decreased distance among shoal mates and the latter did not. Also note that despite that the experimental zebrafish had full access to cues of all modalities about the predator, the presence or absence of the predator had no significant effect. For procedural details, see Section 2. For details of the results of the statistical analysis, see Section 2.
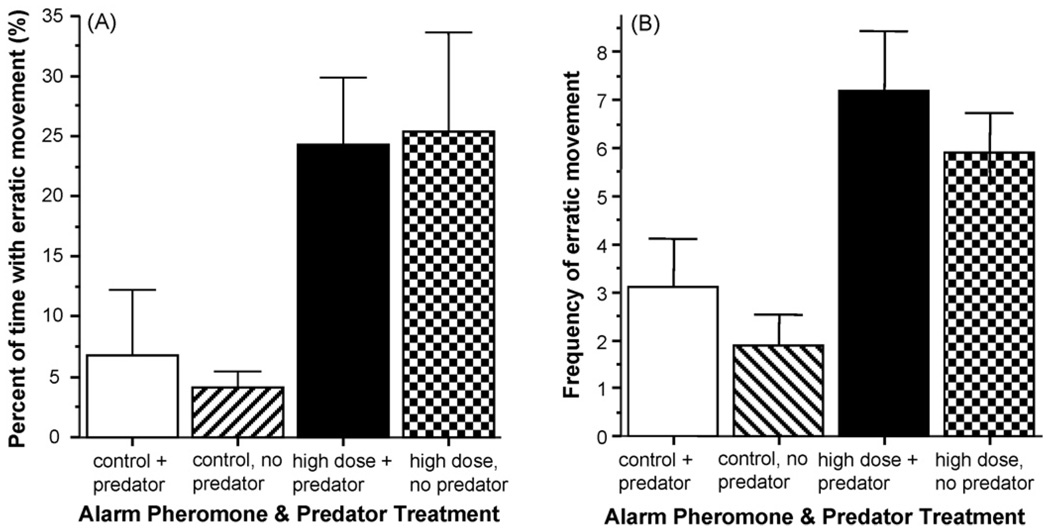
Analysis of the amount (percent of duration relative to session length) of erratic movement (Fig. 7) also demonstrated that while the alarm substance had a significant fear inducing effect (ANOVAF(1, 34) = 26.58, p < 0.001), the presence or absence of the predator had no significant effect (ANOVA F(1, 34) = 0.20, p > 0.05) and there was no interaction between the alarm substance and predator factors (ANOVA F(1, 34) = 0.10, p > 0.05). The results for the frequency of erratic movement are practically the same as for the relative duration (Fig. 7B). Variance analysis showed a significant alarm substance effect (F(1, 34) = 17.36, p < 0.001), no predator effect (F(1, 34) = 1.68, p > 0.05) and no interaction between these factors (F(1, 34) = 0.002, p > 0.05).
Fig. 7.
Alarm substance treatment significantly increased Erratic movement in zebrafish. (Panel A) The percent of time zebrafish performed Erratic movement during the observation session is shown. (Panel B) The number of times (frequency) erratic movement was performed during the observation session is shown. Mean + S.E.M. is shown. Sample sizes (n) represent the number of five-fish shoals tested and are as follows: control (zero alarm substance) with predator present = 10; control (zero alarm substance) with predator absent = 10; high concentration alarm substance with predator present = 10; high concentration alarm substance with predator absent = 10. Note the robust increase in duration as well as frequency of Erratic movement in response to the alarm substance treatment as compared to control. Also note that despite that the experimental zebrafish had full access to cues of all modalities about the predator, the presence or absence of the predator had no significant effect. For procedural details, see Section 2. For details of the results of the statistical analysis, see Section 3.
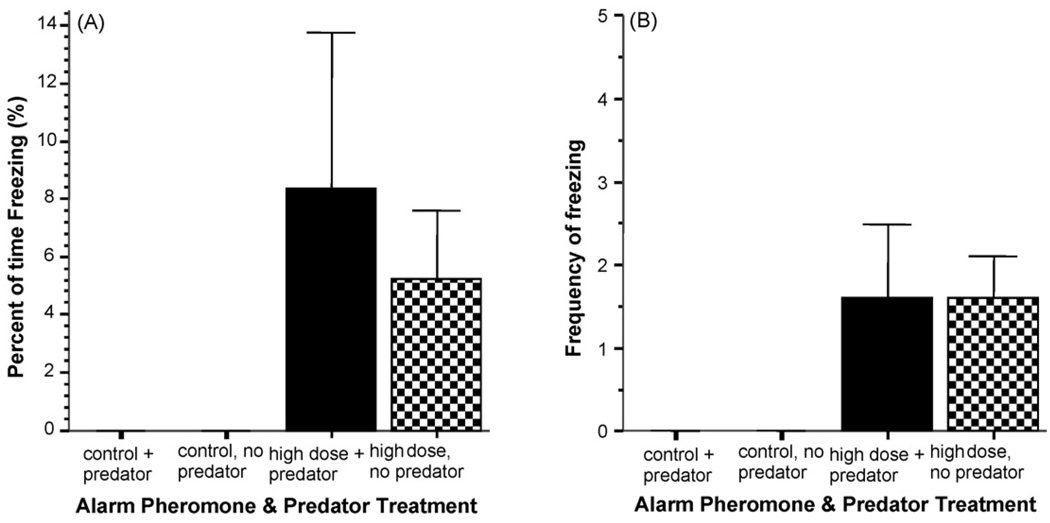
Although similarly to the first experiment freezing occurred rarely and for a short period of time, significant alarm substance treatment was revealed (Fig. 8A) for the duration of this behavior (ANOVA F(1, 34) = 4.73, p < 0.05). The presence or absence of the predator had no significant effect (F(1, 34) = 0.25, p > 0.05) and the interaction between these factors was also non-significant (F(1, 34) = 0.25, p > 0.05). The analysis of the frequency of freezing (Fig. 8B) showed the same results. Although the frequency of freezing was low, a significant alarm substance effect (F(1, 34) = 8.70, p < 0.01) and no significant effect of the predator (F(1, 34) = 0.00, p > 0.05) and no significant interaction (F(1, 34) = 0.00, p > 0.05) was found.
Fig. 8.
Alarm substance treatment significantly increased freezing in zebrafish. (Panel A) The percent of time zebrafish performed freezing during the observation session is shown. (Panel B) The number of times (frequency) freezing was performed during the observation session is shown. Mean + S.E.M. is shown. Sample sizes (n) represent the number of five-fish shoals tested and are as follows: control (zero alarm substance) with predator present = 10; control (zero alarm substance) with predator absent = 10; high concentration alarm substance with predator present = 10; high concentration alarm substance with predator absent = 10. Note the increase in duration as well as frequency of freezing in response to the alarm substance treatment as compared to control but also note that the presence or absence of the predator had no significant effect. For procedural details, see Section 2. For details of the results of the statistical analysis, see Section 3.
4. Discussion
Zebrafish significantly changed their behavior in response to the presentation of alarm substance. Most robust among these changes was the increase of erratic movement. Erratic movement is a stereotypical zig-zagging behavior that has been described in fish species in situations when pain [10] or fear inducing stimuli [18,19] were presented to the experimental subjects. The fear inducing stimuli included novelty or the appearance of predators or predator models [19]. Erratic movement occurs mainly on the bottom of the tank. The fish are slightly oriented downwards and appear to try to “drill” themselves into the bottom glass while performing the zig-zagging. In nature, zebrafish live in small creeks and lakes where the water is stagnant or moves slowly [12]. The bottom of these waters most often has a lot of sediment and is full of vegetation [12]. Although not observed in nature, erratic movement, if performed on the bottom of these waters, would stir up the debris and the cloud of dirt formed would certainly cover the fish. Thus, it is likely erratic movement is an adaptive escape reaction, an antipredatory response that is exhibited by zebrafish in the context of fear inducing stimulation. Thus, we interpret the increase of erratic movement in response to the administration of the alarm substance as an antipredatory behavior likely to represent increased fear reaction.
It is important to note the almost perfect linear dose response relationship between the different concentrations of alarm substance administered and the amount (frequency or duration) of erratic movement recorded. Although we expected that alarm substance would increase antipredatory responses, we had no assumptions about the dose response curve. One could have expected a yes or no type dichotomous response: if the substance is detected it “means” a conspecific is injured, and an antipredatory reaction should result. However, this is not what we found. One could argue that perhaps the alarm substance was not as detectable at lower concentrations as in the highest and that is the reason why less strong alarm reaction was elicited. However, it is clear from the significant changes in behavior found in response to the low and medium concentration of alarm substance that the experimental fish did detect the substance. Briefly, the responses were not all or none, but rather they were proportional to the amount of substance in the water. It is likely that in nature zebrafish exhibit the strongest alarm reaction when an injured fish is nearby and thus the concentration of alarm substance in the water detected by the neighboring fish is high. If the injury occurred farther away, the predator is unlikely to be nearby, and the smaller concentration of the alarm substance elicits a diminished alarm reaction. Also important to note that apart from the possibly adaptive aspects of this finding, the graded and dose dependent response to the alarm substance makes this response amenable to pharmacological or genetic screening. A drug or a mutation that alters sensitivity to the alarm substance or one which alters the strength or amount of antipredatory responses elicited by the substance may be discovered by quantifying these responses.
Another robust change we observed in response to the alarm substance treatment was increased shoal cohesion. The distance among members of the shoal of experimental zebrafish decreased. The response was fast, i.e. occurred within the short 7-min observation session. Although the highest alarm substance dose elicited the strongest response and the control (zero concentration) group showed the smallest group cohesion (largest distance among experimental fish), the intermediate doses gave mixed results: the low dose was almost as effective as the highest dose and the medium dose was less effective than the low dose. We do not have an explanation for this finding. Shoaling behavior is thought to be an antipredatory response [31]. Fish in a shoal represent a more difficult target because the predator’s attention is divided. However, shoaling may also serve other functions, including efficient foraging and reproduction [27]. Thus, shoal cohesion may be sensitive to factors other than those associated with fear. Furthermore, the most direct and the most effective response to the imminent presence of danger appears to be erratic movement, i.e. the breaking up of the shoal. Taken together the above, we suggest that quantification of shoal cohesion may not be the most precise and most direct measure of the response to the alarm substance treatment.
Unexpectedly, in the first set of experiments we found no effect of the alarm substance on freezing. We, and others [29], have observed that when hiding places are available, erratic movement is often followed by immobility, i.e. freezing. Under natural conditions this may serve as an effective camouflage: the debris that was stirred up by the erratically moving fish would efficiently hide the motionless prey staying on the bottom. Notably, however, there were no hiding places in our observation tank. Its bottom was clear glass and the tank was well illuminated in all areas. Thus, the environmental conditions of the test did not favor freezing and indeed this behavior occurred very infrequently and for only very short periods of time.
Similarly to freezing, the measure of bottom dwell time also did not show the expected [29] alarm substance induced changes. Zebrafish did not spend significantly more time on the bottom quarter of the observation tank when given alarm substance as compared to control. The reason why this behavioral measure was unable to detect alarm substance induced changes in behavior may be similar to the reason why freezing did not occur. The well-illuminated observation tank with its bare glass bottom may not have favored this antipredatory strategy.
Experimental analysis of the above speculations will help us develop proper quantification methods for the detection of antipredatory responses. Nevertheless, for now, erratic movement appears to be the most reliable way to quantify alarm substance induced responses. It is notable that in the current study it was measured manually, i.e. by a human observer. The resolution and reliability of human observation based behavioral quantification methods are excellent (see ref. [13] and references therein), however, they are not appropriate for screening type applications because they are time consuming and labor intensive. Briefly, automated quantification methods are a must for drug and mutation screening. We are working on detection algorhythms using videotracking systems that are expected to properly quantify erratic movement based on the speed of the movement (e.g. above 5 cm/s), the bout of the fast swim episodes (less then 10 s), and angular velocity (speed of turning above 90°/s). However, it may be easier to modify experimental conditions so that behavioral parameters simpler to measure would properly reflect the fear responses. Bottom dwell time and freezing would be easy to measure with video-tracking or photocell detectors in an automated manner. We are experimenting with illumination levels (reduced illumination) and/or darker backgrounds and presentation of hiding places (e.g. artificial plants on the bottom) to favor the above behaviors under fear inducing conditions.
Another question this paper studied was whether alarm substance alone is sufficient to induce reliable and robust alarm reactions. The main conclusion one could draw from the second set of experiments was that the presence or absence of predatory fish had no detectable effect on the efficacy of the alarm substance in zebrafish. In this set of experiments we allowed zebrafish not only to see their predator but also to perceive olfactory, auditory and lateral line cues. We argued that perhaps visual cues were not sufficient but when all cues were available to the prey the predator must enhance the effect of the alarm substance. Contrary to our expectations, we found no such effects. While the high dose of the alarm substance showed a robust effect on shoaling (increased shoal cohesion) and on erratic movement (increased duration and frequency), the presence or absence of a predatory fish made no difference with regard to these responses. Importantly, this study was not designed to detect finer effects of predatory fish or the potentially differential effects of a natural predator [1] but the results clearly demonstrate that presentation of the alarm substance alone is sufficient to induce significant and robust alarm reactions.
Interestingly, in this set of experiments the alarm substance was effective to increase freezing, and changes in bottom dwell time were also close to significant. The method of alarm substance extraction was the same in the two experiments, i.e. all procedural details were strictly followed in both. However, the exact amount of alarm substance extracted could not be ascertained and may have varied. This could in principle account for differences in the result. It is notable that the control fish also behaved somewhat differently in the second set of experiments as compared to the first set. This difference cannot be accounted for by alarm substance concentration differences but rather may be due to the fact that in the first set of experiments the control fish could only view the predator but in the second set they either had no predator or had access to cues of all modalities. Irrespective of the exact explanation, however, the results of the second experiment also confirm that in the current experimental set up the most reliable measure of alarm substance induced behavioral changes is the increased amount of erratic movement performed.
The last point we would like to consider is the identification of the alarm substance. Without the knowledge of the chemical composition of the zebrafish alarm substance it may not be possible to precisely manipulate the experimental fish, and the variability inherent in fresh-extraction of the substance may pose problems. Although the exact chemical makeup of the alarm substance has not been identified for zebrafish, alarm substances are known to contain pterin and purine components [36]. Von Frisch [14] believed that alarm substance induced antipredatory responses were characteristic of schooling fish and particularly of Cyprinids. Subsequent studies have shown, however, that alarm responses elicited by the alarm substance occur actually in wider range of species belonging to the super-order Ostariophysi [29]. The prototypical Ostariophysan alarm substance has been identified as hypoxanthine-3-N-oxide, or H3NO [7,30]. This synthetic substance has been shown to share some typical chemical characteristics with the alarm substances of a number of Ostariophysi species, and to be as effective as their skin extracts [6]. Currently, it is not known whether alarm substances identified in other Ostariophysi species or H3NO are equally potent in zebrafish. Nevertheless, H3NO may be effective in eliciting alarm reactions in this species too. This possibility is likely given the empirical data showing cross-species effectiveness of this substance [6,7] and given the argument that narrow selectivity for a particular species-specific alarm substance may be maladaptive in nature. The question of how zebrafish respond to skin extracts of other fish species and, more importantly, to H3NO will be systematically analyzed in the future.
In summary, the findings of the current study suggest that alarm substance treatment is effective in inducing fear responses in zebrafish and these responses are quantifiable using manual observation-based as well as computerized video-tracking-based methods. However, the results also suggest that some aspects of the employed behavioral methods may need to be systematically explored and perhaps refined before this natural behavioral response may be exploited for high throughput screening purposes. These include the development of reliable automated behavioral quantification of alarm reactions and the confirmation of the efficacy of a synthetic alarm substance. Once the above is accomplished, it is likely that zebrafish will be a useful screening tool and model organism for the analysis of fear in vertebrates and anxiety in humans.
Acknowledgements
We would like to thank S. Prajapatti, J. Mella, R. Khrishnannair, M. Sison & C. Buske for their technical help. Supported by NSERC (#311637-06) and NIH/NIAAA (#1R01AA015325-01A2) grants to RG.
References
- 1.Bass SLS, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: the effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 2.Berghmans S, Hunt J, Roach A, Goldsmith P. Zebrafish offer the potential for a primary screen to identify a wide variety of potential anticonvulsants. Epilepsy Res. 2007;75:18–28. doi: 10.1016/j.eplepsyres.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Blanchard DC, Griebel G, Blanchard RJ. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog Neuropsychopharmacol Biol Psychiatr. 2003;27:1177–1185. doi: 10.1016/j.pnpbp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Blaser R, Gerlai R. Behavioral phenotyping in zebrafish: comparison of three behavioral quantification methods. Behav Res Meth. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 5.Bracha HS. Freeze, flight, fight, fright, faint: adaptationist perspectives on the acute stress response spectrum. CNS Spectr. 2004;9:679–685. doi: 10.1017/s1092852900001954. [DOI] [PubMed] [Google Scholar]
- 6.Brown GE, Adrian JC, Jr, Naderi NT, Harvey MC, Kelly JM. Nitrogen oxides elicit antipredator responses in juvenile channel catfish, but not in convict cichlids or rainbow trout: conservation of the ostariophysan alarm substance. J Chem Ecol. 2003;8:1781–1796. doi: 10.1023/a:1024894026641. [DOI] [PubMed] [Google Scholar]
- 7.Brown GE, Adrian JC, Jr, Smyth E, Leet H, Brennan S. Ostariophysan alarm substances: laboratory and field tests of the functional significance of nitrogen oxides. J Chem Ecol. 2000;26:139–154. [Google Scholar]
- 8.Burger J. Antipredator behaviour of hatchling snakes: effects of incubation temperature and simulated predators. Anim Behav. 1998;56:547–553. doi: 10.1006/anbe.1998.0809. [DOI] [PubMed] [Google Scholar]
- 9.Choy Y, Fyer AJ, Goodwin RD. Specific phobia and comorbid depression: a Closer look at the National Comorbidity Survey data. Comp Psychiatr. 2007;48:132–136. doi: 10.1016/j.comppsych.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Csányi V, Gervai J. Behavior-genetic analysis of the paradise fish, Macropodus opercularis. II. Passive avoidance learning in inbred strains. Behav Genet. 1986;16:553–537. doi: 10.1007/BF01066341. [DOI] [PubMed] [Google Scholar]
- 11.Delaney M, Follet C, Ryan N, Hanney N, Lusk-Yablick J, Gerlach G. Social interaction and distribution of female Zebrafish (Danio rerio) in a large aquarium. Biol Bull. 2002;203:240–241. doi: 10.2307/1543418. [DOI] [PubMed] [Google Scholar]
- 12.Engeszer R, Patterson L, Rao A, Parichy D. Zebrafish in the wild: a review of natural history and new notes from the field. Marry Ann Liebert Inc. 2007;4:21–40. doi: 10.1089/zeb.2006.9997. [DOI] [PubMed] [Google Scholar]
- 13.Fitch T, Adams B, Chaney S, Gerlai R. Force transducer-based movement detection in fear conditioning in mice: a comparative analysis. Hippocampus. 2002;12:4–17. doi: 10.1002/hipo.10009. [DOI] [PubMed] [Google Scholar]
- 14.Frisch Kvon. Uber einen schreckstoff der fischhaut und seine biologische Bedeutung. Z vergl Physiol. 1941;29:46–145. [Google Scholar]
- 15.Phenomics: fiction or the future? Trends Neurosci. 2002;25:506–509. doi: 10.1016/s0166-2236(02)02250-6. [DOI] [PubMed] [Google Scholar]
- 16.Gerlai R. Zebra fish: an uncharted behavior genetic model. Behav Genet. 2003;33:461–468. doi: 10.1023/a:1025762314250. [DOI] [PubMed] [Google Scholar]
- 17.Gerlai R, Clayton NS. Analysing hippocampal function in transgenic mice: an ethological perspective. Trends Neurosci. 1999;22:47–51. doi: 10.1016/s0166-2236(98)01346-0. [DOI] [PubMed] [Google Scholar]
- 18.Gerlai R, Csányi V. Genotype environment interaction and the correlation structure of behavioral elements in paradise fish (Macropodus opercularis) Physiol Behav. 1990;47:343–356. doi: 10.1016/0031-9384(90)90153-u. [DOI] [PubMed] [Google Scholar]
- 19.Gerlai R, Lahav S, Guo, Rosenthal A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 20.Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacol Biochem Behav. 2006;85:752–761. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gervai J, Csanyi V. Behavior-genetic analysis of the paradise fish, Macropodus opercularis. I. Characterization of the behavioral responses of inbred strains in novel environments: a factor analysis. Behav Genet. 1985;15:503–519. doi: 10.1007/BF01065447. [DOI] [PubMed] [Google Scholar]
- 22.Grunwald DJ, Eisen JS. Timeline: headwaters of the zebrafish—emergence of a new model vertebrate. Nat Rev Genet. 2002;3(9):717–724. doi: 10.1038/nrg892. [DOI] [PubMed] [Google Scholar]
- 23.Hall D, Suboski MD. Visual and olfactory stimuli in learned release of alarm reactions by zebra danio fish (Brachydanio rerio) Neurobiol Learn Mem. 1995;63:229–240. doi: 10.1006/nlme.1995.1027. [DOI] [PubMed] [Google Scholar]
- 24.Hendrie CA, Weiss SM, Eilam D. Exploration and predation models of anxiety: evidence from laboratory and wild species. Pharmacol Biochem Behav. 1996;54:13. doi: 10.1016/0091-3057(95)02176-0. [DOI] [PubMed] [Google Scholar]
- 25.Laurila A, Pakkasmaa S, Merila J. Population divergence in growth rate and antipredator defences in Rana arvalis. Oecologia. 2006;147:585–595. doi: 10.1007/s00442-005-0301-3. [DOI] [PubMed] [Google Scholar]
- 26.Miklósi A, Csányi V, Gerlai R. Antipredator behavior in paradise fish (Macropodus opercularis) larvae: the role of genetic factors and paternal influence. Behav Genet. 1997;27:191–200. doi: 10.1023/a:1025601828464. [DOI] [PubMed] [Google Scholar]
- 27.Miller N, Gerlai R. Quantification of shoaling behaviour in zebrafish (Danio rerio) Behav Brain Res. 2007;184:157–166. doi: 10.1016/j.bbr.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Palleroni A, Hauser M, Marler P. Do responses of galliform birds vary adaptively with predator size? Anim Cogn. 2005;8:200–210. doi: 10.1007/s10071-004-0250-y. [DOI] [PubMed] [Google Scholar]
- 29.Pfeiffer W. The distribution of fright reaction and alarm substance cells in fishes. Copeia. 1977;4:653–665. [Google Scholar]
- 30.Pfeiffer W, Riegelbauer G, Meier G, Scheibler B. Effect of hypoxanthine-3-N-oxide and hypoxanthine-1-N-oxide on central nervous excitation of the black tetra, Gymnocorymbus ternetzi (Characaidae, Ostariophysi, Pisces) indicated by dorsal light response. J Chem Ecol. 1985;11:507–523. doi: 10.1007/BF00989562. [DOI] [PubMed] [Google Scholar]
- 31.Queiroz H, Magurran AE. Safety in numbers? Shoaling behaviour of the Amazonian red-bellied piranha. Biol Lett. 2005;22:155–157. doi: 10.1098/rsbl.2004.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Searcy YM, Caine NG. Hawk calls elicit alarm and defensive reactions in captive Geoffroy’s marmosets (Callithrix geoffroyi) Folia Primatol (Basel) 2003;74:115–125. doi: 10.1159/000070645. [DOI] [PubMed] [Google Scholar]
- 33.Sison M, Cawker J, Buske C, Gerlai R. Fishing for genes influencing vertebrate behavior: zebrafish making headway. Lab Anim. 2006;35:33–39. doi: 10.1038/laban0506-33. [DOI] [PubMed] [Google Scholar]
- 34.Smith ME, Belk MC. Risk assessment in western mosquitofish (Gambusia affinis): do multiple cues have additive effects? Behav Ecol Sociobiol. 2001;51:101–101. [Google Scholar]
- 35.Tyrer P, Baldwin D. Generalised anxiety disorder. Lancet. 2006;16:2156–2166. doi: 10.1016/S0140-6736(06)69865-6. [DOI] [PubMed] [Google Scholar]
- 36.Waldman B. Quantitative and developmental analysis of the alarm reaction in the zebra Danio Brachydanio rerio. Copeia. 1982;1:1–9. [Google Scholar]
- 37.Westerfield M. A guide for the laboratory use of zebrafish (Danio rerio) 4th ed. Eugene: Univ. of Oregon Press; 2000. The zebrafish book. [Google Scholar]